Abstract
The present work focuses on total concentrations of trace elements such as chromium (Cr), manganese (Mn), cobalt (Co), nickel (Ni), copper (Cu), zinc (Zn), arsenic (As), selenium (Se), cadmium (Cd), and lead (Pb) and their operationally defined speciation in surface sediments from three Ethiopian Rift Valley Lakes (Hawassa, Koka, and Ziway). Total concentrations were determined using inductively coupled plasma–mass spectrometer (ICP-MS). A six-step sequential extraction was also applied to evaluate the mobilization potential of these elements. Compared with the consensus-based sediment quality guidelines (SQGs) developed for freshwater ecosystems, the total Zn concentration in sediments from Lakes Hawassa and Ziway, as well as Cr, Ni, and Zn in sediments from Lake Koka, was higher than the probable effect concentration (PEC), suggesting that these elements could have a negative effect on benthic organisms. The sequential extraction results showed that Cd, Mn, and Se were to a certain extent associated with the reversible fractions (F1–F3) in sediments, and thereby more mobile than the other investigated trace elements. Predictions of mobility of trace elements using the distribution coefficients (Kd) values were also in agreement with the sequential extraction results. Furthermore, multivariate statistical analyses showed that redox-sensitive fractions (F4 and F6) and the residual fraction (F7) were the dominant factors controlling most trace element mobility. Finally, applying the Risk Assessment Code (RAC) classification system, results showed that Mn in Lake Koka could pose a high risk, while Cd and Mn in Lake Hawassa as well as Cd, Mn, and Se in Lake Ziway sediments could pose medium risk to benthic organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Ethiopian Rift Valley Lakes (ERVL), located in the Great East Africa Rift Valley (GEARV), are considered to be very important for the economy of the region, to the livelihood of the local population, and to the biodiversity conservation of the water bodies. The lakes have mostly been used for commercial fisheries, irrigation, recreation, and some domestic purposes (Gebre-Mariam & Desta, 2002; Zinabu, 1998). However, due to increased human activities, there is a concern that severe contamination of these scarce freshwater resources could go unnoticed and eventually lead to irreversible degradation. There are already indications that long-term changes have occurred in the water qualities of some of these lakes (Zinabu et al., 2002). These changes have mainly been attributed to human activities such as intensive agriculture in the watersheds of the lakes and land use and modification as well as municipal and industrial discharges into the lakes and their inflows (Gebre-Mariam & Desta, 2002; Zinabu, 1998).
Evaluation of trace element levels in water, sediment, and biota is an essential component of environmental monitoring and assessment. For the ERVL considered in this study (Hawassa, Koka, and Ziway), information on the total concentrations of trace elements in water (Masresha et al., 2011; Zinabu & Pearce, 2003), aquatic macrophytes (Amare et al., 2014; Kassaye et al., 2016), and different organs of selected fish species (Ataro et al., 2003; Deribe et al., 2014; Desta et al., 2006, 2007, 2008; Dsikowitzky et al., 2013; Kebede & Wondimu, 2004; Samuel et al., 2020; Sorsa et al., 2016; Tadiso et al., 2011; Tessema et al., 2020) have been documented. Speciation of selected trace elements in the lake waters has also been studied (Masresha et al., 2011). Besides, total concentrations of selected trace elements in sediments from Lake Hawassa (Amare et al., 2014; Yohannes et al., 2013), and from Lakes Hawassa and Ziway (Mekonnen et al., 2012, 2015) have also been studied. However, proper assessment of trace elements in lake sediments requires determination of not only their total concentrations, but also of their different geochemical associations controlling their potential mobility and bioavailability (Fytianos & Lourantou, 2004; Gibbs, 1973; Hass & Fine, 2010; Tessier & Campbell, 1987; Tessier et al., 1979).
In this regard, to the best of our knowledge, speciation studies of trace elements in sediments from ERVL in general and Lakes Hawassa, Koka, and Ziway in particular have not been reported to date. Therefore, the main objective of the present study was to apply chemical sequential extractions to assess the mobility, bioavailability, and possible environmental risks associated with selected trace elements in surface sediments from these three freshwater lakes of the Ethiopian rift valley.
Materials and methods
Description of study area
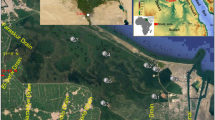
The study area included three lakes (Hawassa, Koka, and Ziway) located in the ERVL region (Fig. 1). The Ethiopian Rift Valley extends over 750 km from the southwest to the northeast and is dominated by acidic silica-rich (rhyolites, ignimbrites, pumices and ash) volcanic rocks (Gizaw, 1996).
Lake Hawassa (lat: 6°58′–7°8′N; long: 38°22′–38°27′E) is a terminal lake with no visible surface outflow. It is located at an altitude of 1680 m above sea level (a.s.l), and has a maximum depth of about 22 m (Wood & Talling, 1988) with surface and catchment areas of 90 and 1250 km2, respectively (Kebede et al., 1994). Lake Hawassa is fed by the River Tikur Wuha, the only perennial inflow, which drains a swampy wetland—a remnant of the former old Lake Shallo (Makin et al., 1975). The Hawassa Industrial Zone which has a cluster of factories, including a textile factory, is situated upstream of the lake and the effluents from these factories are discharged into the swampy area from which River Tikurwhua originates (Gebre-Mariam & Desta, 2002; Samuel et al., 2020). A more detailed description of Lake Hawassa and its catchment can be found in Desta et al. (2006, 2007, 2008).
Lake Koka (lat: 8°17′–8°29′N; long: 38°55′–39°79′E) is an artificial lake situated in the Awash River Basin and has been referred to as the Koka Reservoir in some literature (Mesfin et al., 1988; Zinabu & Pearce, 2003). The lake is located at an altitude of 1660 m a.s.l and has a surface area of 200 km2. The reservoir was created after the construction of the Koka Dam across the River Awash in 1960 (Hughes, 1992) for irrigation and hydroelectric power development purposes. River Mojo and River Awash are the main inflows, while River Awash also drains the lake. The Little and Big Akaki Rivers, being tributaries of the River Awash, have been identified as the most polluted rivers in the country (Alemayehu, 2001). The Ethiopian Tannery, the largest in the country, is also situated within the Lake Koka catchment area.
