Abstract
Nowadays, sewage sludge and water bodies are subjected to heavy pollution due to rapid population growth and urbanization. Heavy metal pollution represents one of the main challenges threatening our environment and the ecosystem. The present work aims to evaluate the contamination state of the sewage sludge and lake sediments in the Republic of Benin. Twenty metallic elements including 15 rare earth elements (Eu, Sb, Cs, Nd, Pr, Gd, La, Ce, Tb, Sm, Dy, Ho, Eu, Yb, and Lu) and five precious elements (Ag, Au, Pd, Pt, and Ru) were investigated using inductive plasma-mass spectrometry. Results showed broad range concentrations of the elements. Ce, La, and Nd were present in both sediments and sewage sludge at concentrations ranging 5.80–41.30 mg/kg dry matter (DM), 3.23–15.60 mg/kg DM, and 2.74–19.26 mg/kg DM, respectively. Pr, Sm, Gd, Tb, Dy, Eu, Er, Yb, Cs, Ho, and Tm concentrations were lower (0.02–5.94 mg/kg DM). Among precious elements, Ag was detected at the highest concentration in all sites (0.43–4.72 mg/kg DM), followed by Pd (0.20–0.57 mg/kg DM) and Au (0.01–0.57 mg/kg DM). Ru and Pt concentrations were < 0.20 mg/kg DM in all samples. Pollution indices and enrichment factor indicated a strong to severe enrichment of the elements, mainly Ce and precious elements in both sediments and sewage sludge. This revealed a growing anthropogenic input which was also implied by principal component analysis. The evaluation of pollution loading index (PLI) indicated a moderate to strong contamination (0.12 ≤ PLI ≤ 0.58; 37 ≤ PLI ≤ 114, respectively, for rare earth elements and precious elements), while the degree of contamination indicated a moderate polymetallic contamination for rare earth elements and significant contamination for precious elements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid and liquid wastes management is a real issue for developing countries. For years, these countries have paid little attention to their natural environment. The observation today is particularly dramatic for aquatic ecosystems. Wastes, which are continually discharged into the water bodies, deposit mostly at the bottom of the river. Sediments constitute a privileged biotope to investigate the state of pollution of an aquatic system. They represent a privileged place where the organic matter is transformed and where trace elements accumulate. A significant fraction of elements present in aquatic environments is reversibly associated with surface sediments. Changes in the physicochemical conditions of the environment can lead to remobilizations of elements associated with sediments (Agarwal et al. 2005) and consequently pose very high risks for organisms living in aquatic ecosystems and for the final consumer, human (Amiard-Triquet 1989; Hope 2006; Yi et al. 2011). The origin of metal pollution is one of major environmental challenges worldwide. Indeed, the concentration of most of these contaminants sometimes reach toxic levels for aquatic life in both water and sediments (Fakayode 2005; Monferran et al. 2016). Physicochemical analysis of lake sediment, wastewater, and sludge is essential to study anthropogenic impacts on ecosystems and to assess the risks from human wastes.
In Africa, particularly in the Republic of Benin, wastewater is mostly discharged into water bodies without any pre-treatment. Nokoué Lake and lagoon in Porto-Novo town are subjected to intensive discharges from hospital effluent, inadequate disposal of expired and/or adulterated drugs from Nigeria, and the dumping of similar wastes (Chouti et al. 2010). Nowadays, there is almost no data available to monitor the pollution level of the lake and sewage sludge in the Republic of Benin. In order to evaluate the quality of sediments and sewage sludge and preserve the Lake, after our preliminary investigation on 22 metallic elements, mostly heavy elements (Suanon et al. under review), we proposed in this study to further evaluate the occurrence and contents of 20 metallic elements including 15 rare earth elements Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu and five precious metals Ag, Au, Pd, Pt, and Ru. We investigated not only in the sediments of the Lake Nokoué but also in the sewage sludge of SIBEAU, the only one wastewater treatment plant (WWTP) in the Republic of Benin. Specifically, we determined the element concentrations in both sewage sludge and lake sediments and evaluated the level of contamination/metal pollution based on the calculation of certain parameters such as the enrichment factor (EF), degree of contamination (DC), and the pollution loading index (PLI).
