Abstract
Forest roads alter the biotic and abiotic components of ecosystems, modifying temperature, humidity, wind speed, and light availability that, in turn, cause changes in plant community composition and diversity. We aim at investigating and comparing the diversity of herbaceous species along main and secondary forest roads in a temperate-managed hornbeam-beech forest, north of Iran. Sixteen transects along main and secondary forest roads were established (eight transects along main roads and eight along secondary roads). To eliminate the effect of forest type, all transects were located in Carpinetum-Fagetum forests, the dominant forest type in the study area. The total length of each transect was 200 m (100 m toward up slope and 100 m toward down slope), and plots were established along it at different distances from road edge. The diversity of herbaceous plant species was calculated in each plot using Shannon-Wiener index, species richness, and Pielou’s index. The results showed that diversity index decreased when distance from road edge increases. This decreasing trend continued up to 60 m from forest road margin, and after this threshold, the index slightly increased. Depending on the type of road (main or secondary) as well as cut or fill slopes, the area showing a statistical different plant composition and diversity measured through Shannon-Wiener, species richness, and Pielou’s index is up to 10 m. The length depth of the road edge effect found in main and secondary forest roads was small, but it could have cumulative effects on forest microclimate and forest-associated biota at the island scale. Forest managers should account for the effect of road buildings on plant communities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Forest roads have many complex effects on surrounding area over time and space (Forman and Alexander 1998). Forest roads can cause severe environmental impacts including road surface erosion (Zemke 2016), sediment yield (Akay et al. 2008), pollution of off-site waters (Forsyth et al. 2006), slope failures and mass movement (Amaranthus et al. 1985), and losses of habitat (Flory and Clay 2006). Therefore, when designing forest roads, managers should weigh cost efficiency decisions against sustainable management practices. Most modern road development projects in managed forests modify existing roads, which provides opportunities for improvement of ecological conditions during the planning, operation, and maintenance phases. To mitigate the effects of forest road modification planning and implementation on ecological processes, impact assessments should be conducted across temporal scales. In fact, the Transportation Research Board and National Research Council (2005) has called for the development of multiple robust ecological indicators to improve cross-scale evaluation of shifting ecological conditions for a broad array of ecosystems.
Understanding the determinants of species diversity patterns is of central interest in ecology (Eycott et al. 2006). For plants, it has been widely recognized that besides classic ecological factors such as climate, energy, and water availability as well as soil (Gatson 2000; Pausas and Austin 2001; Schnitzer et al. 2011; Brown et al. 2015), drivers related to human disturbance play a key role in affecting diversity patterns (Devictor et al. 2008; Catford et al. 2012; MacDougall et al. 2013). There are several pervasive threats against the diversity of plant communities (e.g., habitat loss and fragmentation, exotic species invasion, and pollution) directly or indirectly connected to roadway design, construction, and maintenance (Watkins et al. 2003; Godefroid and Koedam 2004; Flory and Clay 2006; Avon et al. 2013). When imposed on a landscape, roads form a network of disturbance corridors, reducing interior habitats and exposing organisms to what have been termed “edge effects” (Harrison and Bruna 1999; Cadenasso and Pickett 2001; Ewers and Didham 2006; Zurita et al. 2012). Road edges are a fundamental driver of specific composition of plant communities by causing changes in temperature, moisture, light availability, and wind speed (Parendes and Jones 2000; Watkins et al. 2003; Delgado et al. 2007; Flory and Clay 2009). Roadway boundaries, in particular, tend to host plant communities with relatively high species richness, but can be dominated by exotic species or species with a high disturbance tolerance (Tyser and Worley 1992; Forman and Alexander 1998; Marcantonio et al. 2013).
Disturbances to plant communities from roadways are especially evident in forests, with a suite of unique ecological effects (Forman and Alexander 1998; Laurance et al. 2001; Avon et al. 2010) linked to core habitat fragmentation, horizontal natural processes interruption, and natural processes alteration (Hawbaker et al. 2006; Delgado et al. 2007).
Studies conducted in different biomes (e.g., Buckley et al. 2003; Mullen et al. 2003; Pauchard and Alaback 2004; Hosseini et al. 2011; Lotfalian et al. 2012; Lee et al. 2012) highlighted common features of roads effects on herbaceous diversity: (i) roads affect plant species composition along road edge as well as in forest interior (Lotfalian et al. 2012; Neher et al. 2013), (ii) the disturbance has the strongest magnitude in the first 5 m from road margins (Arevalo et al. 2005; Avon et al. 2010; Hosseini et al. 2011; Lotfalian et al. 2012; Marcantonio et al. 2013), (iii) alpha diversity increases with increasing distance from roads (see, e.g., Zeng et al. 2010; Lotfalian et al. 2012; Marcantonio et al. 2013), and (iv) gamma diversity decreases when road density increases (Findlay and Houlahan 1997; Buckley et al. 2003). These studies indicated that herbaceous species richness along main roads is higher than along skid trails and forest interior (Lee et al. 2012). Plant species diversity along roads is increased by the encroachment of generalist and alien species that are adapted to disturbed habitats. Alien species, once established along the forest edges, thanks to the favorable ecological conditions, move very rapidly toward forest interior, not infrequently altering the whole forest system (Watkins et al. 2003; Godefroid and Koedam 2004; Pauchard and Alaback 2004; Honu and Gibson 2006).