Lake Ziway (lat: 7°51′–8°07′N; long: 38°43′–38°56′E) is fed by River Meki from the west and River Katar from the east, and it is drained by the River Bulbula which finally joins Lake Abiyata (Fig. 1). It is situated at 1636 m a.s.l and has a maximum depth of 7 m. The surface and catchment areas of the lake are 442 and 7025 km2, respectively (Kebede et al., 1994). The town of Ziway is situated at the western shore of the lake and, as a result, there is intensive human activity such as the use of lake water for irrigation, excessive expansion of floriculture farms (greenhouses), and commercial fish farming (Ayenew, 2007).
Sample collection and storage
A sampling of sediments from all the three lakes was carried out between July and September 2009. A gravity corer (diameter = 4.5 cm) made from hard plastic (Plexiglass) was used to collect surface sediment samples. Sampling was performed at about the center of each lake in order to minimize any disturbance that could occur from the shore and hence could represent accumulation sites for bottom sediments. The depths of all the sediment cores obtained were less than 10 cm, and results are reported for the upper surface layer (upper 6 cm).
After sampling, the sediment cores were immediately sliced into 2 cm sections with a clean plastic cutter and then transferred to a clean and dry self-sealing polyethylene bags. The samples were then marked and kept in an ice box until they were transported to the laboratory at Hawassa University (within 24 h), where they were kept in the deep freezer at − 20 °C. It is believed that freezing samples at − 20 to − 30 °C is the most dependable method of sediment storage especially for trace elements and potentially for chemical speciation purposes (Förstner & Wittmann, 1981), although perturbations due to freezing must be taken into account (Hlavay et al., 2004; Kersten & Förstner, 1995). The samples were again transferred to an ice box for transport to The Norwegian University of Life Science (NMBU), Norway, and kept in the deep freezer at − 20 °C prior to sequential extractions and the determination of total concentrations of the trace elements.
Sample preparation, pH, and loss on ignition (LOI)
The samples were defrosted and excess water was removed by vacuum filtration. A 1-g sub-sample from each 2 cm section was dried overnight at 105 °C in a muffle furnace to determine the water content. The organic matter content of the sediment was determined by loss on ignition (LOI) using a temperature of 550 °C for 12 h. Subsequently, the wet weight equivalent to the required 1 or 2-g dry weight was determined based on water to dry sediment ratio. The wet sediment weighing was done shortly after vacuum filtration to avoid water loss by evaporation due to exposure to air. The sediment pH was determined by using a glass electrode (Mettler Toledo, SenTix 21) after the samples were mixed with deionized water in a ratio (1:5) and allowed to settle overnight.
For determination of total concentrations of the trace elements, subsamples were freeze-dried, homogenized, and sieved (2 mm) and dried samples of about 0. 2 and 0.3 g were generally collected for analyses.
Sequential extraction procedure (SEP)
The sequential extraction of sediment samples was carried out according to the procedure described by Oughton et al. (1992) with slight modifications (Salbu, 2000; Skipperud et al., 2009). Wet sediment samples were used in order to preserve as much as possible the original trace element speciation, as drying techniques would influence trace element speciation in sediments and should therefore be avoided (Rapin et al., 1986). The sediment samples were accurately weighed in centrifuge tubes (50 mL, Nalgene Centrifuge Ware) and subjected to sequential extraction by applying the operating conditions and reagents summarized in Table 1. For convenience, the fractions were referred to as F1–F7 (Table 1) where F7 was the residue. The supernatants from each step were separated from undissolved sediments by high-speed centrifugation (10,000 g) using the Beckman Avanti 30 Centrifuge. The trace elements in the residual fractions (F7) were determined after acid digestion using UltraClave (Milestone Microwave Laboratory Systems, USA).
The Risk Assessment Code (RAC) system was applied to assess the potential environmental risk of the elements investigated. According to this system, the environment risk caused by a trace element is judged to have no risk, low risk, medium risk, high risk, and very high risk if the relative (%) sum of the water-soluble and exchangeable fraction was < 1, 1–10, 11–30, 31–50, and > 50%, respectively (Jain, 2004).
Chemical analysis
All sediment extracts were heated to dryness on a sand bath at 80–90 °C and the residue left after dryness was re-dissolved using 2 mL ultrapure HNO3. This procedure was repeated three times. The residues left after final drying were later re-dissolved in 2.5 mL ultrapure HNO3 and transferred into 50-mL clean brand new test tubes. Subsequently, 250 µL of internal standard containing 4 mg/L of rhodium (Rh), indium (In), tellurium (Te), and thalium (Tl) was added for measurement purposes, and then the samples were diluted with deionized water (Barnstead, > 18 MΩ.cm−1). Calibration blanks and standard solutions were used for calibrating the inductively coupled plasma–mass spectrometer (ICP-MS) (PerkinElmer Sciex, ELAN 6000). The residues obtained from the 7 M HNO3 acid treatment were further diluted ten times prior to analysis. The total trace element concentrations were determined following acid digestion by the UltraClave (Milestone Microwave Laboratory Systems, USA) using the same procedure as described for the residual fraction. A mixture of 5 mL HNO3 and 1 mL of HF were added to accurately weighed 0.2–0.3 g samples and digested at high temperature (maximum 260 °C) and pressure (max. about 160 bar) in an UltraClave. Samples were taken after about 2 h, and 6 mL of saturated boric acid (H3BO3) was added to each digested sample and heated again at a temperature of 85 °C in an ultrasonic bath for 30 min.
The accuracy of the determination of trace elements was evaluated by using standard reference material (National Institute of Standards and Technology, SRM-1646 estuarine sediment). The precision of the total digestion procedure was estimated from replicates (n = 5) each weighing about 0.2–0.3 g. The accuracy of the sequential extraction procedure was evaluated by comparing the sum of the trace element concentrations of all fractions (∑SEP) with the total concentrations obtained from UltraClave digestion using HF-HNO3-H3BO3 mixture. The precision of the method for the sequential extraction procedure was estimated from replicates (n = 5) of 1 g dry sediment samples subjected to the same sequential extraction procedure as the samples.