Materials and methods
Sampling and study area
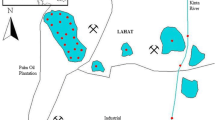
Sludge and sediment samples were taken in June 2016 from 11 sites in Nokoué Lake and wastewater treatment plant in Cotonou town (department of Littoral) and Sèmè-Kpodji (department of Ouémé) (Fig. 1) (Suanon et al. under review). H1, H2, LN1, LN2, LN3, and LN4 are lake’s sediments. H1 and H2 are sites close to the wastewater stream from hospitals (Hospital 1 and Hospital 2). S1, S2, S3, and S4 are treated sewage sludge samples taken from four different discharge sites from SIBEAU station. SS is the soil sample collected by the sea where the treated wastewater from the SIBEAU station is discharged by direct connection. Sediment samples were collected at approximately 5 to 10 cm depth from the surface using cylindrical PVC pipes (2.5 cm of diameter and 3 m long). These pipes can be lengthened by connecting them via joints. Collected sediments were packaged in 50 mL brown colored bottles and sent to the Institute of Urban Environment, Chinese Academy of Sciences for treatment and analysis. During sampling, pH, temperature (T) and electrical conductivity (EC) were measured in situ. The EC and T were measured via a portable multi-parameter (Hach Sension 156) and the pH is measured using a pH meter (WTW pH 3110).
Analytical techniques
Prior to analysis in the laboratory, the sediment and sludge samples were preserved under − 80 °C. The samples were introduced into the lyophilizator (Beijing boyikang Laboratory Instrument Co. Ltd.) and the temperature was set at − 50 °C. After complete drying of samples after 60 h, the samples were removed from the freeze-drying system and then grounded in an agate mortar and sieved (diameter < 2 mm). Elemental analysis: total carbon (C), nitrogen (N), and sulfur (S) contents were determined by pyrolysis on the powdered samples using a macro elemental analyzer (CHNS/O Vario MAX, Elementar, Germany). The calibration of the apparatus was carried out with a soil standard and pyrolysis was carried out at 950 °C. As for the organic matter, it was determined by weight loss via ignition/calcination of samples at 500 °C for 24 h.
For metal concentration determination, the sediment and sludge samples were digested in a previously decontaminated polytetrafluoroethylene tube by washing with hot aqua regia (3:1 mixture of HNO3: HCl) at 90 °C under a microwave MARS 5 for 2 h as recommended by US Standard (US-EPA 3051A). One hundred milligram of the sample (sludge/sediment) was weighted and introduced into the digestion tube with 12 mL of freshly prepared aqua regia. The mixture is placed under microwave for hot digestion at 180 °C and under high pressure according to the US standard (US-EPA 3051A). The digestion program involved two steps: (i) allowing an increase of the temperature from ambient temperature to 180 °C (10 min) and (ii) then 45 min residence time. After complete digestion, samples were cooled, filtered, and collected in a 50 mL plastic centrifuge tube and then diluted to 45 mL with ultra-pure water. Samples were stored at 4 °C until analysis.
Metal detection was performed using inductive plasma-mass spectrometry (ICP-MS Agilent 7500CX). For quality control of elements extraction protocol in the sediments and sludge samples, the experiment was repeated using a certified standard sample (GBW07309, GSD-9, Inspection and Quarantine of the People’s Republic of China). Recovery was between 85.6 and 102.5%.
Pollution indices
In order to evaluate and assess the impact of pollutants in the environment, several parameters such as EF, DC, and PLI were calculated.
Enrichment factor
Elements are mainly associated with fine particles (clays, iron oxides and hydroxides, organic materials, sulphides, etc.), and therefore, it would be necessary to express the concentrations of rare earth elements and precious elements related to the sediment granulometry. For this purpose, we have chosen to normalize the concentrations of the rare earth elements measured in relation to aluminum, which is a major constituent of clay minerals and proves to be a good tracer of the fine fraction. Moreover, this element is found at high concentrations in sediments that artificial enrichment linked to anthropogenic input is less likely, and its massive diffusion to water might not occur, given that it is a refractory element. This normalization, which consists of expressing the ratio of the content of a given element relative to that of the normalizing factor, in this case aluminum, makes it possible to define the EF of a given metallic trace element in the sediment. The EF is calculated as recommended by Sakan et al. (2009) (Eq. 1):
Where [M]samp is the concentration of the metal in the sediment/sludge sample; [Al]samp is the concentration of aluminum in the sediment/sludge sample; [M]crust and [Al]crust are their respective upper crustal concentrations. The EF values were interpreted according to the level of contamination as reported by Sakan et al. (2009): FE < 1, no enrichment; 1 ~ 3, low enrichment; 3 ~ 5, moderate enrichment; 5–10, moderate to strong enrichment; 10 ~ 25, high enrichment; 25 ~ 50, very strong enrichment; and FE > 50, extreme enrichment.
