Abstract
A quick, easy, cheap, effective, rugged and safe (QuEChERS) method was optimized for the extraction of non-steroidal anti-inflammatory drugs (NSAIDs) diclofenac and ibuprofen from sewage sludge. Dispersive-solid phase extraction (d-SPE) was employed for sample clean-up. Instrumental analysis was performed by high-performance liquid chromatography. Ecological risk was assessed for four trophic levels: fish, daphnia, algae and bacteria. The method limits of quantification for diclofenac and ibuprofen were 0.43 µg g− 1 and 0.45 µg g− 1, respectively. Correlation coefficients were above 0.999. Extraction recoveries ranged from 70 to 118 % and satisfactory inter-day reproducibility (% RSD) of < 18 % was obtained. Diclofenac and ibuprofen were measured up to 1.02 µg g− 1 and 6.6 µg g− 1, respectively in sewage sludge from three Nigerian wastewater treatment plants (WWTPs). Ibuprofen posed high risk to fish, daphnia, algae and bacteria. This work presents the first report on the ecological risk assessment of diclofenac and ibuprofen in sewage sludge from Nigerian WWTPs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Diclofenac and ibuprofen are the most commonly used non-steroidal anti-inflammatory drugs (NSAIDs), accounting for 22 % and 51 % respectively of total NSAIDs consumption (Conaghan 2012; Zhang et al. 2020). Diclofenac and ibuprofen are the most commonly detected NSAIDs in the environment due to their large consumption in daily life and incomplete removal during wastewater treatment processes (Zhang et al. 2020). Diclofenac was included in the previous watch list of substances to be established alongside the list of priority substances by the European Union (EU 2013; Vieno and Sillanpää 2014). Wastewater treatment plants (WWTPs) are known to contribute to the spread of pharmaceutical residues in the environment through the disposal of sewage sludge and application of sewage sludge as organic manures. Due to their large consumption, these NSAIDs have been detected worldwide in sewage sludge (Vieno and Sillanpää 2014).
The widespread occurrence of diclofenac and ibuprofen in the aquatic ecosystems raises concerns about their potential risks to aquatic organisms (Zhang et al. 2020). Non-target organisms might be adversely affected by relevant ambient low-level concentrations of these NSAIDs after long-time exposures (Du et al. 2016). Ibuprofen and diclofenac were recently found to impair the cardiovascular development of zebrafish (Danio rerio) at low environmentally relevant concentrations (Zhang et al. 2020). Toxicity data of the target compounds are presented in Table 1.
Diclofenac and ibuprofen are prone to adsorption onto sewage sludge based on the criteria for predicting a compound sorption behavior (Verlicchi and Zambello 2015) and their Log Kow values as shown in Table S1 (Supplementary material).This implies that sewage sludge can serve as an important reservoir for these compounds. Therefore, concentrations of diclofenac and ibuprofen in sludge can give an important indication of their pollution levels. Sewage sludge is such a complex matrix that contains substances that can interfere with the determination of analytes of interest and this interference may have negative impact on the whole analytical process (Pérez-Lemus et al. 2019). It is therefore necessary to develop an efficient extraction protocol and clean-up procedure to eliminate or reduce such interferences.
The most commonly applied extraction techniques for diclofenac and ibuprofen determination in sewage sludge are ultrasound assisted extraction (UAE), pressurized liquid extraction (PLE) and microwave assisted extraction (MAE) (Pérez-Lemus et al. 2019; Lonappan et al. 2016; Guerra et al. 2014). These techniques usually involve long and laborious analytical processes and the use of relatively expensive equipment (Ajibola et al. 2020a; Pérez-Lemus et al. 2019). Liquid chromatography coupled to mass spectrometry or tandem mass spectrometry has been mostly employed for the determination of these NSAIDs due to its high sensitivity and selectivity (Pérez-Lemus et al. 2019; Rossini et al. 2016; Lonappan et al. 2016; Peysson and Vulliet 2013). However, these instruments are not readily available in most laboratories of developing countries due to the associated high acquisition and maintenance cost (Ajibola et al. 2020b). Hence, the applicability of the more readily available and affordable high performance liquid chromatography using ultraviolet or diode-array detection has also been demonstrated for these NSAIDs determination (Ashfaq et al. 2017; Hlengwa and Mahlambi 2020).
One of the green and environment-friendly sample preparation techniques recently applied for the determination of pharmaceuticals in sewage sludge is the quick, easy, cheap, effective, rugged and safe (QuEChERS) extraction (Nannou et al. 2019; Pérez-Lemus et al. 2019). QuEChERS extraction offers advantages such as low solvent consumption, rapidity, low cost and simplicity (Ajibola et al. 2020a; Anastassiades et al. 2003). Despite the aforementioned advantages of QuEChERS extraction only few studies (Rossini et al. 2016; Peysson and Vulliet 2013) have exploited, so far, the applicability of QuEChERS method for the extraction of diclofenac and ibuprofen from sewage sludge. QuEChERS methods were recently developed and applied successfully for determination of multiclass antibiotics in sewage sludge (Ajibola et al. 2020a) and fluoroquinolone antibiotics in wastewater (Ajibola et al. 2020b) from WWTPs in Nigeria. There is currently a paucity of analytical and environmental data on the occurrence and potential ecological risks of diclofenac and ibuprofen residues in sewage sludge from WWTPs in Africa, including Nigeria. To the best of authors’ knowledge only one study (Olarinmoye et al. 2016) reported, till date, the occurrence of diclofenac and ibuprofen in sewage sludge from WWTPs in Nigeria. Furthermore, ibuprofen is an over-the-counter drug in Nigeria and both NSAIDs are commonly prescribed. Consequently, there is high level of human consumption of these drugs and their presence is anticipated in sewage sludge from Nigerian WWTPs. Moreover, the common fates of sewage sludge in the Nigerian WWTPs investigated in the present study are land application for agricultural purposes and disposal into dumpsites. As a result, diclofenac and ibuprofen residues may enter surface water via runoff from soil or leach into groundwater from dumpsites. Therefore, the presence of diclofenac and ibuprofen in sewage sludge samples may pose a potential threat for the soil and aquatic ecosystem.