In spite of extensive studies about forest road ecology in temperate broadleaf mixed forests (see, e.g., Forman and Deblinger 2000; Parendes and Jones 2000; Flory and Clay 2009), few studies have been conducted in the Hyrcanian Mixed Forests ecoregion of Northern Iran (Hosseini 2010; Hosseini et al. 2011; Lotfalian et al. 2012). However, the Hyrcanian forests are unique Arcto-Tertiary forests, where several tree genera (i.e., Pterocarya, Albizia, Parrotia, or Gleditsia) survived the last ice age (Scharnweber et al. 2007). Approximately 60% of Iranian forests have been managed since the early 1970s, and the ecological effects of forest roads on herbaceous diversity have a recent history. This pattern is in contrast with other temperate forests considered in road ecology literature, which have a longer management history (at least 100 years; Honu and Gibson 2006; Avon et al. 2010).
In this study, we aim at investigating the diversity of herbaceous species along main and secondary roads in Hyrcanian forest, north of Iran, taking into account the effect of cut slope (the soil surface that remains above the road after material is removed) and fill slope (the soil surface build below the road). In detail, we aimed to answer the following questions: (i) Is the effect of main and secondary forest roads on herbaceous species diversity and composition the same? (ii) Is the effect of main and secondary forest roads the same on the herbaceous plant communities of the cut and fill slope?
Materials and methods
Study area
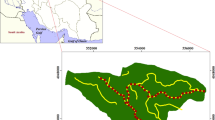
The study took place throughout the Hyrcanian forest, northern Iran; the forest is categorized as temperate deciduous forest and covers about 1.9 million hectares (Marvi-Moahdjer 2012). The study area is located in the Kheyrud forest (latitude 36°33′N, longitude 50°33′E; WGS84), an educational and experimental site of University of Tehran, that covers about 8000 ha (Fig. 1).
There was no meteorological record at the study site; however, a nearby meteorological station (7 km distance), Nowshahr Meteorological Station (36°39′N, 51°30′E), reported that from 1985 to 2008, a mean annual precipitation of 1303 mm. October was the wettest month (average 235 mm), and August was the driest month (average 42 mm). Long-term mean annual air temperature was 16.2 °C, with February and August being the coldest and warmest months, respectively. The main parent material are limestone, dolomite, and to lesser extent granite (Deljouei 2013). According to USDA soil classification, the soil in the study site is Alfisol without any diagnostic horizon. Soils experience Udic moisture conditions and Mesic temperature conditions (Anonymous, Department of Forestry and Forest Economic 1995).
Site selection protocol and sampling design
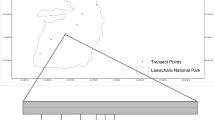
The research was carried out considering the main and secondary forest roads in the second district (Namkhane). Namkhane district covers about 1083 ha, and its elevation ranges from 350 to 1350 m a.s.l. Harvesting operations are conducted in 788 ha of the district, while the rest (295 ha) is considered as protected area. Total road length and road density are 15.8 and 20 m/ha, respectively. The width of the roads is between 5 and 6 m (Deljouei 2013). Main roads are paved by using unsealed aggregate; however, secondary forest roads are recognized as unpaved roads. Furthermore, daily traffic in secondary forest roads is much less than main forest roads. Data was collected in summer 2012 (September) and spring 2013 (April). In accordance with what reported in literature, we set 100 m buffers in both side of the selected roads (e.g., Belinchon et al. 2007; Avon et al. 2010). This also allowed to prevent interaction effects of other roads on sample sites. Sixteen transects along main and secondary forest roads were accomplished (eight transects along main roads and eight along secondary roads). To eliminate the effect of forest canopy, all transects were located in Carpinetum-Fagetum forests, the dominant forest type in the study area (Deljouei 2013). The total length of each transect was 200 m (100 m toward up slope and 100 m toward down slope), and plots were established along it at 0 (road edge), 5, 10, 15, 20, 30, 45, 60, and 100 m distances from road edge (Pauchard and Alaback 2004; 2006; Watkins et al. 2003; Avon et al. 2010). Along each transect, we established 1 × 2 m sampling plots perpendicularity to the road with longer side parallel to the road (Fig. 2). In each plot, we recorded presence and abundance of herbaceous plant species on the forest floor using Londo Scale (Londo 1976). All plants were listed and identified using Flora orientalis (Boissier 1867), Flora of Turkey (Davis 1942), and Flora Iranica (Rechinger 1963–1998). Furthermore, invasive species were identified according to Iranian checklist produced by Shakeri (2012).
Data analysis
Raunkiaer (1934) proposed a life-form classification system based on the place of the plant’s growth-point manner (i.e., buds) during unfavorable seasons. Plant species are often sorted into five classes based on this classification system: phanerophytes, chamaephytes, hemicryptophytes, cryptophytes, and therophytes. As there is no specific life-form class in Raunkiaer’s original system for non-self-supporting plants, these were classified according to the reduction of their aerial parts, as described by Cain (1950). The Raunkiaer system was used to classify species in this study by plant adaptation to ecological conditions (Mera et al. 1999).