Degree of contamination
In order to compare the total metal contents in different sites, the DC was calculated according to the following equation (Eq. 2) (Hakanson 1980):
Where CFm is the contamination factor of a given trace element “m” in the sediment/sludge sample. CF is defined by the normalization, expressing the ratio of the content of a given substance in the studied sample to that of the normalizing factor. This contamination factor for a metal “m” is expressed by the equation below (Eq. 3) (Lee et al. 2006):
Where C s is the concentration of a given metal in the sediment/sludge sample and B n is its corresponding background concentration in the earth crust.
Pollution loading index
The PLI makes it possible to assess the level of pollution considering all the studied elements and hence the impact of anthropogenic activities on sediment/sludge quality. It was determined according to Eq. (4) (Tomlinson et al. 1980; Yu et al. 2012; Lu et al. 2014):
In this study, n = 15 for rare earth elements and n = 5 for precious elements. According to Zhang et al. (2014), PLI = 0 means that, on average, the concentration of every element is below the background level; 0 < PLI ≤ 1 means unpolluted; 1 < PLI ≤ 2 means unpolluted to moderately polluted; 2 < PLI ≤ 3 means moderately polluted; 3 < PLI ≤ 4 means moderately to highly polluted; 4 < PLI ≤ 5 means highly polluted; and PLI > 5 means very highly polluted.
Statistical analysis
Statistical analysis including correlation and principal component analysis (PCA) were performed by SPSS 20.0 and graphical plotting was performed by Origin software version 9.0.
Result and discussion
Physicochemical characteristics
The physicochemical characterization of the studied sediment and sewage sludge samples are given in Table 1. The values provide an idea of the state of the environment during the sampling. The analysis of the table shows that the pH values were neutral to slightly alkaline. The sites under marine influence had pH values greater than 7 while the zones close by hospital stream (H1 and H2) had pH values lower than 7. The lowest temperature was obtained in the sea samples and the highest was in the sludge samples. The lake and marine waters have a relatively low temperature. The average EC measured was 2.4 mS/cm in the sediments and 4.5 mS/cm in the sewage sludge. These values are relatively higher than those of freshwater between 0.1 and 1 mS/cm as reported by Sahli et al. (2014), highlighting a higher concentration of charged particles in the sediments (Wong et al. 2001). Indeed, since the samples were collected during the rainy season, the charged particles could be leached into the water body to increase the conductivity. Moreover, according to Bouih et al. (2005), the various domestic and industrial discharges are generally rich in monovalent and divalent ions, which contribute to the elevated conductivity of sediments. These different environments are subjected to the daily discharges of urban and industrial effluents. As a result, the high conductivity values could also result from the anthropogenic origin. Unlike sediments, sludge samples are very rich in organic matter, carbon, nitrogen, and sulfur. The sludge from the treatment plant would not be recommendable for fertilization, since the value of the EC above 4 would cause an inhibitory effect on plant growth (Wong et al. 2001).
Metallic contents in sediment and sludge
Figure 2 shows the results of analyzed rare earth elements and precious elements in the sediments and sewage sludge samples. Metal concentrations in the samples were generally heterogeneous and varied depending on the metal, site, and type of samples (sediment or sludge). Data indicated that Ce, La, and Nd were the most abundant elements in the samples (5.80–41.30 mg/kg dry matter (DM), 3.23–15.60 mg/kg DM, and 2.74–19.26 mg/kg DM, respectively), followed by Pr, Sm, Gd, Tb, and Dy (0.13–5.94 mg/kg DM). Eu, Er, Yb, Cs, Ho, and Tm were detected at very low concentrations from 0.02 mg/kg DM (Tm) to 1.34 mg/kg DM (Eu). The quantification of precious elements also revealed a broad range of their contents. Ag was the most abundant precious metal detected in all sites (0.43–4.72 mg/kg DM) followed by Pd (0.20–0.57 mg/kg DM) and Au (0.01–0.57 mg/kg DM). Ru and Pt concentrations were < 0.20 mg/kg DM in all samples. The concentrations detected were comparable to those obtained in the sediments of the Hanghe River in China and relatively lower compared to those obtained in the Changjiang River sediments reported in the work of Yang et al. (2002). The sum of all detected metal contents in different sites ranged from 16.64 mg/kg DM (LN2) to 136.54 mg/kg DM (H2), while the sum was between 15.4 and 98.8 mg/kg DM when considering only rare earth elements. The higher values were all obtained in the sediments of Nokoué Lake. Those values for rare earth elements were below the reported range of 97.6–202.0 mg/kg DM found in Bohai Bay sediments in China (Zhang et al. 2014China is a highly industrialized country, and it has more electronic products than the underdeveloped country like Benin. This may explain the higher concentrations of rare earth elements recorded in the environmental samples in China.