Against this background, the objective of this work was to optimize a QuEChERS based extraction method for the analytical determination of diclofenac and ibuprofen in sewage sludge. This work was also aimed at investigating the occurrence and potential ecological risks of diclofenac and ibuprofen residues in sewage sludge from three Nigerian WWTPs. This study expands our knowledge on the applicability of QuEChERS extraction method for determination of target NSAIDs in sewage sludge. To the best of our knowledge, this is the first report on the ecological risk assessment of diclofenac and ibuprofen residues in sewage sludge from municipal and hospital WWTPs in Nigeria.
Materials and Methods
Analytical standards of diclofenac and ibuprofen as well as Na2EDTA.2H2O salt were purchased from Sigma Aldrich (Steinheim, Germany). Acetonitrile and methanol of HPLC grade were obtained from Merck (Darmstadt, Germany). Ultrapure water was provided by a Milli-Q water purification system (Merck, Germany). Sodium chloride was purchased from Acros Organics (Denmark) while magnesium sulphate was purchased from Acros Organics (Germany). Sodium acetate was purchased from Fisher Scientific (United Kingdom). Formic acid of analytical grade was used. Dispersive-SPE sorbent of primary and secondary amine (PSA bulk sorbent) was purchased from Supelco (Bellefonte, USA). Stock standard solutions (100 µg mL− 1) of diclofenac and ibuprofen were prepared in acetonitrile. Standard working solutions were prepared from the stock solutions. Some relevant physicochemical properties of target compounds are presented in Table S1 (Supplementary material).
Sewage sludge samples were collected from three WWTPs in Lagos and Ibadan, South-Western Nigeria: Alausa WWTP in Lagos (GPS: 6.6164143, 3.3609648), Ijaiye WWTP in Lagos (GPS: 6.6434736, 3.325676) and University College Hospital (UCH) WWTP in Ibadan (GPS: 7.4056697, 3.9038453). While Alausa WWTP is a typical urban municipal WWTP, Ijaiye and UCH WWTPs are hospital WWTPs. UCH and Alausa WWTPs employ the activated sludge process of treatment while Ijaiye WWTP incorporates a membrane bioreactor with anaerobic digestion as treatment processes. Details about the characteristics and operational parameters of the investigated WWTPs have been presented in a previous study (Ajibola et al. 2020a). Pre-cleaned amber glass bottles were used for samples collection. The samples were transported immediately into the laboratory in an insulated box (cooler) with ice to keep the temperature below 4 ℃ during transportation. On arrival in the laboratory the samples were stored in the freezer at −20 ℃. Sewage sludge samples were collected from the three WWTPs in September 2017 (first sampling) and September 2018 (second sampling). In September 2017, both primary and secondary sludge samples were collected from UCH WWTP for five consecutive days, secondary sludge samples were collected from Ijaiye WWTP for three consecutive days and one secondary sludge sample was collected from Alausa WWTP. In the second sampling (September 2018), sewage sludge samples were collected for three consecutive days from UCH WWTP and Ijaiye WWTP. Sewage sludge samples could not be collected at Alausa WWTP for the second sampling campaign. Three grab samples collected daily were pooled to give a single sample (approximately 2 L) of sludge per day. Sewage sludge samples were air-dried, ground, sieved, wrapped with aluminum foil and stored in the freezer before QuEChERS extraction. A mixture of representative quantities of all sewage sludge samples (primary and secondary sewage sludge) was used for performing optimization and validation. The spiking procedure of sewage sludge involved the spiking of a mixed standard solution of target NSAIDs, vigorously shaken on a vortex mixer to ensure that the analytes have sufficient contact with the sludge sample. The samples were left in the dark overnight to enable sufficient interaction of analytes with the sewage sludge and to remove the solvent introduced during spiking (Ajibola et al. 2020a; Peysson and Vulliet 2013).
The optimized QuEChERS extraction involves the weighing of 2 g dried and homogenized sludge sample into a 50 mL Falcon® polypropylene centrifuge tube. 200 µL of water was added into the centrifuge tube and shaken vigorously. The samples were left in the dark overnight so as to allow the solvent introduced to evaporate. 10 mL of 0.1 M EDTA was added and manually shaken. Afterwards, 8 mL of acetonitrile was added followed by 2 mL of methanol. The mixture was manually shaken and vortex-mixed for 1 min. Acetate buffer salt (consisting of 1 g CH3COONa and 4 g MgSO4) was added. The centrifuge tube was immediately hand-shaken so as to prevent the coagulation of MgSO4, and immediately vortex-mixed for 2 minutes. Centrifugation of the sample was afterwards carried out for 15 minutes at 3,500 rpm.