The limits of detection (LOD) for the residual fractions and total concentrations of trace elements were determined by 3 times the standard deviation of 18 blanks, while the LOD for the other sequential extracts were determined by 3 times the standard deviation of 13 blanks. The accuracy, i.e., deviations of measured values (n = 2) from the certified value(s) of the reference material (SRM-1646) (Table 2) was < 10% for all elements except Cr (12%), while the precision for total and residual trace element determinations were all less than 5%, indicating that our data should be considered reliable.
Statistics
Arithmetic and geometric mean values were calculated for the trace element concentrations in sediments. If the mean and geometric mean values were similar, the distributions were assumed to be near normal.
Dixon’s Q-test was applied to test outliers, and results showed that one of the replicate values for the H2O2-extractable fractions obtained for precision determination was actually an outlier, and thus not included in the mean calculation. The distribution coefficients (Kd,) between surface sediment and the lake water were calculated according to
where \(C\; sediment\) refers to the concentration (µg/g d.w.) of a trace element in sediment, while \(C\; water\) refers to the concentration (µg/mL) of the same trace element in surface water of the lakes studied. Concentrations of trace elements in water used for the Kd calculation have previously been published by our group (Masresha et al., 2011).
Multivariate analysis was conducted by using the CANOCO 4.5 software package, including the diagram software CanoDraw 4.14. Partial redundancy analysis (pRDA) was performed to evaluate the overall variation and patterns in the data related to the trace element concentrations obtained from the various sequential extraction steps. In addition, an initial pRDA was also performed to identify if there were any differences in trace element concentrations among the different lakes and sediment depths.
The concentrations of trace elements in some of the samples, 19 out of 140 (14%), were below the limits of detection (LOD). Hence, we considered the substitution of samples having trace element concentrations below LOD with half of LOD in our data set as valid, in accordance with Antweiler and Taylor (2008) stating that substituting left-censored data below LOD with LOD*0.5 is an adequate method if the amount of censored data is not too high.
The various samples were classified according to their respective sequential extraction step, lake, and depth. The classifications were further applied to make a set of categorical explanatory variables, i.e., seven sequential extraction steps, three lakes, and three sediment depths. The categorical explanatory variables were coded by dummy values, i.e., 1 and 0. Prior to the analyses, the data was log(x + 1) transformed to reduce the effects of extreme values, and in addition, centered and standardized (i.e., bringing their means to zero and their variance to one).
Since the variables lake and depth are not independent, it was decided to use the interaction between the two variables (lake × depth) as the explanatory variable in the initial pRDA. In addition, the seven sequential extraction steps were included as co-variables (i.e., removing the effect of the various sequential extraction steps). Samples belonging to specific sequential extraction steps were defined as blocks in the permutation set-up (i.e., the blocks were defined by the co-variable). Samples within each block were freely permuted and between-block variation was thus excluded from the test.
To evaluate if there were any overall differences between the various sequential extracts in terms of trace element concentrations, partial redundancy analysis (pRDA) was performed. In this model, the seven sequential extracts were used as explanatory variables and the interaction between lake and sediment depth was used as a co-variable. A total of nine blocks were defined using the co-variable lake × sediment depth as criteria, and the permutations were only performed on samples within these blocks. Hence, between-block variation was excluded from the test.
The permutation tests were performed by the Monte Carlo permutation test and results with p < 0.05 were assigned as statistically significant. We used 499 permutations under reduced model, which is preferable with small data sets.
Results and discussion
Data quality
The precision of the sequential extraction procedure was in the range 2–25% for As, 3–12% for Cd, 1–14% for Cr, 4–11% for Co, 1–20% for Cu, 2–23% for Pb, 1–17% for Mn, 2–11% for Ni, 8–17% for Se, and 4–19% for Zn. The measurement uncertainties varied according to the concentrations of the elements in the different extracted fractions.
The sum of concentrations in seven sequential extracts (∑SEP) is compared with the total concentrations obtained from a single-step dissolution of the same samples in Table 3. The differences between total trace element concentrations and ∑SEP values were mostly < 20%. Higher deviations could occasionally be observed for trace elements with concentrations close to LOD due to higher uncertainties associated with measurements and possibly also due to higher relative loss due to sorption processes during extractions. Taking the lack of sediment homogeneity into account, the agreement between total concentrations from the single-step digestion and sequential extractions (∑SEP) was judged to be acceptable.
Sediment characteristics
Tudorancea and Zullini (1989) reported that the type and size of particles in the deeper sediment parts of Lakes Hawassa, Koka, and Ziway were medium and fine silt, having a particle size in the range 2–63 µm (Förstner & Wittmann, 1981). Sediment characteristics such as water content, percent loss on ignition (% LOI), and pH are presented in Table 4. The mean pH of the sediment samples from all the three lakes varied between 8.0 and 8.5, and the sediments could be categorized as weakly alkaline. The water content (weight %) in the sediment was in general close to or more than 50%, and the 2-cm top sediment layers appeared to have similar water contents as the lakes. The mean LOI (%) values of the sediments from the three lakes varied between 7 and 34%, and the LOI (%) values were the lowest for sediments from Lake Koka (% LOI550 = 7.3–7.6). In agreement with this study, Mesfin et al. (1988) and Tudorancea and Zullini (1989) reported that LOI (%) values for Lake Koka were in the range 7.4–11.5%. Although the mean LOI (%) values reported for Lake Ziway in the present study (8.3–11%) were within the range (4.7–21.4%) reported by Tudorancea and Zullini (1989), they were nearly two times lower than the maximum LOI reported by these authors. The LOI (%) determined in this study for sediments from Lake Hawassa (29–34%) appeared to be higher than the results reported in a previous study (5–23%) (Telford et al., 1999). However, the LOI (%) reported in this study for Lake Hawassa was comparable with the maximum LOI (%) previously reported (3.8–31.4%) by Tudorancea and Zullini (1989).