The presence or absence of a species in a sampling unit is important to the characterization of different communities (Dai et al. 2006). However, not all species contribute equally to identifying groups, making key indicator species valuable in community classification. For this purpose, a number of approaches have been developed and the species indicator value (IV) method by Dufrêne and Legendre's (1997) has been commonly applied (e.g., Dai et al. 2006). The IV method is an explicit approach for detection of indicator species at various distances from forest roads to identify similarities (or groups) in sample compositions by combining the species abundance of each group with the occurrence likelihood of a species in that group (McCune and Grace 2002; Dai et al. 2006). As a consequence, an indicator species is characterized with a high IV (Dai et al. 2006). In this study, we used IV method to find indicator species together with Monte Carlo test to evaluate the statistical significance of IV (Dufrêne and Legendre 1997).
The diversity of herbaceous plant species was calculated in each plot using Shannon-Wiener index, species richness (number of herbaceous species), and Pielou’s index (Magurran 2004). These indices have been broadly used for calculating species diversity in ecological literatures (e.g., Watkins et al. 2003; Elliott and Knoepp 2005).
Past Ver. 2.17 software was used to calculate all the indices for each plot. To explore differences between main and secondary forest roads and between cut and fill slopes, two-way ANOVA was used (Bihamta and Zare Cahouki 2008). To choose the best model describing correlation of diversity indices to distance from the road, we evaluated linear, polynomial, power, logarithmic, and exponential models using the highest adjusted coefficient of determination (R 2) as discriminate, as well as smallest Akaike’s Information Criterion (AIC). AIC was used as it offers an estimate of information lost when a model is used to represent processes generating the observed data (Akaike 2014). All statistical analyses were conducted using R Ver. 3.0.1 software (R Development Core Team 2013).
Results
We recorded 62 herbaceous plant species distributed among 34 families (Table 1). The family with the greater number of species was Cyperaceae (five species). Twenty families (58.8%) were represented by only a single species. The biological spectrum of the study plots had a high proportion of hemicryptophytes (54.8%), followed by cryptophytes (25.8%), therophyte (11.3%), phanerophytes (4.8%), and chamaephytes (3.3%). We identified five exotic species (Arum maculatum L., Vincetoxicum scabrum L., Dentaria bulbifera L., Equisetum telmateia L., and Sanicula europaea L.), which belong to five different families.
Overall, we found 20 indicator species along main and secondary forest roads along the main slopes (Table 2). Most of these species were classified as hemicryptophytes (45%). Near the main roads, 10 species were found to be indicator species (six and four species in up and down slopes); similar results were obtained in the secondary road. Most (60%) of these species were indicators of no distance from road edges (50% of them are hemicryptophytes). The highest mean value of Shannon-Winner index (2.01) and species richness (11.25) were found to be in the down slope of main road, whereas the highest mean value of Pielou’s index (0.86) was found to be in the up slope of the main road. Overall, the mean amount of Shannon-Winner index and species richness in the secondary roads were higher than main roads, and vice versa for the Pielou’s index (Table 3).
Shannon-Wiener diversity index at different distances (F = 9.87, P = 0.05) from the road edge, up and down slopes (F = 66.71, P = 0.019), and interaction between forest roads and slopes (F = 6.04, P = 0.031) were significantly different. Also, the interaction between slope and distance was significant (F = 2.85, P = 0.032).
The results showed how Shannon-Wiener diversity index was generally higher below the road than above the road for both main and secondary forest roads (Fig. 3 and Table 3). The overall trend was a decrease of index values with increasing the distance from the road. The decreasing trend continued up to 60 m from forest road margin, and after that, the index increased or reached a steady value (excepted main road, up slope from the road). Tukey test (P < 0.05) showed a significant difference between road distance and Shannon-Wiener diversity index in up slope of main road (i.e., road edges showed higher values with respect to other distance) and down slope of secondary road (i.e., 20, 30, 60, and 100 m values were significantly lower than those measured in road edges and 5 m distance transects).
As for species richness, our results showed the number of species sampled at different distances from road edges (up to 100 m) was significantly different (F = 9.34, P = 0.013). Also, there was a significant difference in terms of species richness between up and down slopes (F = 33.04, P = 0.041), and the interaction between slope and road was also significant (F = 22.59, P = 0.017). The trends of species richness index in main and secondary forest roads and in down and up slopes are shown in Fig. 4. Species richness index in down slope was generally higher than in up slope (Fig. 4 and Table 3). Species richness index decreases with increasing distance from the road edge. However, for secondary road in down slope, decreasing trend of species richness index continued up to 60 m, then the index value increased. Moreover, Tukey test (P < 0.05) suggested that there was a significant difference between road distances and species richness index in up slope of main road (i.e., 0 m was higher than to another distance) and in down slope of main road (i.e., zero meter was higher than to 45 and 100 m).
Pielou’s index was computed from data collected from different distances from road edge and for the value of this index interaction of slope and road did not show significantly differences (F = 2.02, P = 0.14; F = 8.82, P = 0.23, respectively). Pielou’s index increases when distance from road edge increases in main road in both of up and slopes, and conversely, trends were shown in secondary roads (Fig. 5). Tukey test (P < 0.05) showed that there was a significant difference between road distances and Pielou’s evenness index only in down slope of secondary road (i.e., the index was lower for 20 m than for 0 and 5 m distance).
Discussion
The results achieved in this study highlighted the impacts of main and secondary roads on herbaceous forest plant diversity, supporting the scientific literature concerning road ecology in temperate areas (e.g., Watkins et al. 2003; Hansen and Clevenger 2005; Avon et al. 2010). In this work, we focused on the impact of roads in temperate forests diversity, considering their effects on the plant communities along main and secondary roads (both up and down slopes) in Hyrcanian forest, Northern Iran.