Dispersive-solid phase extraction (d-SPE) was employed for the clean-up of extracts from QuEChERS extraction. The organic phase of QuEChERS extraction was transferred into a 15 mL polypropylene centrifuge tube containing the clean-up sorbent (150 mg PSA + 450 mg MgSO4). The mixture was shaken manually, vortex-mixed for 1 min and centrifuged at 1,000 rpm for 5 minutes. The supernatant solution was decanted into a glass vial and evaporated gently to dryness. The residue was reconstituted with 2 mL mixture of acetonitrile and acidified water (pH of 3.0), 80:20 (v/v), resulting in an enrichment factor of 5. The reconstituted residue was transferred into a 2 mL vial for HPLC analysis.
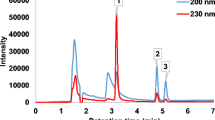
Chromatographic separation and detection of target compounds was performed using an Agilent Series 1100 HPLC system equipped with UV and fluorescence detectors. The HPLC system was equipped with an Agilent degasser, a pump and an auto-sampler. Chromatographic separation of the analytes was achieved with a Zorbax Eclipse XDB-C18 column (4.6 mm x 150 mm, 5 µm) at ambient temperature. The mobile phase is a mixture of acetonitrile and ultrapure water (acidified to pH 3.0 with formic acid), 60:40 (v/v). Isocratic elution was carried out at a flow rate of 0.8 mL min− 1. Three minutes of re-equilibration was programmed between successive injections and the injection volume was 20 µL. The wavelength of 200 nm was used for UV detection. Quantification of target compounds was carried out using the matrix-matched calibration curves constructed by spiking matrix extracts after the extraction.
Analyses of procedural blanks were carried out to ensure that the reagents used were free of the analytes of interest. In order to ascertain if the HPLC mobile phase was free of diclofenac and ibuprofen residues, 20 µL of the mobile phase (acetonitrile/acidified water) was injected into the HPLC instrument. The procedural blanks and mobile phase contained no ibuprofen and diclofenac residues. Between successive injections of samples into the HPLC instrument, solvent blank (consisting of only mobile phase) injections were carried out to avoid carry over from a previous injection.
Linearity was assessed by matrix-matched calibration curves generated by spiking the sludge extracts at four concentration levels between 0.1 µg mL− 1 and 10 µg mL− 1 for ibuprofen and three concentration levels between 0.5 µg mL− 1 and 10 µg mL− 1 for diclofenac. Extraction and overall recoveries were determined by spiking sludge samples at two different concentrations (1 µg g− 1 and 10 µg g− 1). Extraction recovery is the extraction efficiency of the analyte in QuEChERS extraction. Overall recovery is the performance of the entire analytical method which includes the extraction step, d-SPE clean-up and instrumental performance. For the calculation of overall recovery, the responses of pre-extraction spike samples were compared with the responses of the corresponding standard solution while extraction recovery was calculated by comparing the responses of pre-extraction spike samples with those of the post-extraction spike samples at the corresponding concentrations. Mathematically,
Precision of the method was appraised through intra-day and inter-day analyses. The intra-day (repeatability) and inter-day (reproducibility) were performed at 1 µg g− 1 in three replicate determinations and were expressed as relative standard deviation (RSD, %). Limit of detection (MLOD) and limit of quantification (MLOQ) of the method were determined through the matrix-matched calibration curves obtained by spiking sludge samples prior to extractions at a low concentration level (Gago-Ferrero et al. 2015) of 1.0 µg g− 1.The variation (standard deviation) of the responses (concentrations) of the replicate determinations was multiplied by a factor of 3.3 and divided by the slope of the calibration curve for calculating MLOD while the variation (standard deviation) of the responses (concentrations) of the replicate determinations was multiplied by 10 and divided by the slope of the calibration curve for calculating MLOQ.
Risk assessment of ibuprofen and diclofenac residues in sewage sludge (secondary sludge) was evaluated by calculating risk quotient (RQ) values. Toxicity data (EC50) was obtained from literature for four different trophic levels: fish, daphnia, algae and bacteria (Verlicchi et al. 2012; Sanderson et al. 2003; Ra et al. 2008). Details concerning the toxicity data and literature sources are presented in Table 1. Predicted no effect concentrations for water (PNECwater) was calculated by dividing EC50 by an assessment factor of 1000 so as to take into account the effect on other, potentially more sensitive, aquatic species to those used in toxicity studies (Verlicchi et al. 2012; Martin et al. 2012). PNEC for sludge (PNECsludge) was estimated from PNECwater using the following equation according to Martin et al. (2012):
where Kd is the solid–water distribution coefficient of the corresponding pharmaceutical. Kd values of diclofenac (232 L Kg− 1 for secondary sludge) and ibuprofen (7.1 L Kg− 1 for secondary sludge) were obtained from Stasinakis et al. (2013) and Ternes et al. (2004), respectively. Maximum concentration was used as measured environmental concentration (MEC) for calculating RQ value to assess the worst case scenario. In cases where all the measured concentration values for a given matrix or sampling site were below the MLOQ, the value of MLOQ was used as MEC for the RQ calculation. RQ values were calculated as the ratio of MEC to the predicted no effect concentrations PNECsludge of the target NSAIDs in accordance to the European Commission Technical Guidance Document on Risk Assessment (European Commission 2003). The risk ratios were categorized according to Wee et al. (2019) as negligible risk (RQ < 0.01), low risk (0.01 ≤ RQ < 0.1), medium risk (0.1 ≤ RQ < 1), and high risk (RQ ≥ 1).
