Abstract
Elevated levels of chloride concentration due to anthropogenic activities including the road salts, septic effluent and agricultural sources are common in shallow groundwater of the recent glacial deposits north of Toronto, Ontario, Canada. Identifying suitable techniques for discriminating the source of the chloride concentration helps to better plan the protection of groundwater in the area. This paper examines the applicability of geochemical techniques with emphasis on Panno et al. (Ground Water 44: 176–187, 2006) and Mullaney et al. (2009) graphical approaches for discriminating the sources of chloride with known causes of impacts. The results indicated that the graphical methods developed using Cl−, Br− and/or total nitrogen (N) could identify the combined sources of road salts and septic systems. However, discriminating between the road salts, septic effluent or agricultural sources needs to be complemented by other techniques including the artificial sweeteners and isotope tracers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chloride is a minor constituent of the earth’s crust, but a major dissolved constituent of most natural water (Davis and DeWiest 1966; Kelly et al. 2010). According to Mullaney et al. (2009), chloride in groundwater may be due to a number of natural or human sources. Natural sources of chloride include (1) the oceans; (2) the natural weathering of bedrock, surficial materials, and soils; (3) geologic deposits containing halite, or saline groundwater (brines); and (4) volcanic activity. Some of the anthropogenic sources can be road de-icers, septic effluent, municipal wastewater effluent, agricultural activities and landfills.
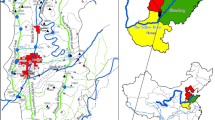
High chloride concentration has been observed in shallow groundwater in an area of approximately 1756 km2 north of Toronto, Canada (Fig. 1). Identifying the sources of chloride would help to better plan for protecting the groundwater. Various authors have used different geochemical methods for determining potential sources. Howard and Beck (1993) used iodide and fluoride as indicators of road salt source and found that they were especially useful for differentiating between road salt and saline bedrock water.
Several authors have used the ratio of chloride to bromide to identify the anthropogenic sources of chloride, including domestic effluent, agricultural activities and road salt in natural water including groundwater (Davis et al. 1998; Thomas 2000; Jagucki and Darner 2001). According to Davis et al. (1998) shallow groundwater will generally have Cl−/Br− ratio between 100 and 200; domestic sewage, between 300 and 600; and, water affected by dissolution of halite, between 1000 and 10,000. Both Cl− and Br− ions are not affected by redox processes or precipitation-dissolution reactions in groundwater (Davis et al. 1998), although they report that desorption from clays and breakdown of organic matter can be sources of Br−. Pristine groundwater containing Cl− and Br− sourced from atmospheric deposition will have a Cl−/Br− ratio of between 50 and 100 (Katz et al. 2011). Cl− can be added from fertilizers (KCl), landfills and domestic sewage. The addition of Cl− from road salt is analogous to the dissolution of halite or rocksalt, leading to a large increase in the Cl−/Br− ratio.
Panno et al. (2006) applied graphical techniques using Cl−, Br−, I− and total nitrogen (N) to graphically discriminate among the sources of chloride in groundwater affected by septic effluent, animal waste, landfill leachate, sea water, Illinois Basin brine, road salt and agricultural activities in Illinois. According to Panno et al. (2006), the graphical techniques were useful for discriminating between the sources of Cl− contamination in groundwater in Illinois and should be more widely applicable because of the similarities of the sources of contamination and groundwater and surface water composition. Mullaney et al. (2009) developed mixing curves to represent binary mixtures of halite, sewage or animal waste, potassium chloride fertilizer, landfill leachate, Illinois Basin brines, and seawater sources of chloride for the glacial aquifer system (GAS) in the USA. They plotted data from various sources for GAS wells and surface water in forest, agricultural and urban on the mixing curve to evaluate if any of the samples were representative of end members of simple mixing.