A total of 62 herbaceous species were identified in the study area, in agreement to what was reported by Esmailzadeh et al. (2012) in the east of Hyrcanian forest, who found 58 herbaceous species in Gorgan forest. The most represented family in the collected set of species was Cyperaceae. This is not surprising, since Cyperaceae is a large family, being the third largest family of monocotyledons occurring across a wide range of altitudes, 0 to 5000 m A.M.S.L. (Mline and Mline 1975; Ghosh and Maiti 2014). Moreover, many species in this family have a high tolerance for extreme temperatures and poor soils (Mline and Mline 1975).
The biological spectrum of plants reflects their adaptive response to the environment and provides an ecological classification that may be indicative of habitat conditions (Siadati et al. 2010). The highest proportion of biological spectra of this study was allocated to hemicryptophytes (54.8%). This result is in agreement with what found by Morre (2008) in temperate forests. It is important to note that hemicryptophyte-dominated flora has also been found to be typical in moist to humid temperate regions (Raunkiaer 1934) and in deciduous and mid-altitudinal forests (Raju et al. 2014).
Since roads can act as both corridors for invasion and suitable habitat, they facilitate the spread of exotic plants (e.g., Tyser and Worley 1992; Watkins et al. 2003; Godefroid and Koedam 2004; Pauchard and Alaback 2004; Flory and Clay 2006; Honu and Gibson 2006). In this study, we found five exotic plant species whose distribution appeared to be restricted to the highly light environment, up to 10 m far from main road and 5 m along secondary road. The combination of high propagule pressure and favorable growing conditions allows exotic plants to establish themselves along roadside edges in many ecosystems (Tyser and Worley 1992; Forman and Deblinger 2000; Parendes and Jones 2000; Gelbard and Belnap 2003; Watkins et al. 2003; Barton et al. 2004; Flory and Clay 2006). It is well known that at their borders, forests face changed light conditions and increased disturbance. These abiotic conditions generally promote higher plant species diversity by encouraging the establishment of more exotic species (e.g., Bernhardt-Römermann et al. 2006). This edge effect was also found in the current study, even though the encroachment of exotic species was limited to the 0 and 10 m plots (in main road) and 0 to 5 m (in secondary road; occurrence of exotic species, e.g., A. maculatum L., V. scabrum L., and Sanicula europea L.). S. europea may prove to be one of the more aggressive exotic competitors of native plant species in the study area due to its size and history of rapid colonization of ditches, swamps, and other aquatic area. A. maculatum is another highly invasive exotic species widespread across most of Europe and Turkey (Pritchard et al. 1993). Light availability along the forest road and in the adjacent forest is only one of the reasons underlying the presence of A. maculatum in the sampled plots. Indeed, warmer temperature linked with more solar radiation influence seed development on the parent plants with respect to dormancy status (Pritchard et al. 1993). This species’ shorter duration of dormancy has been observed during exposure to one or a combination of drought, high nitrogen, and warm temperature conditions (Fenner 1991). All these environmental conditions have been reported in previous work regarding forest roadway impacts on the proximal forest interior habitat (<10 m away from road edge, e.g., Parendes and Jones 2000; Weathers et al. 2001). For example, Weathers et al. (2001) showed that forest edge (0 to 25 m) have received greater amount of nitrogen than forest interior. V. scabrum is another exotic species that we observed along forest roads. This species is native of the Himalayan region and has been indicated with a high invasion potential (Eivazi 2013). D. bulbifera is naturally distributed in northern and central Europe, usually found in calcareous soils (Hultén 1971). We found the species mainly on calcareous soils (Deljouei 2013), confirming what reported in the literature. E. telmateia, another exotic species collected in this study, has been reported in literature as a potential invasive species (Hyde et al. 1978; Eivazi 2013). Found in seepage lines and open woodlands, E. telmateia commonly forms large clonal colonies. The auto-ecology of this exotic species indicates that the low light levels inherent to forest interior conditions may provide an invasion barrier (Brothers and Spingarn 1992; Buckley et al. 2003). Therefore, any non-natural alteration of physical, environmental, and biological barriers allowing more solar radiation to reach the ground facilitated the establishment of exotic plant along the road side up to 5 and 10 m in secondary and main roads, respectively. We hypothesize that, in our study area, processes driving the arrival and establishment of exotic species include: (1) an initial mechanical damage to forest structure during the road building and utilization; (2) the creation of suitable seedbeds with bare mineral soil and high level of light moisture, and nutrients that are required by some exotic species; and (3) the presence of multiple human and natural dispersal vectors.
The analysis based on the species indicator value (IV) method provided by Dufrêne and Legendre's (1997) allowed us to find a set of indicator species for each road distance class (Table 2). In particular, we found that Festuca drymeia and Circaea lutetiana were indicator species for 60 and 100 m distance classes in secondary roads’ up slopes, and Solanum kiesertzkii was found to be an indicator species for the 60-m distance in secondary roads’ down slope. These species established themselves in closed canopy areas, suggesting higher shade tolerance than species situated only 5–10 m from the edge of forest roads. Forest interior plots (i.e., 60 and 100 m plots) appear to be influenced greatly by high shade, leaf litter, and moisture. Geum urbanum, Mentha aquatica, Microstegium vimineum, Poa nemoralis, Prunella vulgaris, Microstegium vimineum, Sambucus ebolus, Setaria viridis, and Feragaria vesca were all found to be as indicator species of forest edges. There are likely more occurrences of this species at road edge due to the high availability of light and high tolerant to road-associated disturbance (Eivazi 2013).