Results and Discussion
The QuEChERS method is based on two sequential steps: a solid–liquid (buffered) partitioning with a salting-out effect followed by a clean-up using dispersive solid-phase extraction (d-SPE) (Nannou et al. 2019). For method development and optimization, 2 g dried and homogenized sludge sample was spiked with standard solution of diclofenac and ibuprofen at 10 µg g− 1 fortification level. The optimization of QuEChERS extraction was carried out by optimizing the extracting solvents composition and buffer salt. The influence of Na2EDTA on the recoveries of target compounds from sewage sludge was also investigated. Acetonitrile was chosen as organic based solvent. In some experiments, acetonitrile (organic solvent) was modified with methanol (20 %) while aqueous solvent was modified with 0.1 M Na2EDTA. The buffer salt compositions evaluated were: unbuffered salt consisting of 4 g MgSO4 + 1 g NaCl and acetate buffer salt consisting of 4 g MgSO4 + 1 g CH3COONa. Duplicate determinations were carried out for each set of experiments. Results of the optimization of extraction solvents and buffer salt composition are presented in Fig. 1.
The extraction recoveries of both diclofenac and ibuprofen were < 10 % when only acetonitrile was used as organic solvent in combination with unbuffered salt. Modification of acetonitrile content with methanol (20 %), still in combination with unbuffered salt, yielded the extraction recoveries of 38 % and 35 % for diclofenac and ibuprofen, respectively. This implies that the addition of methanol to the organic solvent slightly improved the extraction recoveries of both compounds. Moreover, when acetate buffer salt was experimented using only acetonitrile as organic solvent the extraction recoveries of diclofenac and ibuprofen were 43 % and 37 %, respectively. There was a considerable improvement in the extraction recoveries of both compounds in comparison with when unbuffered salt was used (< 10 %). This shows that acetate buffer salt was more efficient than unbuffered salt. With the use of acetate buffer, the pH gets close to 4.8 facilitating the extraction of pH dependent compounds (Lehotay et al. 2010) like diclofenac and ibuprofen which are weakly acidic (Table S1, supplementary material). Modification of acetonitrile content with methanol (20 %), in combination with acetate buffer salt, 57 % extraction recovery was obtained for diclofenac while 54 % extraction recovery was obtained for ibuprofen. Again, modification of acetonitrile with methanol improved the extraction efficiency of both compounds. The use of the polar co-solvent methanol increased the polarity of the organic phase (Guo et al. 2016) thereby improving the solubility of diclofenac and ibuprofen in the organic phase. This resulted into improved extraction recoveries of the compounds. Hlengwa and Mahlambi (2020) recently demonstrated that a mixture of water, methanol and acetonitrile provided good extraction recoveries for these NSAIDs in soil using ultrasonic extraction. Also, an improvement in the extraction of antibiotics was observed when a mixture of acetonitrile and methanol was used rather than only acetonitrile (Ajibola et al. 2020a). Therefore, acetonitrile modified with methanol (20 %) was used for subsequent optimization experiments.
In order to further improve the extraction recoveries of both compounds, modification of the aqueous solvent was investigated by considering the influence of Na2EDTA. In this regard, some experiments were conducted using only water or 0.1 M Na2EDTA in water as the aqueous solvent. Acetonitrile modified with methanol (20 %) in combination with either acetate buffer salt or unbuffered salt was used. The results of the extraction recoveries, reflecting the influence of Na2EDTA, are also shown in Fig. 1. Again, acetate buffer salt was more efficient than unbuffered salt for the extraction of diclofenac and ibuprofen (Fig. 1). Moreover, modification of the aqueous solvent with Na2EDTA considerably improved the extraction of both compounds using either acetate buffer salt or unbuffered salt. For the unbuffered salt, diclofenac extraction recovery improved from 38–43 % while ibuprofen extraction recovery improved from 35–52 %. Results with the acetate buffer salt showed that diclofenac extraction recovery improved from 57–71 % while ibuprofen extraction recovery from 54–80 %. Overall, addition of Na2EDTA improved the extraction efficiency of both compounds from sewage sludge. Addition of Na2EDTA as chelating agent can facilitate the extraction of analytes by preventing their complexation with divalent cations such as Mg2+ and Ca2+ (Ajibola et al. 2020a; Peysson and Vulliet 2013). Diclofenac and ibuprofen have the capacity to form complexes with cations due to the carboxylate group (structures are shown in Table S1, Supplementary information) which can act as a ligand (Psomas 2020). This can lead to reduction in the recovery of the compounds. To prevent this, Na2EDTA acted as a chelating agent to bind the metal ions, resulting in the improvement of the extraction recoveries of the compounds. Peysson and coworker also observed a general improvement (of 50 %) in the recoveries of pharmaceuticals from sewage sludge when water was replaced by 0.1 M EDTA (Peysson and Vulliet 2013).
The recoveries (means) of the different extraction procedures were compared using the standard error of the means according to Nicholls 2016. All recoveries (means) of the different extraction procedures were statistically different. The efficiency of the clean-up procedure was estimated by comparing the extraction recoveries and overall recoveries for each compound. From Table 2 the d-SPE clean-up procedure was very efficient for diclofenac and moderately efficient for ibuprofen probably due to its incomplete desorption from the d-SPE sorbent or matrix interference. The method workflow is presented in Fig S1 (supplementary material).