Pollution indices
Index EF
Table 2 shows the EF values of elements in sediment and sludge samples. Results showed that all samples were enriched with the targeted rare earth elements (Cs, Ce, La, Nd, Pr, Sm, Gd, Tb, Dy, Eu, Er, Yb, Sb, Ho, and Tm) as EF > 1. Cs was the element having the highest EF between 528 and 2102 followed by terbium (Tb) with EF between 2.8 and 88. Dy, Er, Yb, Ho, and Tm had the lowest EF values (1 < EF < 3). According to classification by Sakan et al. (2009), it can be stated that all sites were extremely enriched by Cs (EF > 50). The sites S4 and SS were also extremely enriched by Tb (EF = 73.3 and 88, respectively). S1, S2, S3, LN2, and H2 were very severely enriched (25 < EF < 50) and the others were moderately enriched. Regarding Sb, the sites LN1 and S1 had a strong enrichment (FE = 12.2 and 14.7, respectively). The sites H2, LN2, LN3, LN4, and S2 showed moderate enrichment by Sb (5 < FE < 10). LN1 and SS sites were moderately enriched by Ce, La, Nd, Pr, Sm, Gd, and Tb. Moderate enrichment by Gd (FE = 5) was also noted at the sites LN3 and S1, while the sites H1, LN3, and S4 were enriched by Eu. Precious elements (Ag, Pd, Au, Ru, and Pt) also exhibited very high EF values: Ag (45 < EF < 6546), Pd (3775 < EF < 5133), Au (79 < EF < 5719), Ru (1325 < EF < 8590), and Pt (62 < EF < 394). All sites were extremely enriched with the five studied precious elements. It is generally accepted that EF < 2 reflects the natural variability inherent to the mineralogical composition of the sample. Beyond this, significant enrichment is suspected (Sutherland 2000; Hernandez et al. 2003). However, the use of the local geochemical background in the calculation of the EF leads to a reduction of this threshold to 1.5, as this approach greatly reduces the risk of natural sediment variability that could be caused by crustal values. Therefore, according to previous studies (Zhang and Liu 2002; Sakan et al. 2009; Zhang et al. 2009), an EF between 0.05 and 1.5 indicates that the metal is completely crystallized in the sediment whereas an EF greater than 1.5 suggests an anthropogenic origin. In this study, the average EFs of all rare earth elements and precious elements detected were far beyond 1.5, suggesting anthropogenic origin of rare earth elements and precious elements in sediment and sludge.
Index DC
The DC evaluated from the contamination factor is associated with four quality classes (Hakanson 1980): DC < 6 (low contamination), 6 ≤ DC < 12 (moderate contamination), 12 ≤ DC < 24 (considerable contamination), 24 ≤ DC (very high contamination). Analysis of the results in Table 3 revealed a very high polymetallic contamination for all precious elements. Indeed, these elements showed a DC far beyond the inferior limit of a high contamination (594 < DC < 2858). For rare earth elements, the DC values do not exceed the maximum level of the second class defined by Hakanson (1980), indicating moderate contamination except the site S4 where the DC is 13.9, thus indicating considerable polymetallic contamination. Generally, metal ions in wastewater and sewage sludge which end up in water bodies are originated from various anthropogenic activities (mining, metallurgical operations, burning fossil fuels, cement production, electroplating, leather tanning) and products (manufacturing plastics, fertilizers, pesticides, anticorrosive agents, Ni-Cd batteries, paints, pigments, dyes, photovoltaic devices) (Law and Gordon 1979; Sörme and Lagerkvist 2002; Rule et al. 2006; Fu and Wang 2011). These critical DC values were mainly due to cerium, lanthanum, and neodymium, whose concentrations predominated in the samples. This could be due to inputs to the studied site/environment from certain human activities (catalysis, glasses, ceramics, and also certain lamps).
Index PLI
The results of the pollution factor and the PLI are also recorded in Table 3. Results revealed that all rare earth elements have a PLI lower than 1, while the precious elements have a PLI values between 37.6 and 302.6. According to Zhang et al. (2011), these results revealed that the sampling sites were classified as unpolluted when considering rare earth elements. However, all sites were heavily contaminated with precious elements. When considering all elements combined together, the PLI values were in the range 0.54 to 2.62, and the results indicated moderate contamination of the sites (Tomlinson et al. 1980; Yu et al. 2012).