All the considered diversity indices decreased when distance from road edge increases. This decreasing trend continued up to 60 m from forest road margin, and after this threshold, the index slightly increased (except for main road in up slope). Avon et al. (2010) found construction of forest roadways impacted plant community composition, resulting in different communities between road verge and forest interior that extended <5 m into the forest. Frequent disturbances are intrinsic to road verges, including nutrient rich soil (Trumbulak and Frissell 2000; Flory and Clay 2006) and sunlight exposure (Parendes and Jones 2000; Watkins et al. 2003; Flory and Clay 2006). Enhanced sunlight exposure, storm water runoff, and vehicular traffic along roads also aid transportation of seeds via wind, water, and animal movements (Forman and Alexander 1998; Trumbulak and Frissell 2000; Myers et al. 2004; Flory and Clay 2006). Previous results showed that diversity indices decreased with increasing distance from roads (Zeng et al. 2010; Lotfalian et al. 2012). Our results are in agreement with this trend, showing that road construction may contribute to an increased diversity and richness of plant species along roads in the study area. Furthermore, our results find forest roads can strongly influence the diversity of plant communities. Roadway disturbances (e.g., traffic) and maintenance have been shown to decrease the nearby plant community’s species richness (Godefroid and Koedam 2004; Neher et al. 2013). The decrease of species richness index (S) with increased distance from the road could be temporary, with plant species richness declining with the eventual closing of the canopy and recovery of compacted soils, as found by Buckley et al. (2003). This is in accordance with past research, which showed that species richness increased in the vicinity of roads (Laurance 2000; Fahrig and Rytwinski 2009; Berenji Tehrani et al. 2015).
The road type also impacted the overall diversity of plant communities, with a higher average of Shannon-Winner index and species richness associated to secondary roads compared to main roads. The traffic along secondary roads is usually much less than along main roads, providing less disturbance, which favors introduced generalist species. Traffic impacts on environmental conditions surrounding roadways were a result of the construction disturbance and road material- and traffic-related pollution (Angold 1997; Spellerberg 1998).
Conclusions
The results achieved in this work showed significant differences in terms of plant composition and diversity up to 10 m from forest road margins, similar to what was reported by other studies. Moreover, we found five exotic plant species spread in the forest habitats up to 10 m far from main roads and 5 m along secondary roads. We suggest that a thorough monitoring of vegetation after road construction should be included in local forestry plans for a sustainable management of forest systems. Indeed, only careful planning of forest road building activities and a forest management based on ecological grounds will allow to minimize the effect of road construction on forest structure. When considering road impact buffers and managing the areas surrounding roads (e.g., through exotic weed control, selective cuts as well as road closure), road planners and managers should monitor microclimatological shifts along road edges, test their spatio-temporal stability, and assess their relationships with ecological processes. This will aid the design of road schemes less affecting for forest plant diversity, and the integration of road management and construction practices into forest conservation. More attention should be focused on predicting, planning, monitoring, and assessing the cumulative impacts of roadway design, construction, maintenance, and use in forests. Currently, environmental assessment methods and data are insufficient to meet rapid assessment objectives. However, in situ and remote sensing monitoring equipment as well as data compilation, analysis, and modeling techniques are continually being introduced and improved. Advances in computer technology grant practitioners quick access to these monitoring and data handling tools. These new tools have substantially improved environmental assessment of roadway impacts to plant communities (Transportation Research Board and National Research Council 2005).
References
Akaike, H. (2014). Akaike’s information criterion. Hoboken: International Encyclopedia of Statistical Science.
Akay, A. E., Erdas, O., Reis, M., & Yuksel, A. (2008). Estimating sediment yield from a forest road network by using a sediment prediction model and GIS techniques. Building and Environment, 43(5), 687–695.
Amaranthus, M. P., Rice, R. M., Barr, N. R., & Ziemer, R. R. (1985). Logging and forest roads related to increased debris slides in southwestern Oregon. Journal of Forestry, 83(4), 229–233.
Angold, P. G. (1997). The impact of a road upon adjacent heathland vegetation: effects on plant species composition. Journal of Applied Ecology, 34, 409–417.
Anonymous, Department of Forestry and Forest Economic. (1995). First revision of Forest management plan for Namkhaneh District in Kheyrud Educational and Research Forest. Faculty of Natural Resources. Iran: University of Tehran.
Arevalo, J. R., Delgado, J. D., Otto, R., Naranjo, A., Salas, M., & Fernandez-Palacios, J. M. (2005). Exotic species in the roadside plant communities through an altitudinal gradient in Tenerife and Canaria (Canary Islands). Plant Ecology, 7, 185–202.
Avon, C., Berge, L., Dumas, Y., & Dupouey, J.-L. (2010). Does the effect of forest roads extend a few meters or more into the adjacent forest? A study on understory plant diversity in managed oak stands. Forest Ecology and Management, 259(8), 1546–1555.
Avon, C., Dumas, Y., & Bergès, L. (2013). Management practices increase the impact of roads on plant communities in forests. Biological Conservation, 159, 24–31.
Barton, A. M., Brewster, L. B., Cox, A. N., & Prentiss, N. K. (2004). Non-indigenous woody invasive plants in a rural New England town. Biological Invasions, 6, 205–211.