It should be noted that all the experiments during the optimization stage were performed at a spiking concentration level of 10 µg g− 1. For method validation, a lower spiking concentration level of 1 µg g− 1 was included. Better extraction recoveries were obtained for both diclofenac and ibuprofen at a lower spiking concentration level of 1 µg g− 1 (Table 2). Finally, the optimized QuEChERS extraction solvents involved 10 mL of 0.1 M Na2EDTA (in water), 8 mL acetonitrile and 2 mL methanol. Acetate buffer salt (1 g CH3COONa and 4 g MgSO4) was chosen for liquid-liquid partitioning. Dispersive-solid phase extraction (d-SPE) sorbent (150 mg PSA + 450 mg MgSO4) was used for the clean-up of QuEChERS extracts.
The performance parameters of the analytical method are presented in Table 2. Extraction recoveries and overall recoveries of diclofenac and ibuprofen in sewage sludge were evaluated at two different fortification levels (1.0 µg g− 1 and 10 µg g− 1). Extraction recoveries of diclofenac at 1.0 µg g− 1 and 10 µg g− 1 fortification levels were 118 ± 6 % and 80 ± 4 %, respectively whereas the extraction recoveries for ibuprofen at 1.0 µg g− 1 and 10 µg g− 1 fortification levels were 81 ± 7 % and 70 ± 5 %, respectively. Overall recoveries of diclofenac at 1.0 µg g− 1 and 10 µg g− 1 fortifications were 98 % and 76 %, respectively while the overall recoveries of ibuprofen were 44 % and 53 %. The obtained lower overall recoveries of ibuprofen compared to its extraction recoveries could be a result of matrix interference or incomplete desorption from d-SPE sorbent. Hence, matrix-matched calibration was applied for the quantification of both NSAIDs. Better recovery values were achieved in this present work than the recoveries reported by Rossini et al. (2016) who obtained recoveries range of 37 % to 55 % for diclofenac and recoveries range of 4–49 % for ibuprofen. Saleh et al. (2011) reported similar recoveries to our study for diclofenac (90.3 %) and ibuprofen (47.8 %) using pressurized hot water extraction (PHWE).
The method MLOD for both diclofenac and ibuprofen was 0.13 µg g− 1 while MLOQ values were 0.43 µg g− 1 and 0.45 µg g− 1 for diclofenac and ibuprofen, respectively. MLOQs of 0.05 µg g− 1 and 3.0 µg g− 1 were reported for diclofenac and ibuprofen, respectively by Peysson and Vulliet (2013) using a mass spectrometer for detection. Despite the use of a less sensitive detector (ultraviolet detector) in this study, lower MLOQ value was achieved for ibuprofen than the MLOQ reported by Peysson and Vulliet (2013).This indicates the efficiency of the developed QuEChERS extraction coupled with the applied d-SPE clean-up. Comparable MLOQ values were reported for diclofenac and ibuprofen by Martin et al. 2010 using UAE + HPLC-FLD/DAD (ibuprofen – 0.355 µg g− 1; diclofenac − 0.10 µg g− 1) and Morales-Toledo et al. 2016 using MAE + UHPLC-FLD (ibuprofen – 0.288 µg g− 1). Intra-day repeatability (%RSD) values of diclofenac and ibuprofen were 4.5 % and 6.8 %, respectively while inter-day precision (reproducibility) for both compounds were less than 18 %. Correlation coefficients of 0.9997 and 0.9993 were obtained for diclofenac and ibuprofen respectively, indicating good linearity for both compounds.
The developed method was applied for the determination of diclofenac and ibuprofen in sewage sludge samples collected from three WWTPs in Nigeria. Table 3 shows the concentration profiles of target NSAIDs in sewage sludge samples. For the first sampling campaign, concentration of diclofenac in UCH primary sludge ranged from < MLOQ to 0.6 µg g− 1 and < MLOQ to 0.5 µg g− 1 for UCH secondary sludge). Diclofenac concentration in Ijaiye sludge ranged from < MLOQ to 0.6 µg g− 1. Diclofenac was detected below MLOQ in Alausa sludge. Moreover, for the first sampling the highest concentration of ibuprofen was measured in Ijaiye sludge (6.6 µg g− 1). Ibuprofen concentration in UCH primary sludge ranged from < MLOQ to 0.7 µg g− 1 and < MLOQ in UCH secondary sludge. 0.6 µg g− 1 of ibuprofen was quantified in Alausa sludge. For the second sampling, diclofenac in UCH primary sludge ranged from < MLOQ to 1.02 µg g− 1 and < MLOQ to 0.5 µg g− 1 for UCH secondary sludge.
Diclofenac concentration in Ijaiye sludge was < MLOQ. Ibuprofen concentration was below MLOQ in UCH primary sludge and ranged from < MLOQ – 3.7 µg g− 1 in UCH secondary sludge. Ijaiye sludge concentration for ibuprofen ranged from < MLOQ to 2.4 µg g− 1 while Alausa sludge had 0.6 µg g− 1 of ibuprofen. There was no remarkable difference in the concentrations of diclofenac and ibuprofen in sewage sludge collected during the first sampling and second sampling.