Correlation and principal components analysis
The correlation matrix is given in Table 4. An analysis of this matrix revealed a strong correlation within rare earth elements (0.65 ≤ r ≤ 0.99) and within the precious elements (0.40 ≤ r ≤ 0.78). No correlation was found between rare earth elements and precious elements. Most of the correlations observed were very significant (p < 0.01) between the elements of the same group. This would explain the possibility that these elements came from common pollution sources. On the other hand, no correlation was observed between the environmental factors and most of the studied elements, except for Tb, Ru, and Pt with which a certain correlation was noted.
PCA in Fig. 3 shows that the elements Eu, Sb, Cs, Nd, Pr, Gd, and Sm were well correlated positively with the first component (PCA1) explaining 62.83% of total variance; elements such as Pt, Au, Ag, Pd, Tb, and Ru were much more correlated with the second component (PCA2) explaining 18.17% of total variance. Referring to the EF data, the elements Pt, Au, Ag, Pd, Tb, and Ru were highly enriched in the samples. This revealed the influence of anthropogenic activities on their occurrence in the samples. In other words, anthropogenic sources would contribute more to the enrichment of Pt, Au, Ag, Pd, Tb, and Ru in the studied sediment and sludge. Consequently, the PCA1 could be interpreted as natural factors and the PCA2 could be associated with anthropogenic factors.
Conclusion
The contents of rare earth elements and precious elements in the lake sediment and urban WWTP sludge and the state of metallic pollution were assessed. It could be stated that the contamination of sediments and sewage sludge by rare earth elements was not that alarming since no calculated PLI exceeded 1. However, the values for the DC indicated moderate polymetallic contamination of the sediments and sludge regarding rare earth elements and considerable contamination when considering precious elements. The calculated EFs revealed worrisome situations in several sites for several elements. The high values of the EFs revealed an increasingly anthropogenic contamination mainly for precious elements. Although these indices give us an idea on the current status of metallic contamination in the aquatic environment, for the assessment of the actual impact of metallic contamination on the aquatic ecosystem, further biological monitoring studies will still be essential.
References
Agarwal, A., Singh, R. D., Mishra, S. K., & Bhunya, P. K. (2005). ANN-based sediment yield models for Vamsadhara river basin (India). Water SA, 31(1), 85–100.
Amiard-Triquet, C. (1989). Bioaccumulation et nocivité relatives de quelques polluants métalliques à l’égard des espèces marines. Bulletin d’Ecologie, 20(2), 129–151.
Bouih, H. B., Nassali, H., Leblans, M., & Srhiri, A. (2005). Contamination en métaux traces des sédiments du lac Fouarat (Maroc). Afrique Science: Revue Internationale des Sciences et Technologie, 1(1), 109–125.
Chouti, W., Mama, D., & Alapini, F. (2010). Etude des variations spatio-temporelles de la pollution des eaux de la lagune de Porto-Novo (sud Bénin). International Journal of Biological and Chemical Sciences, 4(4), 1017–1029.
EPA Standard 3051A. Microwave assisted acid digestion of sediments, sludge, soils, and oils. https://www.epa.gov/sites/production/files/2015-12/documents/3051a.pdf. Accessed 3/11/2017.
Fakayode, S.O. (2005). Impact of industrial effluents on water quality of the receiving Alaro River in Ibadan, Nigeria (pp. 1–13).
Fu, F., & Wang, Q. (2011). Removal of heavy metal ions from wastewaters: a review. Journal of Environmental Management, 92(3), 407–418.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control. A sedimentological approach. Water Research, 14(8), 975–1001.
Hernandez, L., Probst, A., Probst, J. L., & Ulrich, E. (2003). Heavy metal distribution in some French forest soils: evidence for atmospheric contamination. Science of the Total Environment, 312(1), 195–219.
Hope, B. K. (2006). An examination of ecological risk assessment and management practices. Environment International, 32(8), 983–995.
Law, S. L., & Gordon, G. E. (1979). Sources of elements in municipal incinerator emissions. Environmental Science & Technology, 13(4), 432–438.
Lee, C. S. L., Li, X., Shi, W., Cheung, S. C. N., & Thornton, I. (2006). Metal contamination in urban, suburban, and country park soils of Hong Kong: a study based on GIS and multivariate statistics. Science of the Total Environment, 356(1), 45–61.