Belinchon, R., Martinez, I., Escudero, A., Aragon, G., & Valladares, F. (2007). Edge effects on epiphytic communities in a Mediterranean Quercus pyrenaica forest. Journal of Vegetation Science, 18(1), 81–90.
Berenji Tehrani, F., Majnounian, B., Abdi, E., & Zahedi Amiri, G. (2015). Impacts of forest road on plant species diversity in a Hyrcanian forest, Iran. Croat J For Eng, 36(1), 63–71.
Bernhardt-Römermann, M., Kirchner, M., Kudernatsch, T., Jakobi, G., & Fischer, A. (2006). Changed vegetation composition in coniferous forests near to motorways in southern Germany: The effects of traffic-born pollution. Environmental Pollution, 143, 572–581.
Bihamta, M. R., & Zare Cahouki, M. (2008). Principles of statistics for the natural resources science. Iran: University of Tehran.
Boissier, P. E. (1867). Flora Orientalis, 5 vols. and Suppl. Basel-Geneva-Lyon.
Brothers, T. S., & Spingarn, A. (1992). Forest fragmentation and alien plant invasion of central Indiana old-growth forests. Conservation Biology, 6, 91–100.
Brown, K. A., Parks, K. E., Bethell, C. A., Johnson, S. E., & Mulligan, M. (2015). Predicting plant diversity patterns in Madagascar: understanding the effects of climate and land cover change in a biodiversity hotspot. PloS One. doi:10.1371/journal.pone.0122721.
Buckley, D. S., Crow, T. R., Nauertz, E. A., & Schulz, K. E. (2003). Influence of skid trails and haul roads on understory plant richness and composition in managed forest landscape in upper Michigan, USA. Forest Ecology and Management, 175, 509–520.
Cadenasso, M. L., & Pickett, S. T. A. (2001). Effect of edge structure on the flux of species into forest interiors. Conservation Biology, 15, 91–98.
Cain, S. A. (1950). Life forms and phytoclimate. Botanical Review, 16, 1–32.
Catford, J. A., Daehler, C. C., Murphy, H. T., Sheppard, A. W., Hardesty, B. D., Westcott, D. A., Rejmánek, M., Bellingham, P. J., Pergl, J., Horvitz, C. C., & Hulme, P. E. (2012). The intermediate disturbance hypothesis and plant invasions: Implications for species richness and management. Perspect Plant Ecol Evol Syst, 14, 231–241.
Dai, X., Page, B., & Duffy, K. J. (2006). Indicator value analysis as a group prediction technique in community classification. South African Journal of Botany, 72, 589–596.
Davis, P. H. (1942). Flora of Turkey and the East Aegean Islands. Edinburgh: Edinburgh University Press.
Delgado, J. D., Arroyo, N. L., Arevalo, J. R., & Fernandez-Palacios, J. M. (2007). Edge effects of roads on temperature, light, canopy cover, and canopy height in laurel and pine forests (Tenerife, Canary Islands). Landscape and Urban Planning, 81(4), 328–340.
Deljouei, A. (2013). Effect of main and secondary forest roads on herbaceous diversity. M.Sc. Thesis, University of Tehran, Iran.
Devictor, V., Julliard, R., & Jiguet, F. (2008). Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos, 11, 507–514.
Dufrêne, M., & Legendre, P. (1997). Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs, 67, 345–366.
Eivazi, M. R. (2013). The role of forest roads in introduction and rehabilitation of invasive plant species (case study: Kheyrud forest, Patom Series), M.Sc. thesis, Department of Forestry and Forest Economics, University of Tehran, Iran.
Elliott, K. J., & Knoepp, J. D. (2005). The effects of three regeneration harvest methods on plant diversity and soil characteristics in the southern Appalachians. Forest Ecology and Management, 211, 296–317.
Esmailzadeh, O., Hosseini, S. M., Asadi, H., Ghadiripour, P., & Ahmadi, A. (2012). Plant biodiversity in relation to physiographical factors in Afratakhteh yew (Taxus baccata L.) habitat, NE Iran. J Plant Biol, 4(12), 1–12.
Ewers, R. M., & Didham, R. K. (2006). Continuous response functions for quantifying the strength of edge effects. Journal of Applied Ecology, 43, 527–536.
Eycott, A. E., Watkinson, A. R., & Dolman, P. M. (2006). Ecological patterns of plant diversity in a plantation forest managed by clearfelling. Journal of Applied Ecology, 43, 1160–1171.
Fahrig, L., & Rytwinski, T. (2009). Effects of roads on animal abundance: an empirical review and synthesis. Ecology and Society, 14(1), 21.
Fenner, M. (1991). The effects of the parent environment on seed germinability. Seed Science Research, 1, 75–84.
Findlay, C. S., & Houlahan, J. (1997). Anthropogenic correlates of species richness in southeastern Ontario wetlands. Conservation Biology, 11(4), 1000–1009.
Flory, S. L., & Clay, K. (2006). Invasive shrub distribution varies with distance to roads and stand age in eastern deciduous forests in Indiana, USA. Plant Ecology, 184, 131–141.
Flory, S. L., & Clay, K. (2009). Effects of roads and forest successional age on experimental plant invasions. Biological Conservation, 142, 2531–2537.
Forman, R. T. T., & Alexander, L. E. (1998). Roads and their major ecological effects. Annual Review of Ecology and Systematics, 29, 207–231.