The application of artificial sweeteners to identify sources of domestic effluent represents another approach. Artificial sweeteners are used in food, beverage and other products as alternatives to sugar, most commonly in soft drinks, to provide the sweet taste without the added caloric requirements to synthesize. As a result, these compounds can readily pass through the human body with little chemical transformation. Some of these compounds have been detected in domestic wastewater that affected the groundwater through septic systems, leaking sewers or storage lagoons (Buerge et al. 2009; Scheurer et al. 2009; Van Stempvoort et al. 2011a; Tran et al. 2014). Acesulfame, sucralose, saccharin and cyclamate are among the artificial sweeteners that have been used as anthropogenic markers for domestic wastewater impact (Van Stempvoort et al. 2011a, 2011b, 2013; Mawhinney et al. 2011; Wolf et al. 2012; Oppenheimer et al. 2011). There is general agreement among the authors that due to their higher resistant nature to transformation and absorption on soil, acesulfame and sucralose are ideal markers for evaluating the impact of domestic wastewater contamination on receiving water bodies, particularly groundwater (Oppenheimer et al. 2011; Lange et al. 2012; Tran et al. 2014).
Although the geochemical techniques discussed above could be widely applicable in identifying sources of chloride, multiple sources of chloride as well as the hydrology and hydrogeology of an area may complicate the interpretation of the affected water (Panno et al. 2006). This paper extends the geochemical graphical techniques developed by Panno et al. (2006) and Mullaney et al. (2009) by including artificial sweeteners among the discriminants in these techniques and applies them to investigate several sites in the shallow aquifer north of Toronto. The septic source of chloride at the impacted sites was confirmed by identifying the presence of artificial sweeteners (acesulfame and sucralose).
Potential sources of chloride in the study area
In the study area, the quaternary glacial deposits form shallow and deep aquifers. Natural sources of chloride are not considered as a probable source for the high chloride concentration in the area. According to Howard and Beck (1993) in quaternary sediments of Southern Ontario, there is very little chloride enrichment associated with natural chemical evolutionary processes and the background concentration is in the range 15–20 mg/L. The investigation by Dyke (1999) also indicated that chloride and nitrate in the glacial sediments probably originated from non-natural sources and are indicators of urbanization and agricultural activities respectively. The study of Khazaei (2013, 2014a, 2014b, 2015)) indicates where the shallow aquifer is protected by a thick layer of low hydraulic conductivity (e.g. A-9 and B-6S sites in Table 1) and it is not under impact of anthropogenic activities, the chloride concentration is in the range of 0.29 to 1.0 mg/L. This is in agreement with Dyke (1999) who demonstrated that chloride and nitrate in the glacial sediments likely derived from non-natural sources and are indicators of urbanization and agricultural activities respectively. The lack of anthropogenic impact was confirmed by the absence of tritium (<0.8 TU). Tritium is a commonly used radiogenic isotope that provides the ability to identify the time of groundwater recharge relative to the period of thermonuclear bomb testing in 1950s and 1960s. The interpretation of tritium in terms of groundwater recharge dates is shown in Table 2. The absence of tritium provides an indicator of absence of recent recharge (recharged prior to 1952) occurrence (Clark and Fritz 1997; Henkemans 2010). Therefore, the high chloride levels in shallow groundwater in the study area reflect land-based human activity including, but not limited to, road salt, sewage effluent and/or agricultural activities. It should be noted that in the study area precipitation has typically a low chloride concentration. The average chloride concentration from two rainfall events collected during 2003 to 2004 in an urban area of Toronto, approximately 20 km south of study area, was 0.35 mg/L (Toronto Region Conservation Authority 2006).
Road salt is used for de-icing to improve the safety of winter driving on urban and rural roads as well as on highways. MMM and Golders (2006) found that the average usage of road salts was 14,630 t in the winter of 2000/2001 and 8710 t in the winter of 2001/2002 within the study area. The composition of the salt applied is sodium chloride (40 to 70%), potassium chloride (3 to 7%) and calcium chloride (15 to 40%). The salts can easily be mobilized by rain and enter rivers, lakes and shallow groundwater systems where they can cause serious degradation of water quality (Howard and Beck 1993). Thus, road salt is a potential source of high chloride concentration in the study area.