Overall, in this present study, the highest concentration of diclofenac (1.02 µg g− 1) was measured in UCH primary sludge while ibuprofen maximum concentration of 6.6 µg g− 1 was quantified in Ijaiye sludge. Sorption of compounds to sludge takes place through absorption and adsorption processes (Vieno and Sillanpää 2014; Ternes et al. 2004) and these two processes account for the sorption of a compound to sludge. Sorption to sludge can be estimated by Kd value. For an efficient sorption, Kd value should be over 300 L Kg− 1 (Vieno and Sillanpää 2014). According to Stasinakis et al. (2013), Kd values for diclofenac in primary and secondary sludge are 459 L Kg− 1 and 232 L Kg− 1, respectively. This implies that diclofenac has more tendencies to accumulate in primary sludge than in secondary sludge during wastewater treatment processes. This was evident in UCH sludge for which the highest concentration was measured in primary sludge. Lonappan et al. (2016) also determined higher concentration of diclofenac in primary sludge than in secondary sludge. In spite of the higher Kd (232 L Kg− 1) of diclofenac than that of ibuprofen (7.1 L Kg− 1), ibuprofen concentrations were higher than diclofenac concentrations in most sewage sludge samples.
With the lower Kd value of ibuprofen, sorption of ibuprofen to sewage sludge was expected to be lower than that of diclofenac. This occurrence of ibuprofen at higher concentrations than diclofenac in most sewage sludge samples could be as a result of much higher prescription and dispensing rate of ibuprofen at UCH and Ijaiye hospitals and higher consumption rate of ibuprofen by the residents around Alausa WWTP.
Comparable or lower concentrations of diclofenac and ibuprofen in sewage sludge were quantified in some studies in Europe and North America (Rossini et al. 2016; Peysson and Vulliet 2013; Gago-Ferrero et al. 2015; Lonappan et al. 2016; Guerra et al. 2014). In a study in Pakistan, concentrations of up to 6.046 µg g− 1 and 4.968 µg g− 1 were quantified in sludge for ibuprofen and diclofenac, respectively (Ashfaq et al. 2017). Studies on the occurrence of diclofenac and ibuprofen in sewage sludge from WWTPs in Africa are still limited. Hence, there are scarce data from Africa to compare with our results. Up to 1.110 µg g− 1 of diclofenac was measured in sewage sludge from Agbara WWTP (in Nigeria) which treats industrial wastewater (Olarinmoye et al. 2016). In comparison with this present work, Olarinmoye et al. (2016) obtained similar concentrations of diclofenac in sewage sludge from UCH and Alausa WWTPs (UCH sludge: 0.111 µg g− 1, Alausa sludge: 0.635 µg g− 1) but lower concentrations of ibuprofen were quantified in sewage sludge from the same WWTPs (UCH sludge: 0.06 µg g− 1, Alausa sludge: < 0.1 µg g− 1) .
Ecological risk assessment of pharmaceuticals in sewage sludge is important for evaluating their potential adverse impacts to the ecosystem. The common fates of sewage sludge in the studied Nigerian WWTPs are land application for agricultural purposes and disposal into dumpsites. Pharmaceuticals may enter surface water via runoff from soil or leach into groundwater from dumpsites. Therefore, the presence of diclofenac and ibuprofen in the sewage sludge samples represent a potential threat for the soil and aquatic ecosystems. Consequently, these compounds can enter the food chain through transfer from soil to plants (Martin et al. 2012). The RQ values of diclofenac and ibuprofen in secondary sewage sludge are presented in Fig. 2. Risk assessment of secondary sewage sludge indicated that diclofenac in all sewage sludge samples posed negligible risk to fish, daphnia and algae; and medium risk to bacteria. Ibuprofen posed high risk to all the four organisms (fish, daphnia, algae and bacteria) in all secondary sewage sludge samples, with the highest RQ values (13–189) observed for fish. Ibuprofen was found in previously published studies to pose high ecotoxicological risk in digested sludge (Martin et al. 2012; Verlicchi and Zambello 2015). Considering the high RQ values of ibuprofen in the sewage sludge samples and the aforementioned common fates of sewage sludge in the studied Nigerian WWTPs the soil and aquatic ecosystems especially fish, daphnia, algae and bacteria, are threatened with respect to the presence of ibuprofen residues.
In conclusion, a rapid, safe and low-cost method based on QuEChERS extraction and HPLC-UV has been developed and successfully applied for determination of diclofenac and ibuprofen in sewage sludge from three Nigerian WWTPs. The developed QuEChERS method is simple and affordable for routine monitoring of diclofenac and ibuprofen residues in sewage sludge. Diclofenac and ibuprofen were detected in all sewage sludge samples. Diclofenac and ibuprofen were quantified up to 1.02 µg g− 1 and 6.6 µg g− 1, respectively. Ibuprofen posed high risk to fish, daphnia, algae and bacteria in all secondary sewage sludge samples. The high RQ values of ibuprofen indicate serious threats to the soil and aquatic ecosystem especially fish, daphnia, algae and bacteria. The present study expands our knowledge on the applicability of QuEChERS extraction for pharmaceuticals determination in sewage sludge. The findings regarding the occurrence and ecological risk of target NSAIDs call for the adoption of adequate and efficient advanced treatment processes for the treatment of sewage sludge prior to disposal and use as manures for agricultural applications in Nigeria.