Total concentrations of trace elements
The vertical distribution of the trace element concentrations in the 6 cm core was quite even (Table 3). For that reason, the concentrations in the top 2 cm were chosen for comparisons. The average total concentrations of trace elements decreased in the order Mn > Zn > Cr > Ni > Pb > As > Cu > Co > Se > Cd in Lake Hawassa, Mn > Zn > Cr > Ni > Cu > Co > Pb > As > Cd > Se in Lake Koka, and Mn > Zn > Cr > Ni > Cu > Pb > Co > As > Se > Cd in Lake Ziway (Table 3). The sequence was surprisingly similar, especially in sediments from Lakes Koka and Ziway, possibly reflecting sources of natural origin of the trace elements in these lakes.
The mean concentrations of Cr and Co in the three lakes, and Cu in Lakes Koka and Ziway were higher than values reported for five Kenyan Rift Valley Lakes (KRVL) (Ochieng et al., 2007) by factors of about 5–20, 5–14, and 2, respectively. However, the average concentrations of Cd, Pb, Mn, and Zn in sediments from all the three lakes and Ni and Cu in sediments from Lake Hawassa were all within the ranges reported for the five KRVL (Ochieng et al., 2007). As both the Kenyan and the Ethiopian lakes are located within the same geological settings, higher concentrations of some of the trace elements could be attributed to inputs from both local point and diffuse sources.
When comparing the present data with values assigned to unpolluted sediments (Förstner & Wittmann, 1981), the concentrations of Cr, Mn, Ni, and Zn in Lake Koka and Mn, Zn, and Se in Lakes Hawassa and Ziway were higher than concentrations considered to be unpolluted. Similarly, a comparison of our data with consensus-based freshwater sediment quality guidelines (SQGs) (Table 3) developed by MacDonald et al. (2000) indicated that the concentrations of Cr, Ni, and Zn in sediments from Lake Koka and Zn in sediments from Lakes Hawassa and Ziway could potentially induce harmful effects to the benthic organisms as the values exceeded probable effect concentrations (PEC). The total concentrations of As, Cd, and Pb were all lower than the threshold effect concentration (TEC) suggesting that it was unlikely that these trace elements could cause any negative effects on benthic organisms. The consensus-based SQGs are derived from sediment quality guidelines developed by matching sediment chemistry with toxicity tests which implies that the bioavailability has been taken into account. However, SQGs developed for North America cannot directly be utilized at other continents due to (1) the influence of temperature variation on biochemical reaction rates, metabolic rates, and biological activity (Chapman et al., 2006), (2) differences in benthic communities in different regions, and (3) variations in sediment chemistry (mixtures of contaminants), etc. (De Deckere et al., 2011). Since there are no SQGs developed for Ethiopia, information on the mobility of the trace elements in bottom sediments, rather than total concentrations could therefore be used for ecological risk assessment.
Sequential extraction of sediments
The relative distribution (%) of trace elements in the different sequential extracts (F1–F7), as compared to the total concentrations of all extracts, is presented in Fig. 2. The relative fractions of trace elements extracted by H2O (F1) were in general low (0–2%) for all elements investigated; however, somewhat higher in sediments from Lake Koka (0.3–1.8%) as shown in Fig. 2. The relative fraction of trace elements in the F2 extract (pH 7 NH4OAc) was also low (< 1%), except for Cd (6%) and Mn (17%) in sediments from Lake Koka (Fig. 2). The low trace element levels in the F1 and F2 reflected low mobility of the elements. In this study, the mean pH of the lake sediments was between 8.0 and 8.5 indicating that the sediments were slightly alkaline. Generally, the solubility of many trace metals in water decreases as a result of increasing pH (Avila-Pérez et al., 1999).
The relative fraction of trace elements in the F3 fraction (pH 5 NH4OAc) varied from 0.1 to 22% which suggested a large variation among the different trace elements (Fig. 2). The dominating trace elements in this fraction were Mn (16–20%), Cd (10–17%), and Se (5–17%) indicating that a considerable proportion of these elements were reversibly associated to the lake sediments. Trace elements in the F1–F3 fractions are normally considered to be reversibly associated (physical and/or electrostatic sorption) to sediment surfaces (Salbu, 2000). In contrast, trace elements in the F4–F7 fractions are assumed to be irreversibly associated with sediments due to irreversible chemisorption (Salbu, 2000) as redox agents are needed for dissolution.
The relative fraction of trace elements in the F4 extract (NH2OH.HCl) varied from 1 to 33%, and the dominating trace elements were Mn (20–30%), Cd (15–33%), Se (8–15%), Ni (10–15%), and Pb (7–14%). For Co (2–22%), the highest extractable fraction was observed in Lake Koka, while for As (2–15%), the highest fraction was observed in sediments from Lake Hawassa. As a weak reducing agent was applied in F4, the fraction extracted was assumed to be associated with amorphous LOI materials or hydroxides/oxides of elements such as Fe, Mn, or Al.
The relative fraction of trace elements in the F5 extract, where H2O2 was used as a weak oxidizing agent, varied from 0.1 to 87% in the different lakes. The dominating trace elements were Se (48–87%) and Ni (10–39%), while large variations were found for As (1–36%) and Co (2–22%) among the study lakes. The highest fractions were in general found in sediments from Lake Hawassa, probably due to the relatively higher LOI (%) values of the sediments (Table 4) indicating association to organic material. In general, lake sediments with high organic matter due to the presence of stable high molecular mass humic substances could release relatively higher fractions of trace metals under oxidizing conditions (Jain et al., 2007; Singh et al., 1998). The sum of the F6 (7 M HNO3) fraction and the F7 (residual fraction) was 44–96% for As, 40–65% for Cd, 91–95% for Cr, 68–75% for Co, 88–96% for Cu, 76–90% for Pb, 30–56% for Mn, 47–72% for Ni, 0–24% for Se, and 91–95% for Zn, indicating that most of the trace elements were strongly bound to the sediments. Similar to our findings, most of the trace elements investigated in the sediment of Badvci lake (Kosovo) were found in the residual fraction (Malsiu et al., 2020). This metal fraction was of detrital and lattice origin which originates from natural sources (Salomons & Förstner, 1980). It is very unlikely that these trace element fractions would be mobilized and become bioavailable to aquatic biota under the natural conditions found in the lakes. If the sediments are used as food sources by bottom-dwelling organisms, bioavailability could be controlled by assimilation efficiency which, in turn, depends on the type of metal, the physico-chemical form, and distribution in prey, species digestive physiology, environmental conditions, food quality, food ingestion rate, and metal concentration in the diet (Wang & Fisher, 1999). In such a case, consensus-based SQGs could be of significance for detritus-feeding organisms.