Chloride increase in shallow groundwater could be attributed also to domestic wastes in the study area since there are still urban and rural areas with private septic systems. The amount of chloride is between 30 and 100 mg/l in typical domestic wastes (Metcalf and Eddy, Inc 1979). Panno et al. (2006) indicated that the chloride concentrations in the monitoring wells adjacent to the facilities septic field were as much as 16,400 mg/L. Stantec (2010) mapped the location of septic systems in the study area and found a dense concentration in some parts. Thus, septic effluent can be considered as a potential source for the high chloride concentrations measured in shallow groundwater in the study area.
There are also agricultural activities in some areas. Application of potassium chloride (KCl) as a fertilizer can increase chloride concentrations in soil water and underlying shallow groundwater (Panno et al. 2006; Mullaney et al. 2009). Other sources of chloride include manure application as fertilizer and dissolution of salts resulting from irrigation. It should be noted that the agricultural impact on soil water and groundwater quality is less than the more concentrated chloride applications, such as road salt. According to Kelly et al. (2012), chloride concentrations in samples collected from tile drains in east-central Illinois ranged from 10 to 37 mg/L. Therefore, agricultural areas can be considered as a potential source for the high chloride concentrations in the shallow groundwater of the study area. More generally, road salts, septic effluents and agricultural activities are potential sources of high chloride concentration in the area studied in this investigation.
Methods
To carry out the investigation, shallow wells in the vicinity of a major road and within a radius of 1000 m from the active septic systems were identified using the data provided by the local municipalities and Stantec (2010). Water quality data for inorganics (chloride and nitrate) and tritium was reviewed using the data from Khazaei (2013, 2014a, 2014b, 2015). Based on this information and the provincial well records database, 13 sites susceptible to the impact of anthropogenic activities (mainly road salt and septic effluent) were identified in the study area. The susceptibility of these sites was assessed from the hydrogeology, groundwater chemistry and environmental factors. The hydrogeological criterion was the absence of a thick clay layer above the aquifer. Groundwater chemistry criteria were chloride concentration higher than the background of 15–20 mg/L accompanied by a high level of nitrogen compounds, and the occurrence of tritium (if applicable). The environmental factors included the presence of septic systems within a radius of 1000 m and the vicinity to a major road. Two sets of analysis from most of the sites were selected for inorganics data. If more than two sets of analyses were available for a site, the two most recent analyses were selected (Table 1). In addition, two sites where the shallow aquifers were not under anthropogenic impacts, confirmed by lack of tritium, were also identified and all available data for inorganic water quality analysis was collected to have a basis for comparison (Table 3).
Chloride concentration versus sodium was plotted to confirm that all of the 13 sites were under anthropogenic impact (Fig. 2). According to Henkemans (2010), samples falling above the sodium-chloride molar equilibrium line indicate an anthropogenic source. If the Na+ and Cl− ions are ionized in the solution by congruent dissolution, for example, the dissolution of halite, the cations and anions occur in proportion to their stoichiometry in the solid phase. Anthropogenic sources tend to add excess Cl− and affected samples plot above the line, whereas natural effects like ion exchange tend to increase Na+ over Cl− and samples plot below the line.
The tritium data (if available) was used to support the impacts of anthropogenic activities. The sites where the shallow aquifers are not under anthropogenic impacts were confirmed based on the hydrogeology and absence of tritium.
In addition, the Cl−/Br− ratio was examined to also confirm the sites under anthropogenic impacts by comparing the ratio with the sites that were not impacted. According to Davis et al. (1998) most shallow groundwater impacted by anthropogenic activities generally has a Cl−/Br− ratio of more than 200.
Groundwater analysis for artificial sweeteners from 12 of the 13 sites was available (Khazaei 2014b) and used to confirm whether the sites were impacted by septic systems. The results shown in Table 4 will be used as a basis for comparison.