References
Ajibola AS, Tisler S, Zwiener C (2020) Simultaneous determination of multiclass antibiotics in sewage sludge based on QuEChERS extraction and liquid chromatography tandem mass spectrometry. Analytical Methods 12:576–586. https://doi.org/10.1039/c9ay02188d
Ajibola AS, Amoniyan OA, Ekoja FO, Ajibola FO (2020) QuEChERS Approach for the Analysis of Three Fluoroquinolone Antibiotics in Wastewater: Concentration Profiles and Ecological Risk in Two Nigerian Hospital Wastewater Treatment Plants. Arch Environ Contam Toxicol. https://doi.org/10.1007/s00244-020-00789-w
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and dispersive solid-phase extraction for the determination of pesticide residues in produce. J AOAC Int 86:412–431
Ashfaq M, Khan KN, Saif Ur Rehman M, Mustafa G, Nazar MF, Sun Q, Yu CP (2017) Ecological risk assessment of pharmaceuticals in the receiving environment of pharmaceutical wastewater in Pakistan. Ecotoxicol Environ Saf 136:31–39. https://doi.org/10.1016/j.ecoenv.2016.10.029
Conaghan PG (2012) A turbulent decade for NSAIDs: update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol Int 32:1491–1502. https://doi.org/10.1007/s00296-011-2263-6
Du J, Mei C, Ying G, Xu M (2016) Toxicity Thresholds for Diclofenac, Acetaminophen and Ibuprofen in the Water Flea Daphnia magna. Bull Environ Contam Toxicol 97:84–90. https://doi.org/10.1007/s00128-016-1806-7
EU (2013) Directive 2013/39/EU of the European Parliament and of the Council. Available in www format: URL: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:226:0001:0017:EN:PDF
European Commission (2003) Technical guidance document in support of commission directive 93/67/EEC on risk assessment for new notified substances and commission regulation (EC) no 1488/94 on risk assessment for existing substances, part II, (Brussels, Belgium)
Gago-Ferrero P, Borova V, Dasenaki ME, Thomaidis NS (2015) Simultaneous determination of 148 pharmaceuticals and illicit drugs in sewage sludge based on ultrasound-assisted extraction and liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 407: 4287–4297. https://doi.org/10.1007/s00216-015-8540-6
Guerra P, Kim M, Shah A, Alaee M, Smyth SA (2014) Occurrence and fate of antibiotics, analgelsics/anti-inflammatory and antifungal compounds in wastewater treatment processes. Sci Total Environ 473–474:235–243. https://doi.org/10.1016/j.scitotenv.2013.12.008
Guo C, Wang M, Xiao H, Huai B, Wang F, Pan G, Liao X, Liu Y (2016) Development of a modified QuEChERS method for the determination of veterinary antibiotics in swine manure by liquid chromatography tandem mass spectrometry. J Chromatogr B 1027:110–118. https://doi.org/10.1016/j.jchromb.2016.05.034
Hlengwa NB, Mahlambi PN (2020) Ultrasonic followed by solid phase extraction and liquid chromatography-photodiode array for determination of pharmaceutical compounds in sediment and soil. Bull Environ Contam Toxicol 104:464–470. https://doi.org/10.1007/s00128-020-02829-6
Lehotay SJ, Son KA, Kwon H, Koesukwiwata U, Fu W, Mastovska K, Hoh E, Leepipatpiboon N (2010) Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J Chromatogr A 1217:2548–2560. https://doi.org/10.1016/j.chroma.2010.01.044
Lonappan L, Pulicharla R, Rouissi T, Brar SK, Verma M, Surampalli RY, Valero JR (2016) Diclofenac in municipal wastewater treatment plant: quantification using laser diode thermal desorption—atmospheric pressure chemical ionization-tandem mass spectrometry approach in comparison with an established liquid chromatography electrospray ionization–tandem mass spectrometry method. J Chromatogr A 1433:106–113. https://doi.org/10.1016/j.chroma.2016.01.030
Martin J, Camacho-Muñoz D, Santos JL, Aparicio I, Alonso E (2012) Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: Removal and ecotoxicological impact of wastewater discharges and sludge disposal. J Hazard Mater 239–240:40–47. https://doi.org/10.1016/j.jhazmat.2012.04.068
Martín J, Santos JL, Aparicio I, Alonso E (2010) Multi-residue method for the analysis of pharmaceutical compounds in sewage sludge, compost and sediments by sonication-assisted extraction and LC determination. J Sep Sci 33:1760–1766. https://doi.org/10.1002/jssc.200900873
Morales-Toledo A, Afonso-Olivares C, Montesdeoca-Esponda S, Guedes-Alonso R, Sosa-Ferrera Z, Santana-Rodríguez JJ (2016) Optimization and development of SPE and MAE combined with UHPLC-FD for the determination of acetylsalicylic acid, naproxen, ibuprofen and gemfibrozil in sewage and sludge samples. Curr Anal Chem 12:545–552. https://doi.org/10.2174/1573411012666160113235153
Nannou C, Ofrydopoulou A, Heath D, Heath E, Lambropoulou D (2019) QuEChERS—A green alternative approach for the determination of pharmaceuticals and personal care products in environmental and food samples. In: Płotka-Wasylka J, Namieśnik J (eds) Green Analytical Chemistry, Green Chemistry and Sustainable Technology. Springer, Singapore, pp 395–430. https://doi.org/10.1007/978-981-13-9105-7_14
Nannou CI, Kosma CI, Albanis TA (2015) Occurrence of pharmaceuticals in surface waters: analytical method development and environmental risk assessment. Int J Environ Anal Chem 95:1242–1262. https://doi.org/10.1080/03067319.2015.1085520