To evaluate the relationship between the various fractions (F1–F7) and the trace element concentrations, multivariate statistical analysis using partial redundancy analysis (pRDA) was performed. The pRDA showed that the differences in concentrations among the various sequential extraction steps could significantly (p = 0.002) explain 80% of the total variation in the sequential extraction data from all study lakes. Lake and sediment depth interactions significantly (p = 0.002) explained only 5.5% of the variance.
The relationship between the concentrations of trace elements and the respective fractions is shown using the RDA bi-plot (Fig. 3). The RDA bi-plot (Fig. 3) shows that the first extraction steps were generally associated with low leachability/mobility (small-sized sample symbols), whereas the later extraction steps were associated with trace elements strongly bound to the sediment phases that were dissolved using agents of high dissolution power (large sized sample symbols). In particular, the concentrations of most of the trace elements were negatively correlated with their concentrations extracted in F1, F2, and F5, but positively correlated with the concentrations extracted in F4, F6, and F7, and partially to F3. This implies that relatively large concentrations of the trace elements were found in F4 (NH2OH.HCl-extractable), F6 (HNO3-extractable), and F7 (residual) fractions, i.e., the redox and temperature-influenced fractions. Cadmium (Cd) and Mn displayed another release pattern compared to the other trace elements, i.e., were more related to F3 (pH 5 NH4OAc-extractable) and F4 (NH2OH.HCl-extractable) and agreed with results (Fig. 2) that these trace elements were more mobile than the others.
Sources, mobility, and bioavailability of the trace elements
In previous studies (Izquierdo et al., 1997; Turki, 2007), it was reported that the relative distribution between residual and non-residual fractions (exchangeable, “carbonates,” reducible and oxidizable fractions) could be used to predict whether the primary sources of the trace elements would be of anthropogenic or lithogenic origin. According to Samanidou and Fytianos (1987), trace metals from anthropogenic sources are usually present in relatively more mobile forms than those from natural sources. Thus, trace elements found mainly in the residual fraction could be considered to originate from lithogenic minerals, and as a result, did not have a remobilization potential under normal changing environmental conditions encountered in nature. Applying this approach to the present data, the non-residual (F1–F5) fractions (sum total of H2O-, pH 7 NH4OAc-, pH 5 NH4OAc-, NH2OH.HCl-, and H2O2-extractable fractions) of As, Cd, Ni, and Se in sediments from Lake Hawassa, Cd, Mn, and Se in sediments from Lake Koka, and Se in sediments from Lake Ziway exceeded 50% of the total concentrations, indicating a contribution from local anthropogenic sources possibly such as agricultural runoff and municipal and industrial wastes.
The assumed reversible fractions could also be used to assess the potential mobility of trace metals (Salbu, 2000; Skipperud et al., 2009). Therefore, it can be assumed that the F1–F3 fractions (H2O-, pH 7 NH4OAc-, and pH 5 NH4OAc-extractable fractions) represent the mobile fractions in the present study. Accordingly, the sum of the concentrations of trace elements in the first three sequential extracts accounted for 2–6% for As, 11–24% for Cd, < 1% for Cr, 1–8% for Co, 1–3% for Cu, 2–6% for Pb, 20–35% for Mn, 3–4% for Ni, 5–17% for Se, and < 1% for Zn in all the three lakes considered in this study. These results suggested that most of the trace elements investigated were strongly associated to the sediments, indicating that their remobilization potentials were very low. Thus, based on results obtained from sequential extraction, trace elements of highest mobility were Cd, Mn, and Se, while Zn and Cr were elements of extremely low mobilization potential.
Application of risk assessment code (RAC)
According to the RAC classification system, a scale was developed based on the sum of the relative (%) fraction of trace elements in the assumed exchangeable and “carbonate” fractions (Jain, 2004; K. P. Singh et al., 2005) in the upper 6-cm sediment as this is expected to be the most reactive part of the sediment with overlying surface water (Sallade & Sims, 1997). Accordingly, trace elements accumulated in sediments could be considered safe to the environment if the sediment releases < 1% of their total concentrations in the assumed exchangeable and “carbonate” fractions. In addition, trace elements in sediments are considered to easily enter the food chain and pose a risk to the aquatic environment if the sediments could release more than 50% of total concentrations in these fractions.
Based on the RAC criteria, the risk from the trace elements in the study lakes could be arranged as follows:
Lake Hawassa: Cd and Mn (medium risk) > As, Co, Cu, Pb, Ni, and Se (low risk) > Cr and Zn (no risk).
Lake Koka: Mn (High risk) > Cd (medium risk) > As, Co, Cu, Pb, Ni, and Se (low risk) > Cr and Zn (no risk).
Lake Ziway: Cd, Mn, and Se (medium risk) > As, Co, Cu, Pb, and Ni (low risk) > Cr and Zn (no risk).
It is important to emphasize that these conclusions are derived barely from sequential extraction results which are contradictory to the predictions made by comparing total concentrations with consensus-based SQGs. However, diet-related exposure of mobile trace elements at high total concentrations may be of importance if the benthic organisms use sediments as a source of food.
The distribution coefficients (Kd)
To estimate the transfer of trace elements from sediment to the overlying waters, the distribution coefficients, Kd, based on total concentrations in sediments and water are frequently applied (Lee et al., 1997; AaPGCC Tessier & Campbell, 1987) in impact assessment models. The mean Kd values for the trace elements included in this study are shown in Table 5. The distribution coefficients ranged in general from 103 to 105d.w. with the highest transfer (Kd of 103d.w.) of Se, As, and Cu in Lake Hawassa and Se and most other elements in Lake Koka and Se as well as As, Cu, and Cd in Lake Ziway. Low mobility (Kd of 105d.w.) was obtained for Cr, Cu, and Zn in Lakes Hawassa and Ziway. As the Kd concept is based on the assumption that equilibrium/pseudoequilibrium exists between the sediment surface and the water phase, the data from Lake Koka was recalculated as the water contained a high load of particles. Thus, the total concentration of trace elements in water was replaced with the concentration of potentially interacting low molecular mass (LMM) species in water (Table 5, column 4). Recalculation showed that As and Se could be considered mobile (103d.w.), while Cu, Cr, and Zn were inert (Kd of 105d.w.) which was in agreement with results from the other lakes. Therefore, it appeared that Kd values based on total concentrations of trace elements in sediments (∑SEP) and the concentrations of LMM (< 10 kDa) species in water rather than total sediment concentrations and total water concentrations should better predict the potential sediment–water transfer of trace elements in the studied lakes.