The Cl−, Br− and total nitrogen (N) were used to graphically discriminate among the sources of chloride by applying the Panno et al. (2006) and Mullaney et al. (2009) diagrams as shown in Figs. 3, 4 and 5. These diagrams are based on binary mixing curves. For example, Davis et al. (1998) plotted the curve resulting from simple mixtures of 1 L of precipitation, with an original Cl− concentration in of 0.5 mg/L and Cl−/Br− ratio of 100, with small but increasing volumes of brine containing an initial Cl− concentration of 10,000 mg/L and Cl−/Br− ratio of 10,000. The brine was added after the initial precipitation had been concentrated slightly by evaporation. This binary mixing approach demonstrated that recharge estimated by the chloride budget method could be affected by externally derived sources of Cl− (Davis et al. 1998). The diagrams in Panno et al. (2006) and Mullaney et al. (2009), modified as Figs. 3 and 5, show how the Cl− concentration and the Cl−/Br− ratio in precipitation and pristine aquifers can evolve to the various end members by mixing contributions from various anthropogenic sources. Similar diagrams are presented by Katz et al. (2011). The addition of the total nitrogen versus the chloride/bromide ratio, as shown in Fig. 4, helps to distinguish between similar Cl−/Br− ratios found in road salt and affected waters and the landfill leachate group of sources. The results were compared with the presence and/or absence of artificial sweeteners (acesulfame and/or sucralose) at each site to derive the appropriate conclusions.
Cl/Br ratio versus Cl− concentrations of background samples, sources, and affected water samples showing complete and partial separation (overlap) of each group of samples and end member locations. The trend of the data points following lines of evolution/mixing originating from precipitation or pristine groundwater and ending at the sources shows the effects of anthropogenic contamination. Lines drawn on the graph indicate approximate regions where certain groups of samples tend to plot. Endmember samples are indicated with the following letters: B = Illinois Basin brine; BK = evaporite basin brine from Kansas; E = commercially available salt for water softeners and Morton-brand iodized salt; SW = sea water; RS = road salt; and KCI = potassium chloride fertilizer. (modified after Panno et al. 2006).
Total nitrogen (NO3 + TKN) versus Cl/Br ratio. These ratios tend to separate samples from N-enriched sources (mostly landfill, animal waste and septic effluent) from most other samples. Because of their small N component and large Cl/Br ratios, road salt-contaminated samples plot separately from most other types of contaminated groundwater samples. The road salt endmember is indicated as RS (an arbitrarily low 0.01 mg/L N was added to this sample to include it in the plot). Total nitrogen for most of the septic effluent and affected samples is actually NO3 − + NH4 + (TKN was not determined for these samples) (modified after Panno et al. 2006).
Binary mixing curves (modified after Mullaney et al. 2009)
Results and discussions
Plotting chloride concentration versus sodium in Fig. 2 confirmed that all of the 13 sites, including those with no acesulfame and/or sucralose, were under anthropogenic impact because the data points plot either above the sodium-chloride molar equilibrium line or on it. These plotting positions indicate an anthropogenic source according to Henkemans (2010). The sources of anthropogenic impacts could be the road salts, septic effluent and/or agricultural activities. The two samples from the sites known being not impacted by the anthropogenic activities fell below the sodium-chloride molar equilibrium line.
The Cl−/Br− ratio for the sites known being not impacted by anthropogenic activities is less than 100 and is more than 300 for the impacted sites (Tables 1 and 3). This is in agreement with the finding of Davis et al. (1998) that Cl−/Br− ratio for shallow groundwater not impacted by anthropogenic activities generally is less than 200. As can be seen in Table 1, samples at W60 site have low chloride concentrations of 8.9 and 9.05 mg/L, respectively, that are less than the suggested natural background range of 15 to 20 mg/L. However, the Cl−/Br− ratio indicates the impact of anthropogenic activities that is also supported by the presence of tritium (Table 3). Therefore, the Cl−/Br− ratio helps to identify the impact of anthropogenic activity even in the cases of low chloride concentration. However, the ratio cannot discriminate the sources of chloride in the shallow groundwater impacted by more than one source such as septic effluent and road salt.