Nicholls A (2016) Confidence limits, error bars and method comparison in molecular modeling. Part 2: comparing methods. J Comput Aided Mol Des 30:103–126. https://doi.org/10.1007/s10822-016-9904-5
Olarinmoye O, Bakare A, Ugwumba O, Hein A (2016) Quantification of pharmaceutical residues in wastewater impacted surface waters and sewage sludge from Lagos. Nigeria Journal of Environmental Chemistry Ecotoxicology 8:14–24. DOI:https://doi.org/10.5897/JECE2015.0364
Pérez-Lemus N, López-Serna R, Pérez-Elvira SI, Barrado E (2019) Analytical methodologies for the determination of pharmaceuticals and personal care products (PPCPs) in sewage sludge: a critical review. Anal Chimica Acta. https://doi.org/10.1016/j.aca.2019.06.044
Peysson W, Vulliet E (2013) Determination of 136 pharmaceuticals and hormones in sewage sludge using quick, easy, cheap, effective, rugged and safe extraction followed by analysis with liquid chromatography–time-of-flight-mass spectrometry. J Chromatogr A 1290:46–61. https://doi.org/10.1016/j.chroma.2013.03.057
Psomas G (2020) Copper(II) and zinc(II) coordination compounds of non-steroidal anti-inflammatory drugs: Structural features and antioxidant activity. Coord Chem Rev 412:213259. https://doi.org/10.1016/j.ccr.2020.213259
Ra JS, Oh SY, Lee BC, Kim SD (2008) The effect of suspended particles coated by humic acid on the toxicity of pharmaceuticals, estrogens, and phenolic compounds. Environ Int 34:184–192. https://doi.org/10.1016/j.envint.2007.08.001
Rossini D, Ciofi L, Ancillotti C, Checchini L, Bruzzoniti MC, Rivoira L, Fibbi D, Orlandini S, Del Bubba M (2016) Innovative combination of QuEChERS extraction with on-line solid-phase extract purification and pre-concentration, followed by liquid chromatography-tandem mass spectrometry for the determination of non-steroidal anti-inflammatory drugs and their metabolites in sewage sludge. Anal Chim Acta 935:269–281. https://doi.org/10.1016/j.aca.2016.06.023
Saleh A, Larsson E, Yamini Y, Jönsson J (2011) Hollow fiber liquid phase microextraction as a preconcentration and clean-up step after pressurized hot water extraction for the determination of non-steroidal anti-inflammatory drugs in sewage sludge. J Chromatogr A 1218:1331–1339. https://doi.org/10.1016/j.chroma.2011.01.011
Sanderson H, Johnson DJ, Wilson CJ, Brain RA, Solomon KR (2003) Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol Lett 144:383–395. https://doi.org/10.1016/S0378-4274(03)00257-1
Stasinakis AS, Thomaidis NS, Arvaniti OS, Asimakopoulos AG, Samaras VG, Ajibola A, Mamais D, Lekkas TD (2013) Contribution of primary and secondary treatment on the removal of benzothiazoles, benzotriazoles, endocrine disruptors, pharmaceuticals and perfluorinated compounds in a sewage treatment plant. Sci Total Environ 463–464:1067–1075. https://doi.org/10.1016/j.scitotenv.2013.06.087
Ternes TA, Herrmann N, Bonerz M, Knacker T, Siegrist H, Joss A (2004) A rapid method to measure the solid–water distribution coefficient (Kd) for pharmaceuticals and musk fragrances in sewage sludge. Water Res 38:4075–4084. https://doi.org/10.1016/j.watres.2004.07.015
Verlicchi P, Al Aukidy M, Zambello E (2012) Occurrence of pharmaceutical compounds in urban wastewater: removal, mass load and environmental risk after a secondary treatment - a review. Science. of the Total Environment 429:123–155. https://doi.org/10.1016/j.scitotenv.2012.04.028
Verlicchi P, Zambello E (2015) Pharmaceuticals and personal care products in untreated and treated sewage sludge Occurrence and environmental risk in the case of application on soil — A critical review. Science of the Total Environment 538:750–767. https://doi.org/10.1016/j.scitotenv.2015.08.108
Vieno N, Sillanpää M (2014) Fate of diclofenac in municipal wastewater treatment plant-A review. Environment International 69:28–39. https://doi.org/10.1016/j.envint.2014.03.021
Wee SY, Aris AZ, Yusoff FMd, Praveena SM (2019) Occurrence and risk assessment of multiclass endocrine disrupting compounds in an urban tropical river and a proposed risk management and monitoring framework. Sci Total Environ 671:431–442. https://doi.org/10.1016/j.scitotenv.2019.03.243
Zhang K, Yuan G, Werdich AA, Zhao Y (2020) Ibuprofen and diclofenac impair the cardiovascular development of zebrafish (Danio rerio) at low concentrations. Environ Pollut 258:113613. https://doi.org/10.1016/j.envpol.2019.113613
Acknowledgements
The authors acknowledge the efforts of O. Atanlusi, V.I. Adeyemo and M.O. Ajao. Officials at the investigated wastewater treatment plants are acknowledged for assistance in the collection of sewage sludge samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ajibola, A.S., Fawole, S.T., Ajibola, F.O. et al. Diclofenac and Ibuprofen Determination in Sewage Sludge Using a QuEChERS Approach: Occurrence and Ecological Risk Assessment in Three Nigerian Wastewater Treatment Plants. Bull Environ Contam Toxicol 106, 690–699 (2021). https://doi.org/10.1007/s00128-021-03139-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-021-03139-1