Predictions of mobility of trace elements using the Kd values and sequential extraction results were partly in agreement. Trace elements such as Se as well as also Cd in Lake Ziway were predicted to be the most mobile elements by both methods. On the other hand, Mn was predicted as one of the most mobile together with Cd and Se using sequential extraction but was not supported by the Kd values. A possible reason could be that the concentrations of trace elements in surface waters did not only reflect the sediment transfer concentrations, but also includes contributions from other sources.
Conclusions
The present study provides baseline information with respect to the total concentrations and operationally defined speciation of a series of trace elements in surface sediments from Lakes Hawassa, Koka, and Ziway situated in the Rift Valley. This information is of paramount importance as this study is the first of its kind from these tropical lakes that are exposed to many different anthropogenic sources. Comparisons of total concentrations with both reference values for unpolluted sediments and consensus-based SQGs gave predictions that trace elements such as Cr, Ni, and Zn in Lake Koka could bring about adverse biological effects in benthic organisms. As sequential extraction demonstrated that desorption of these elements was rather limited, the expected biological effects may occur only if the sediments are directly ingested by bottom-feeding organisms. The results obtained from sequential extraction indicated also that the mobility of the trace elements present in relatively high concentrations (e.g., Cr, Ni, and Zn) was very low and the corresponding Kd values were very high (Kd of 105 d.w.), except for Mn being somewhat more mobile (Kd of 104 d.w.). The low mobility of the trace elements investigated could also suggest that natural sources were more relevant than anthropogenic sources in these lakes, although a series of anthropogenic sources are situated in the catchments. Cadmium has concentrations corresponding to unpolluted sediments, but such results must be interpreted with caution as Cd has a high remobilization potential (F1 + F2 + F3 = 11–24%, Kd of 104 d.w.). Based on the risk assessment code (RAC) system for sediments, Cd, Mn, and Se were predicted to represent medium to high risk to bottom-dwelling organisms in the lakes. Although sequential extraction and calculation of Kd values seem most useful to predict the potential mobility of trace elements from sediments to pore and overlying waters, exposure of trace elements to biota such as benthic organisms depends not only on mobilization and speciation, but also on ingestion of sediments and uptake of bioavailable trace elements. Therefore, future work should compare results from toxicity tests of relevant sediment living exposed organisms with predictions associated with trace elements in sediments and their potential adverse biological effects derived from the consensus-based SQGs.
Availability of data and materials
All data generated or analyzed during this study are included in this manuscript.
Change history
06 January 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10661-022-10753-x
References
Alemayehu, T. (2001). The impact of uncontrolled waste disposal on surface water quality in Addis Ababa, Ethiopia. SINET: Ethiopian Journal of Science, 24(1), 93–104.
Amare, T. A., Yimer, G. T., & Workagegn, K. B. (2014). Assessment of metals concentration in water, sediment and macrophyte plant collected from Lake Hawassa, Ethiopia. Journal of Environmental & Analytical Toxicology, 4(247), 2161–2525.
Antweiler, R. C., & Taylor, H. E. (2008). Evaluation of statistical treatments of left-censored environmental data using coincident uncensored data sets: I. Summary Statistics. Environmental Science & Technology, 42(10), 3732–3738.
Ataro, A., Wondimu, T., & Chandravanshi, B. S. (2003). Trace metals in selected fish species from lakes Awassa and Ziway, Ethiopia. SINET: Ethiopian Journal of Science, 26(2), 103–114.
Avila-Pérez, P., Balcázar, M., Zarazúa-Ortega, G., Barceló-Quintal, I., & Dıaz-Delgado, C. (1999). Heavy metal concentrations in water and bottom sediments of a Mexican reservoir. Science of the Total Environment, 234(1–3), 185–196.
Ayenew, T. (2007). Water management problems in the Ethiopian rift: Challenges for development. Journal of African Earth Sciences, 48(2–3), 222–236.
Chapman, P. M., McDonald, B. G., Kickham, P. E., & McKinnon, S. (2006). Global geographic differences in marine metals toxicity. Marine Pollution Bulletin, 52(9), 1081–1084.
de Deckere, E., De Cooman, W., Leloup, V., Meire, P., Schmitt, C., & Peter, C. (2011). Development of sediment quality guidelines for freshwater ecosystems. Journal of Soils and Sediments, 11(3), 504–517.
Deribe, E., Masresha, A. E., Gade, P. A., Berger, S., Rosseland, B. O., Borgstrøm, R., et al. (2014). Bioaccumulation of mercury in fish species from the Ethiopian Rift Valley Lakes. International Journal of Environmental Protection, 4(1), 15.
Desta, Z., Borgstrøm, R., Rosseland, B. O., & Dadebo, E. (2007). Lower than expected mercury concentration in piscivorous African sharptooth catfish Clarias gariepinus (Burchell). Science of the Total Environment, 376(1–3), 134–142.
Desta, Z., Borgstrøm, R., Rosseland, B. O., & Zinabu, G. M. (2006). Major difference in mercury concentrations of the African big barb, Barbus intermedius (R.) due to shifts in trophic position. Ecology of Freshwater Fish, 15(4), 532–543.
Desta, Z., Borgstrøm, R., Zinabu, G. M., & Rosseland, B. O. (2008). Habitat use and trophic position determine mercury concentration in the straight fin barb Barbus paludinosus, a small fish species in Lake Awassa, Ethiopia. Journal of Fish Biology, 73(3), 477–497.
Dsikowitzky, L., Mengesha, M., Dadebo, E., de Carvalho, C. E. V., & Sindern, S. (2013). Assessment of heavy metals in water samples and tissues of edible fish species from Awassa and Koka Rift Valley Lakes, Ethiopia. Environmental Monitoring and Assessment, 185(4), 3117–3131.