The artificial sweetener concentrations at the 12 sampled sites indicated that acesulfame and/or sucralose was present in 10 out of 12 sites (Table 4). The review of the hydrogeology of the sites along with the septic systems information indicated that, scarcity of the artificial sweeteners sources, their distance from the samplings location and the thickness of low hydraulic conductivity layer overlying the aquifer, depth to the screen, and groundwater flow direction were among the factors controlling the presence of acesulfame and/or sucralose. Both acesulfame and sucralose were present in the groundwater samples taken from three sites in different domestic areas with a dense concentration of active septic systems at a distance of less than 100 m, a shallow depth of screen (4.3 to 14 m) and an absence of thick low hydraulic conductivity layer (less than 4.5 m) overlying the aquifers. Sucralose was detected where the acesulfame concentration was more than 400 ng/L. The concentration of acesulfame was approximately 1.5 to 3.0 times higher than sucralose. The reason for higher concentration of acesulfame is its higher resistance to degradation in comparison with sucralose (Scheurer et al. 2009; Burge et al. 2009; Robertson et al. 2016). Study of Robertson et al. (2016) indicated that 40,000 ng/L of sucralose degraded to 1000 ng/L after plum (septic system) travel time of about 1–7 years, whereas acesulfame remained consistently high. Therefore, presence of sucralose could be an indication of stronger impact of domestic wastewater effluent on the shallow groundwater in comparison with areas with no sucralose.
The presence of acesulfame and/or sucralose at 10 sampling sites (Table 4) confirmed the influence of septic effluent but their absence at other sites does not unequivocally exclude septic impact on the shallow groundwater. The sweetener could be attenuated through the passage within the geologic formations. All the 12 sites with samples for artificial sweeteners in addition to their proximity to septic system are close to the road and therefore, potentially susceptible to the impacts of both road salt and septic effluent. However, the sweeteners only indicate the impact of septic effluent.
The plot of Cl−/Br− versus Cl− on the Panno et al. (2006) graph indicates that most of the 13 susceptible sites fall in the area identified as impacted by combined sources of road salt and septic effluent (Fig. 3). This is reasonable since all the sites were selected to have potential for both road salt and septic effluent impacts. The samples from S-22 plot above the upper border of the area impacted by combined sources of road salt and septic effluent. Tables 2 and 3 show that this site has the highest chloride concentration of 1170 mg/L as well as the highest concentration of acesulfame (1900 ng/L) and sucralose (580 ng/L). The presence of the highest levels of both acesulfame and sucralose indicates a strong impact by septic effluent. In addition, the hydrogeology of the site (absence of a layer of low hydraulic conductivity above the water table) and its close vicinity to the main road makes the site strongly vulnerable to road salt impact, resulting in the very high chloride concentration. Therefore, the Panno et al. (2006) Cl−/Br− versus Cl− diagram may need further improvement where the sites in the study area, such as S-22, are under strong impacts. In general, the diagram is useful for characterizing the combined impact of both road salts and septic effluent in the study area but it does not discriminate among the sources. However, to discriminate among the two sources, supplementary artificial sweeteners could be helpful in identifying the septic sources.
When the graph for plotting total N (NO3 − + TKN) against Cl−/Br− are used most of the samples fall in the area assigned as Road Salt and Affected Water (Fig. 4). However, both samples from S-22 again fall further outside of the Road Salts & Affected Water. The graph in Fig. 4 has been developed to separate samples affected by Landfill Leachate, Septic Effluent, Animal Wastes, & Affected Waters from those affected by road salt (Panno et al. 2006). The area identified as Landfill Leachate, Septic Effluent, Animal Wastes, & Affected Waters in Fig. 4 most probably is based on plotting of samples from septic effluent that often has a high nitrate concentration. Although most of the impacted groundwater samples collected in this study are also susceptible to septic effluent impact (as confirmed by the acesulfame and/or sucralose), the effluent likely has been diluted during its movement and mixing with the groundwater. Therefore, the nitrate concentrations are not enriched enough (concentration of ~10 mg/L) and the samples do not plot in the area typically indicating impact of septic effluent (Fig. 4). Consequently, the graph of total N against Cl−/Br− introduced by Panno et al. (2006) does not discriminate the septic effluent source of chloride in the groundwater samples impacted by both road salts and septic effluent in the study area. The inclusion of artificial sweeteners in the determinants could resolve the uncertainty.