Lu, X., Zhang, X., Li, L. Y., & Chen, H. (2014). Assessment of elements pollution and health risk in dust from nursery schools in Xi’an, China. Environmental Research, 128, 27–34.
Monferran, M. V., Garnero, P. L., Wunderlin, D. A., & de los Angeles Bistoni, M. (2016). Potential human health risks from elements and As via Odontesthes bonariensis consumption and ecological risk assessments in a eutrophic lake. Ecotoxicology and Environmental Safety, 129, 302–310.
Rule, K. L., Comber, S. D. W., Ross, D., Thornton, A., Makropoulos, C. K., & Rautiu, R. (2006). Diffuse sources of heavy elements entering an urban wastewater catchment. Chemosphere, 63(1), 64–72.
Sahli, L., El Okki, M. E. H., Afri-Mehennaoui, F. Z., & Mehennaoui, S. (2014). Utilisation d’indices pour l’evaluation de la qualite des sediments: cas du Bassin Boumerzoug (Algerie). European Scientific Journal, 10(35), 333–343.
Sakan, S. M., Đorđević, D. S., Manojlović, D. D., & Predrag, P. S. (2009). Assessment of heavy metal pollutants accumulation in the Tisza river sediments. Journal of Environmental Management, 90(11), 3382–3390.
Sörme, L., & Lagerkvist, R. (2002). Sources of heavy elements in urban wastewater in Stockholm. Science of the Total Environment, 298(1), 131–145.
Sutherland, R. A. (2000). Bed sediment-associated trace elements in an urban stream, Oahu, Hawaii. Environmental Geology, 39(6), 611–627.
Tomlinson, D. L., Wilson, J. G., Harris, C. R., & Jeffrey, D. W. (1980). Problems in the assessment of heavymetal levels in estuaries and the formation of a pollution index. Helgoland Marine Research, 33(1–4), 566–575.
Wong, J.W.C., Mak, K.F., Chan, N.W., Lam, A., Fang, M., Zhou, L.X., … & Liao, X.D. (2001). Co-composting of soybean residues and leaves in Hong Kong. Bioresource Technology, 76(2), 99–106.
Yang, S. Y., Jung, H. S., Choi, M. S., & Li, C. X. (2002). The rare earth element compositions of the Changjiang (Yangtze) and Huanghe (Yellow) river sediments. Earth and Planetary Science Letters, 201(2), 407–419.
Yi, Y., Yang, Z., & Zhang, S. (2011). Ecological risk assessment of heavy elements in sediment and human health risk assessment of heavy elements in fishes in the middle and lower reaches of the Yangtze River basin. Environmental Pollution, 159(10), 2575–2585.
Yu, S., Zhu, Y. G., & Li, X. D. (2012). Trace metal contamination in urban soils of China. Science of the Total Environment, 421, 17–30.
Zhang, J., & Liu, C. L. (2002). Riverine composition and estuarine geochemistry of particulate elements in China—weathering features, anthropogenic impact and chemical fluxes. Estuarine, Coastal and Shelf Science, 54(6), 1051–1070.
Zhang, W., Feng, H., Chang, J., Qu, J., Xie, H., & Yu, L. (2009). Heavy metal contamination in surface sediments of Yangtze River intertidal zone: an assessment from different indexes. Environmental Pollution, 157(5), 1533–1543.
Zhang, C., Qiao, Q., Piper, J. D., & Huang, B. (2011). Assessment of heavy metal pollution from a Fe-smelting plant in urban river sediments using environ-mental magnetic and geochemical methods. Environmental Pollution, 159, 3057–3070.
Zhang, Y., Gao, X., & Chen, C. T. A. (2014). Rare earth elements in intertidal sediments of Bohai Bay, China: Concentration, fractionation and the influence of sediment texture. Ecotoxicology and Environmental Safety, 105, 72–79.
Funding
This work was funded by Hundred Talents Program of Fujian Province, China, National Natural Science Foundation of China (31370503, 41673099), STS Project of Fujian-CAS (2016 T3004). The authors also acknowledged the support of the Ministry of Higher Education and Scientific Research of the Republic of Benin.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yessoufou, A., Ifon, B.E., Suanon, F. et al. Rare earth and precious elements in the urban sewage sludge and lake surface sediments under anthropogenic influence in the Republic of Benin. Environ Monit Assess 189, 625 (2017). https://doi.org/10.1007/s10661-017-6331-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-6331-6