Förstner, U., & Wittmann, G. T. (1981). Metal pollution in the aquatic environment (2nd ed.). Springer-Verlag.
Fytianos, K., & Lourantou, A. (2004). Speciation of elements in sediment samples collected at lakes Volvi and Koronia, N. Greece. Environment International, 30(1), 11–17.
Gebre-Mariam, Z., & Desta, Z. (2002). The chemical composition of the effluent from Awassa textile factory and its effects on aquatic biota. SINET: Ethiopian Journal of Science, 25(2), 263–274.
Gibbs, R. J. (1973). Mechanisms of trace metal transport in rivers. Science, 180(4081), 71–73.
Gizaw, B. (1996). The origin of high bicarbonate and fluoride concentrations in waters of the Main Ethiopian Rift Valley, East African Rift system. Journal of African Earth Sciences, 22(4), 391–402.
Hass, A., & Fine, P. (2010). Sequential selective extraction procedures for the study of heavy metals in soils, sediments, and waste materials—a critical review. Critical Reviews in Environmental Science and Technology, 40(5), 365–399.
Hlavay, J., Prohaska, T., Weisz, M., Wenzel, W. W., & Stingeder, G. J. (2004). Determination of trace elements bound to soil and sediment fractions (IUPAC Technical Report). Pure and Applied Chemistry, 76(2), 415–442.
Hughes, R. H. (1992). A directory of African wetlands. IUCN.
Izquierdo, C., Usero, J., & Gracia, I. (1997). Speciation of heavy metals in sediments from salt marshes on the southern Atlantic coast of Spain. Marine Pollution Bulletin, 34(2), 123–128.
Jain, C. K. (2004). Metal fractionation study on bed sediments of River Yamuna, India. Water Research, 38(3), 569–578.
Jain, C. K., Malik, D. S., & Yadav, R. (2007). Metal fractionation study on bed sediments of Lake Nainital, Uttaranchal, India. Environmental Monitoring and Assessment, 130(1), 129–139.
Kassaye, Y. A., Skipperud, L., Einset, J., & Salbu, B. (2016). Aquatic macrophytes in Ethiopian Rift Valley lakes; Their trace elements concentration and use as pollution indicators. Aquatic Botany, 134, 18–25.
Kebede, A., & Wondimu, T. (2004). Distribution of trace elements in muscle and organs of tilapia, Oreochromis niloticus, from Lakes Awassa and Ziway, Ethiopia. Bulletin of the Chemical Society of Ethiopia, 18(2).
Kebede, E., Mariam, Z. G., & Ahlgren, I. (1994). The Ethiopian Rift Valley lakes: Chemical characteristics of a salinity-alkalinity series. Hydrobiologia, 288(1), 1–12.
Kersten, M., & Förstner, M. (1995). Speciation of trace metals in sediments and combustion waste. In A. M. Ure, & C. M. Davidson (Eds.), Chemical Speciation in the Environment. Blackie Academic and Professional, London, 238.
Lee, P.-K., Baillif, P., & Touray, J.-C. (1997). Geochemical behaviour and relative mobility of metals (Mn, Cd, Zn and Pb) in recent sediments of a retention pond along the A-71 motorway in Sologne, France. Environmental Geology, 32(2), 142–152.
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39(1), 20–31.
Makin, M. J., Kingham, T. J., Waddams, A. E., Birchall, C. J., & Teferra, T. (1975). Development prospects in the Southern Rift Valley, Ethiopia. Development prospects in the Southern Rift Valley, Ethiopia, (21).
Malsiu, A., Shehu, I., Stafilov, T., & Faiku, F. (2020). Assessment of heavy metal concentrations with fractionation method in sediments and waters of the Badovci Lake (Kosovo). Journal of Environmental and Public Health, 2020.
Masresha, A. E., Skipperud, L., Rosseland, B. O., Zinabu, G. M., Meland, S., Teien, H.-C., & Salbu, B. (2011). Speciation of selected trace elements in three Ethiopian Rift Valley Lakes (Koka, Ziway, and Awassa) and their major inflows. Science of the Total Environment, 409(19), 3955–3970.
Mekonnen, K. N., Ambushe, A. A., Chandravanshi, B. S., Redi-Abshiro, M., & McCrindle, R. I. (2015). Occurrence, distribution, and ecological risk assessment of potentially toxic elements in surface sediments of Lake Awassa and Lake Ziway, Ethiopia. Journal of Environmental Science and Health, Part A, 50(1), 90–99.
Mekonnen, K. N., Ambushe, A. A., Chandravanshi, B. S., Abshiro, M. R., McCrindle, R. I., & Panichev, N. (2012). Distribution of mercury in the sediments of some freshwater bodies in Ethiopia. Toxicological & Environmental Chemistry, 94(9), 1678–1687.
Mesfin, M., Tudorancea, C., & Baxter, R. M. (1988). Some limnological observations on two Ethiopian hydroelectric reservoirs: Koka (Shewa administrative district) and Finchaa (Welega administrative district). Hydrobiologia, 157(1), 47–55.
Ochieng, E. Z., Lalah, J. O., & Wandiga, S. O. (2007). Analysis of heavy metals in water and surface sediment in five rift valley lakes in Kenya for assessment of recent increase in anthropogenic activities. Bulletin of Environmental Contamination and Toxicology, 79(5), 570–576.
Oughton, D. H., Salbu, B., Riise, G., Lien, H., Østby, G., & Nøren, A. (1992). Radionuclide mobility and bioavailability in Norwegian and Soviet soils. The Analyst, 117(3), 481–486.
Rapin, F., Tessier, A., Campbell, P. G., & Carignan, R. (1986). Potential artifacts in the determination of metal partitioning in sediments by a sequential extraction procedure. Environmental Science & Technology, 20(8), 836–840.
Salbu, B. (2000). Speciation of radionuclides in the environment. In Encyclopedia of Analytical Chemistry. Chichester: John Wiley & Sons Ltd.
Sallade, Y. E., & Sims, J. T. (1997). Phosphorus transformations in the sediments of Delaware’s agricultural drainageways: I. Phosphorus forms and sorption. Wiley Online Library.