Use of the mixing curve developed by Mullaney et al. (2009) to identify sources of chloride showed most of the impacted samples plotted in the area between the curves of dilute groundwater/halite used for de-icing, high range (curve 1) and halite used for de-icing /sewage or animal waste high range (Curve 5) (Fig. 5). This could indicate that the samples are under combined impacts of road salts and septic effluents. Similar to that observed in Figs. 3 and 4, the S-22 samples that are strongly impacted by septic effluent and road salts site have much higher Cl−/Br− and fall above the dilute groundwater/halite used for de-icing, high range (curve 1) in Fig. 5. The samples from N-4S and N-1S fall below halite used for de-icing /sewage or animal waste low range (Curve 6). At N-1S site the septic impact is confirmed by the presence of 169 ng/L acesulfame (Table 4). The N-1S site is also susceptible to road salt impact because of the site hydrogeology as well as its vicinity to the road. No sweeteners concentration from samples at N-8S was recorded (Table 4) but the Cl−/Br− indicated impact of anthropogenic activities that could include the road salts sewer effluent. Sweeteners at N-8S site may also have been attenuated due to the hydrogeological condition and their absence does not necessarily indicate any impact of anthropogenic activities. The graphical method developed by Mullaney et al. (2009) was able to identify most of sites impacted by the combined sources of road salts and septic systems in the study area but the graph does not discriminate among the sources.
It should be mentioned that the impacted site with low chloride concentration (W60) does not fall within the area identified as impacted by road salts and septic effluent in any of the graphical techniques. The most likely sources of anthropogenic impact at this site are road salt and/or septic effluent. Additionally, some of the sampling sites, for example S-5 and S-6, are located in the agricultural areas and they may be susceptible to these activities. However, an agricultural source cannot be identified with the Panno et al. (2006) and Mullaney et al. (2009) methods. An effective method for assessing the agricultural impact is to use isotopes such as 15N, 13C and 37Cl (Clark and Fritz 1997; Annable et al. 2007).
In general, the graphical methods developed by Panno et al. (2006) and Mullaney et al. (2009) are reasonably effective for identifying the combined chloride sources of road salts and septic systems in the study area. However, for the study area, further modification could be made based on the collected data. The methods are not capable of discriminating among the chloride sources. To discriminate the sources, the methods should be complemented by the use of artificial sweeteners tracers including the acesulfame and sucralose. Agricultural sources of chloride could be identified with isotope techniques.
Summary and conclusions
This study has revealed that natural background chloride concentration in shallow groundwater of the recent glacial deposits can be as low as 0.29 to 1.0 mg/L, supporting Dyke (1999) understanding that chloride in the glacial sediments is probably originated from non-natural sources. There is shallow groundwater, impacted by anthropogenic activities confirmed by the presence of tritium, with chloride concentration as low as 9 mg/L. The Cl−/Br− ratio in shallow groundwater that was not impacted by anthropogenic activities is less than 100 and in the impacted sites is above 300. The Cl−/Br− ratio helps identifying impact of anthropogenic activity even in the cases of low chloride concentration. However, the ratio cannot discriminate the actual sources of chloride in the shallow groundwater impacted by more than one sources such as septic effluent and road salt.
The graphical methods developed by Panno et al. (2006) and Mullaney et al. (2009) are generally adequate for identifying sites impacted by combined sources of road salts and septic systems in the study area with small modification. However, discriminating between the sources is not feasible. Artificial sweeteners provide a complementary method for tracking the source of domestic effluent since they are source specific. However, sweeteners may also be attenuated due to hydrogeological conditions. Agricultural sources of chloride could not be discriminated using the above method and isotope techniques should be used. Therefore, an ensemble of approaches needs to be applied to discriminate the different sources of chloride including road salt, domestic effluent and agricultural activities.