Forman, R. T. T., & Deblinger, R. D. (2000). The ecological road effect zone of a Massachusetts (USA) suburban highway. Conservation Biology, 14, 36–46.
Forsyth, A. R., Bubb, K. A., & Cox, M. E. (2006). Runoff, sediment loss and water quality from forest roads in a southeast Queensland coastal plain Pinus plantation. Forest Ecology and Management, 221(1–3), 194–206.
Gatson, K. J. (2000). Global patterns in biodiversity. Nature, 405, 220–227.
Gelbard, J. L., & Belnap, J. (2003). Roads as conduits for exotic plant invasions in a semiarid landscapes. Conservation Biology, 17, 420–432.
Ghosh, A., & Maiti, G. (2014). Taxonomy and present distribution of different species of Carex L., Cyperaceae in Darjeeling and Sikkim Himalayas, India. Int J Life Sci Pharmacol Res, 4(3), 33–56.
Godefroid, S., & Koedam, N. (2004). The impact of forest paths upon adjacent vegetation: effects of the path surfacing material on the species composition on soil compaction. Biological Conservation, 119, 405–419.
Hansen, M., & Clevenger, A. P. (2005). The influence of disturbance and habitat on the frequency of non-native plant species along transportation corridors. Biological Conservation, 125, 249–259.
Harrison, S., & Bruna, E. (1999). Habitat fragmentation and large-scale conservation: what do we know for sure? Ecography, 22, 225–232.
Hawbaker, T. J., Radeloff, V. C., Clayton, M. K., Hammer, R. B., & Gonzalez-Abraham, C. E. (2006). Road development, housing growth, and landscape fragmentation in northern Wisconsin: 1937-1999. Ecological Applications, 16, 1222–1237.
Honu, Y., & Gibson, D. J. (2006). Microhabitat factors and the distribution of exotic species across forest edges in temperate deciduous forest of southern Illinois, USA. J Torrey Bot Soc, 133, 255–266.
Hosseini, S. A. (2010). The effect of forest road clearing limit on regeneration composition. Agriculture and Biology Journal of North America, 1(4), 487–490.
Hosseini, S. A., Jalilvand, H., Pourmajidian, M. R., & Parsakhoo, A. (2011). Effects of forest road clearings on understory diversity beneath Alnus subcordata L. stands in Iran. Maejo International Journal of Science and Technology, 5(2), 241–251.
Hultén, E. (1971). Atlas över växternas utbredning inorden. Stockholm: Generalstabens litografiska anstalts förlag.
Hyde, H. A., Wade, A. E., & Harrison, S. G. (1978). Welsh Ferns. Cardiff: National Museum of Wales.
Laurance, W. F. (2000). Do edge effects occur over large spatial scales? Trends Ecol Evol, 15, 134–145.
Laurance, W. F., Cochrane, M. A., Bergen, S., Fearnside, P. M., Delamônica, P., Barber, C., D'Angelo, S., & Fernandes, T. (2001). The future of the Brazilian Amazon. Science, 291, 438–439.
Lee, M. A., Davies, D., & Power, S. A. (2012). Effects of roads on adjacent plant community composition and ecosystem function: an example from three calcareous ecosystems. Environmental Pollution, 163, 273–280.
Londo, G. (1976). The decimal scale for releves of permanent quadrates. Vegetation, 33(1), 61–64.
Lotfalian, M., RiahiFar, N., Fallah, A., & Hodjati, S. M. (2012). Effects of roads on understory plant communities in a broadleaved forest in Hyrcanian zone. Journal of Forest Science, 58(10), 446–455.
MacDougall, A. S., McCann, K. S., Gellner, G., & Turkington, R. (2013). Diversity loss with persistent human disturbance increases vulnerability to ecosystem collapse. Nature, 494, 86–89.
Magurran, A. E. (2004). Measuring biological diversity. Oxford: Blackwell.
Marcantonio, M., Rocchini, D., Geri, F., Bacaro, G., & Amici, V. (2013). Biodiversity, roads, & landscape fragmentation: two Mediterranean cases. Applied Geography, 42, 63–72.
Marvi-Moahdjer, M. R. (2012). Silviculture. Iran: University of Tehran.
McCune, B., & Grace, J. B. (2002). Analysis of ecological communities. Gleneden Beach: MjM Software Design.
Mera, A. G., Hagen, M. A., & Oreliana, J. A. V. (1999). Aerophyte, a new life form in Raunkiaer’s classification? Journal of Vegetation Science, 10, 65–68.
Mline, L. J., & Mline, M. J. G. (1975). Living plants of the world. New York City: Random House.
Morre, P. D. (2008). Tundra. New York.
Mullen, K., Fahy, O., & Gormally, M. (2003). Ground flora and associated arthropod communities of forest road edges in Connemara, Ireland. Biodiversity and Conservation, 12, 87–101.
Myers, J. A., Vallend, M., Gardescu, S., & Marks, P. L. (2004). Seed dispersal by white-tailed deer: Implications for long distance dispersal, invasion, and migration of plants in eastern North America. Oecologia, 139, 35–44.
Neher, D. A., Amussen, D., & Lovell, S. T. (2013). Roads in northern hardwood forest affect adjacent plant communities and soil chemistry in the proportion to the maintained roadside area. Science of the Total Environment, 449, 320–327.
Parendes, L. A., & Jones, J. A. (2000). Role of light availability and dispersal in exotic plant invasion along roads and streams in the H. J. Andrews Experimental Forest, Oregon. Conservation Biology, 14, 64–75.