Salomons, W., & Förstner, U. (1980). Trace metal analysis on polluted sediments: Part II: Evaluation of environmental impact. Environmental Technology, 1(11), 506–517.
Samanidou, V., & Fytianos, K. (1987). Partitioning of heavy metals into selective chemical fractions in sediments from rivers in northern Greece. Science of the Total Environment, 67(2–3), 279–285.
Samuel, B., Sorsa, S., Daniel, F., Riise, G., & Zinabu, G. M. (2020). Heavy metals in fish muscle from an Ethiopian Rift-Valley Lake (Hawassa) and a neighboring stream (Boicha): Assessment of human health risks. Journal of Applied Sciences and Environmental Management, 24(8), 1409–1418.
Singh, K. P., Mohan, D., Singh, V. K., & Malik, A. (2005). Studies on distribution and fractionation of heavy metals in Gomti river sediments—a tributary of the Ganges, India. Journal of Hydrology, 312(1–4), 14–27.
Singh, S. P., Tack, F. M., & Verloo, M. G. (1998). Heavy metal fractionation and extractability in dredged sediment derived surface soils. Water, Air, and Soil Pollution, 102(3), 313–328.
Skipperud, L., Brown, J., Fifield, L. K., Oughton, D. H., & Salbu, B. (2009). Association of plutonium with sediments from the Ob and Yenisey Rivers and Estuaries. Journal of Environmental Radioactivity, 100(4), 290–300.
Sorsa, S., Gezahagn, A., & Dadebo, E. (2016). Bioaccumulation of heavy metals in two morphotypes of African large barb Labeobarbus intermedius (Osteichthyes: Cyprinidae) in Lake Hawassa, Ethiopia. African Journal of Aquatic Science, 41(4), 427–434.
Tadiso, T. M., Borgstrøm, R., & Rosseland, B. O. (2011). Mercury concentrations are low in commercial fish species of Lake Ziway, Ethiopia, but stable isotope data indicated biomagnification. Ecotoxicology and Environmental Safety, 74(4), 953–959.
Telford, R. J., Lamb, H. F., & Mohammed, M. U. (1999). Diatom-derived palaeoconductivity estimates for Lake Awassa, Ethiopia: Evidence for pulsed inflows of saline groundwater. Journal of Paleolimnology, 21(4), 409–422.
Tessema, K., Mamaru, B., Fetahi, T., & Kebede-Westhead, E. (2020). Accumulation of heavy metals in the physical and biological systems of Lake Koka, Ethiopia: Implications for potential health risks, 25, 314–325.
Tessier, AaPGCC, & Campbell, P. G. C. (1987). Partitioning of trace metals in sediments: Relationships with bioavailability. In Ecological effects of in situ sediment contaminants (pp. 43–52). Springer.
Tessier, A., Campbell, P. G., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51(7), 844–851.
Tudorancea, C., & Zullini, A. (1989). Associations and distribution of benthic nematodes in the Ethiopian Rift Valley lakes. Hydrobiologia, 179(1), 81–96.
Turki, A. J. (2007). Metal speciation (cd, cu, Pb and Zn) in sediments from Al Shabab lagoon, Jeddah, Saudi Arabia. Marine Scienes, 18(1).
Wang, W.-X., & Fisher, N. S. (1999). Assimilation efficiencies of chemical contaminants in aquatic invertebrates: A synthesis. Environmental Toxicology and Chemistry: An International Journal, 18(9), 2034–2045.
Wood, R. B., & Talling, J. F. (1988). Chemical and algal relationships in a salinity series of Ethiopian inland waters. In Saline lakes (pp. 29–67). Springer.
Yohannes, Y. B., Ikenaka, Y., Saengtienchai, A., Watanabe, K. P., Nakayama, S. M., & Ishizuka, M. (2013). Occurrence, distribution, and ecological risk assessment of DDTs and heavy metals in surface sediments from Lake Awassa—Ethiopian Rift Valley Lake. Environmental Science and Pollution Research, 20(12), 8663–8671.
Zinabu, G. M. (1998). Human interactions and water quality in the Horn of Africa. In Science in Africa- Emerging Water Problems. Presented at the Science in Africa- Emerging Water Problems, Philadelphia.
Zinabu, G. M., Kebede-Westhead, E., & Desta, Z. (2002). Long-term changes in chemical features of waters of seven Ethiopian rift-valley lakes. Hydrobiologia, 477(1), 81–91.
Zinabu, G. M., & Pearce, N. J. (2003). Concentrations of heavy metals and related trace elements in some Ethiopian rift-valley lakes and their in-flows. Hydrobiologia, 492(1–3), 171–178.
Acknowledgements
The first author is grateful to Hawassa University for offering a generous PhD study leave to Norway and providing necessary support during the fieldwork, and Lånekassen, Norway, for covering his living expenses during the study leave. The authors are also indebted to Karl-Andreas Jensen, Norwegian University of Life Sciences (NMBU), for measurement of the samples by ICP-MS. Finally, the authors would like to thank Mr. Melaku Getachew at the Ethiopian Environment and Forest Research Institute for preparing a map of the study area.
Funding
This work was supported by NUFU (Norwegian Programme for Development, Research and Education) (NUFUPRO-2007/10115) and by the Research Council of Norway through its Centers of Excellence Funding Scheme, project number 223268/F50.
Author information
Authors and Affiliations
Contributions
This work was principally carried out by Alemayehu Esayas Masresha. Brit Salbu, Lindis Skipperud, and Zinabu Gebremariam closely supervised the work. Sondre Meland did the multivariate statistical analysis. The first draft of the manuscript was written by Alemayehu Esayas Masresha. All authors have commented on previous versions of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
The authors confirm that this manuscript has not been published elsewhere and is not under consideration by another journal. All authors have approved the manuscript and agree with its submission to Environmental Science and Pollution Research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to error in Figure 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Masresha, A.E., Skipperud, L., Gebremariam, Z. et al. Mobile trace elements in sediments from lakes Hawassa, Koka, and Ziway in the Ethiopian Rift Valley. Environ Monit Assess 195, 11 (2023). https://doi.org/10.1007/s10661-022-10512-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10512-y