References
Annable, W. K., Frape, S. K., Shouakar-Stash, O., Shanoff, T., Drimmie, R. J., & Harvey, F. E. (2007). 37Cl, 15N, 13C isotopic analysis of common agro-chemicals for identifying non-point source agricultural contaminants. Applied Geochemistry, 22, 1530–1536.
Buerge, I. J., Buser, H.-R., Kahle, M., Müller, M. D., & Poiger, T. (2009). Ubiquitous occurrence of the artificial sweetener acesulfame in the aquatic environment: an ideal chemical marker of domestic wastewater in groundwater. Environmental Science & Technology, 43(12), 4381–4385.
Clark, I., & Fritz, P. (1997). Environmental isotopes in hydrogeology (p. 328). Boca Raton: Lewis Publishers.
Davis, S. N., & DeWiest, J. M. (1966). Hydrogeology. New York: Willey.
Davis, S. N., Whittemore, D. O., & Fabryka-Martin, J. (1998). Uses of chloride/bromide ratios in studies of potable water. Groundwater, 36(2), 338–350.
Dyke, L. (1999). Regional groundwater and stream chemistry survey, Oak Ridges Moraine, Ontario. Current Research 1999-E; Geological Survey of Canada, 111–121.
Henkemans, E. (2010). A geochemical and isotope study of Oak Ridges Moraine, Southern Ontario. A project report presented to the University of Waterloo in partial fulfillment of the thesis requirement for the degree of Master of Science in Earth Science, Waterloo, Ontario, Canada.
Howard, K. W. F., & Beck, J. B. (1993). Hydrogeochemical implication of groundwater contamination by road de-icing chemicals. Journal of Contaminant Hydrology, 2(3), 245–268.
Jagucki, M. L. and Darner, R. A. (2001). Ground-water quality in Geauga County, Ohio; review of previous studies, status in 1999, and comparison of 1986 and 1999 data. USGS Publication, Water-Resources Investigations Report 2001–4160.
Katz, B. G., Eberts, S. M., & Kauffman, L. J. (2011). Using Cl/Br ratios and other indicators to assess potential impacts on groundwater quality from septic systems: a review and examples from principal aquifers in the United States. Journal of Hydrology, Volume, 397, 151–165.
Kelly, W. R., Panno, S. V., Hackley, K. C., Hwang, H. H., & Martinsek, A. T. (2010). Using chloride and other ions to trace sewage and road salt in the Illinois waterway. Applied Geochemistry, 25, 661–673.
Kelly, W. R., Panno, S. V., Hackley, K. C. (2012). The sources, distribution, and trends of chloride in the waters of Illinois. Illinois State Water Survey, Bulletin B-74.
Khazaei, E. (2013). High chloride at Stouffville MW22. Technical memorandum, Regional Municipality of York, Newmarket, Ontario, Canada.
Khazaei, E. (2014a). High chloride at Newmarket MW18S. Technical memorandum, Regional Municipality of York, Newmarket, Ontario, Canada.
Khazaei, E. (2014b). Artificial sweeteners in shallow wells at York Region. Technical memorandum, Regional Municipality of York, Newmarket, Ontario, Canada.
Khazaei, E. (2015). High chloride concentration at Nobleton PW3. Technical memorandum, Regional Municipality of York, Newmarket, Ontario, Canada.
Lange, F., Scheurer, M., & Brauch, H.-J. (2012). Artificial sweeteners-a recently recognized class of emerging environmental contaminants: a review. Analytical and Bioanalytical Chemistry, 403(9), 2503–2518.
Mawhinney, D. B., Young, R. B., Vanderford, B. J., Borch, T., & Snyder, S. A. (2011). Artificial sweetener sucralose in U.S. drinking water system. Environmental Science & Technology, 45, 8716–8722.