Pauchard, A., & Alaback, P. B. (2004). Influence of elevation, land use, and landscape context on patterns of alien plants invasions along roadsides in protected areas of south-central Chile. Conservation Biology, 18(1), 238–248.
Pausas, J. G., & Austin, M. P. (2001). Patterns of plant species richness in relation to different environments: an appraisal. Journal of Vegetation Science, 12, 153–166.
Pritchard, H. W., Wood, J. A., & Manger, K. R. (1993). Influence of temperature on seed germination and the nutritional requirements for embryo growth in Arum maculatum L. New phytologist, 123(4), 801-809.
R Development Core Team. (2013). R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Raju, V. S., Krishna, P. G., & Suthari, S. (2014). Environmental assessment of climate of a habitat through floristic life-form spectra, a case study of Warangal north forest division, Telangana, India. J Nat Sci, 2(1), 77–93.
Raunkiaer, C. (1934). The life forms of plants and statistical plant geography. Oxford: Clarendon Press.
Rechinger, K. H. (1963–1998). Flora Iranica (Vol. Vol. 1–173). Graz: Akademisch Druck-U Verlagsanstalt.
Scharnweber, T., Rietschel, M., & Manthey, M. (2007). Degradation stages of the Hyrcanian forests in southern Azerbaijan. Archiv Für Naturschutz und Landschafts Forschung, 46, 133–156.
Schnitzer, S. A., Klironomos, J. N., Lamberes, J. H. R., Kinkel, L. L., Reich, P. B., Xiao, K., Rillig, M. C., Sikes, B. A., Callaway, R. M., Mangan, S. A., Van Nes, E. H., & Scheffer, M. (2011). Soil microbes drive the classic plant diversity-productivity pattern. Ecology, 92(2), 296–303.
Shakeri, Z. (2012). Invasion plant species from disturbance in Fagetum forests in northern Iran (Case study: Kheyroud forest). Doctoral thesis, department of Forestry and Forest Economics, University of Tehran, Iran.
Siadati, S., Moradi, H., Attar, F., Etemad, V., Hamzeh’HH, B., & Naqinezhad, A. (2010). Botanical diversity of Hyrcanian forests; a case study of a transect in the Kheyrud protected lowland mountain forests in northern Iran. Phytotaxa, 7, 1–18.
Spellerberg, I. F. (1998). Ecological effects of roads and traffic: a literature review. Global Ecology and Biogeography Letters, 7(5), 317–333.
Transportation Research Board and National Research Council. (2005). Assessing and managing the ecological impacts of paved roads. Washington, DC: The National Academies Press. doi:10.17226/11535.
Trumbulak, S. C., & Frissell, C. A. (2000). Review of ecological effects of roads on terrestrial and aquatic communities. Conservation Biology, 14, 18–30.
Tyser, R. W., & Worley, C. A. (1992). Alien flora in grasslands adjacent to road and trail corridors in Glacier National Park, Montana, USA. Conservation Biology, 6, 253–262.
Watkins, R. Z., Chen, J., Pickens, J., & Brosofske, K. D. (2003). Effects of forest roads on understory plants in a managed hardwood landscape. Conservation Biology, 17(2), 411–419.
Weathers, K. C., Cadenasso, M. L., & Pickett, S. T. A. (2001). Forest edges as nutrient and pollutant concentrators: potential synergisms between fragmentations, forest canopies, and the atmosphere. Conservation Biology, 150, 1506–1514.
Zemke, J. J. (2016). Runoff and soil erosion assessment on forest roads using a small scale rainfall simulator. Hydrology, 3(3), 1–25.
Zeng, S., Zhang, T., Gao, Y., Ouyang, Z., Chen, J., Li, B., & Zhao, B. (2010). Effects of road disturbance on plant biodiversity. World Academy of Science, Engineering and Technology, 66, 437–448.
Zurita, G., Pe’er, G., Bellocq, M. I., & Hansbauer, M. M. (2012). Edge effects and their influence on habitat suitability calculations: a continuous approach applied to birds of the Atlantic forest. Journal of Applied Ecology, 49, 503–512.
Acknowledgments
Dr. Kevin Boston is thanked for his supportive comments and reviews in early version of this paper and also English improvement. Dr. John Toland Van Stan is thanked for English improvement. The authors also express their sincere appreciation to the two anonymous reviewers for their helpful and valuable detailed comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
Careful planning of road building would allow to minimize the negative effect of road construction. Roads cause changes in ecosystems at multiple scales, from the microclimatic processes in the road corridor to the population dynamics and dispersal possibilities of different species. The effects of roads can be measured as the distance from the road, within which changes in species diversity and abundance as well as in hydrological flows, erosion and sedimentation rates can be observed, relative to a control location. Hence, it is plausible that forest plants in adjacent of road and forest interior have different characteristics. In this study, we aim at investigating the diversity of herbaceous species along main and secondary roads in Hyrcanian forest, north of Iran, taking into account the effect of cut slope (the soil surface that remains above the road after material is removed) and fill slope (the soil surface build below the road).
Rights and permissions
About this article
Cite this article
Deljouei, A., Abdi, E., Marcantonio, M. et al. The impact of forest roads on understory plant diversity in temperate hornbeam-beech forests of Northern Iran. Environ Monit Assess 189, 392 (2017). https://doi.org/10.1007/s10661-017-6105-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-6105-1