Metcalf and Eddy, Inc. (1979). Wastewater engineering treatment, disposal and reuse. New York: McGraw Hill.
MMM and Golders (2006). York Region water use assessment potential contaminant source inventory and initial threat assessment. Regional Municipality of York.
Mullaney, J. R., Lorenz, D. L. and Arntson, A. D. (2009). Chloride in Groundwater and Surface Water in Areas Underlain by the Glacial Aquifer System, Northern United States. USGS, Scientific Investigations Report 2009–5086.
Oppenheimer, J., Eaton, A., Badruzzaman, M., Haghani, A. W., & Jacangelo, J. G. (2011). Occurrence and suitability of sucralose as an indicator compound of wastewater loading to surface waters in urbanized regions. Water Research, 45(13), 4019–4027.
Panno, S. V., Hackley, H. H., Greenberg, S. E., Krapac, I. G., Landsberger, S., & Kelly, D. J. (2006). Characterization and identification of Na-Cl sources in ground water. Ground Water, 44, 176–187.
Robertson, W. D., Van Stempvoort, D. R., Spoelstra, J., Brown, S. J., & Schiff, S. L. (2016). Degradation of sucralose in groundwater and implication for age dating contaminated groundwater. Water Research, 88, 653–660.
Scheurer, M., Brauch, H. J., & Lmge, F. T. (2009). Analysis and occurrence of seven artificial sweeteners in German waste water and surface water and in soil aquifer treatment (SAT). Journal of Analytical and Bioanalytical Chemistry, 394(6), 1585–1594.
Stantec (2010). Assessment of drinking water quality threats municipal groundwater supplies. Regional Municipality of York.
Thomas, M. A. (2000). The effect of residential development on ground-water quality near Detroit, Michigan. JAWRA, 36(5), 1023–1038.
Toronto and Region Conservation Authority (2006). Evaluation of an extensive greenroof, York University, Toronto, Ontario. Prepared by: Toronto and Region Conservation Authority.
Tran, N. H., Hu, J., Li, J., & Ong, S. L. (2014). Suitability of artificial sweeteners as indicators of raw wastewater contamination in surface water and groundwater. Water Research, 48, 443–456.
Van Stempvoort, D. R., Roy, J. W., Brown, S. J., Bickerton, G., (2011a). Artificial sweeteners as potential tracers in groundwater in urban environments http://www.sciencedirect.com/science/article/pii/S002216941100120X. Journal of Hydrology, Volume 401, Issues 1–2, 20, Pages 126–133.
Van Stempvoort, D. R., Robertson, W. D., & Brown, S. J. (2011b). Artificial sweeteners in a large septic plume. Groundwater Monitoring & Remediation, 31(4), 95–102.
Van Stempvoort, D. R., Roy, J. W., Grabuski, J., Brown, S. J., Bickerton, G., & Sverko, E. (2013). An artificial sweetener and pharmaceutical compounds as co-tracers of urban wastewater in groundwater. Science of the Total Environment, 461-462(2013), 348–359.
Wolf, L., Zwiener, C., & Zermann, M. (2012). Tracking artificial sweeteners and pharmaceuticals introduced into urban groundwater by leaking sewer networks. Science of the Total Environment, 430, 8–19.
Acknowledgements
The data and information used in this paper is from the Regional Municipality of York (York Region) and obtained through Freedom of Information Request No. 16-121. The author is grateful to Ghislaine Boulianne, Access and Privacy Officer at York Region, for facilitating access of the data and information. The views and conclusions contained in this document are those of the authors and should not be interpreted as necessarily representing the official policies or views, either expressed or implied, of The Regional Municipality of York, its elected and appointed officials or employees.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khazaei, E., Milne-Home, W. Applicability of geochemical techniques and artificial sweeteners in discriminating the anthropogenic sources of chloride in shallow groundwater north of Toronto, Canada. Environ Monit Assess 189, 218 (2017). https://doi.org/10.1007/s10661-017-5927-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-5927-1