Abstract
With economic development and the increase of energy consumption, surface water acidification has been a potential environmental concern in China. Here, we analyzed variations and trends in surface water pH of 73 sites from ten river basins in China from 2004 to 2014 with nonparametric Seasonal Kendall test method. Our analysis showed that the variations of surface water pH in China ranged from 6.5 to 9.0 in the past decade (2004–2014), which satisfied the water quality criteria in pH for protection of aquatic ecosystems in China (6.0–9.0) and USA (6.5–9.0). However, significant decreasing trends in surface water pH were found in 31 monitoring sites, which were mainly located in Haihe River, Taihu Lake and Yangtze River, while the pH value showed significant increasing trends in 22 sites, which mainly were located in Songhua River and Pearl River. Our results suggested the increased potential acidification of susceptible water bodies in China. Besides the control policy of sulfur dioxide (SO2) emissions, the emissions of nitrous oxides (NOx) should also be reduced to protect the aquatic systems in China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surface water acidification caused by acidic deposition could lead to soil acidification (Matzner 1995), forest vitality reduction (Fischer 2007), disturbance of aquatic animals’ metabolic systems, reduction of hatchability, and even the extinction of fish population (Haines and Baker 1986). In addition to biological effects, pH can also affect the solubility of chemical constituents such as nutrients and heavy metal toxicity in the water (EPA 2012). Therefore, pH is an important parameter for understanding biological consequences of acid deposition (Matsubara et al. 2009).
Acidification in surface water has been an environmental problem in Europe and North America. However, the acidity of precipitation has declined over large portions of eastern North America and Western Europe due to more stringent air pollution emission laws, widespread availability of improved pollutant removal technologies, and energy conservation in the past two decades (Skjelkvale et al. 1998; Stoddard et al. 1999; Driscoll 2001; Evans et al. 2001; Kvaeven et al. 2001). The goals of these acid deposition-related emission laws, such as the Clean Air Act Amendments of 1990 in the USA and the 1994 Oslo Protocol in Europe, are to reduce the acidity of precipitation and allow the chemical and biological recovery of previously impacted waters and ecosystems (Burns et al. 2006). Decreases in pH have been clarified in lakes and rivers of northern USA (Charles et al. 1987), Canada (Watt et al. 1983), and Scandinavia (Wright et al. 1976) by analyzing the long-term records.
Due to the increased emissions from mining and fossil fuel consumption in the past 30 years, China has become the third largest acid rain district after Europe and North America (Nordberg 2003), and acid rain pollution is one of the important environmental problems currently in China (Zhang et al. 2007; Ouyang et al. 2008). The economic loss caused by acid rain pollution was about 110 billion yuan every year in China (Zhang et al. 2010). Several studies have been conducted on regional surface water acidification in recent years in China (Zhang et al. 2007; Duan et al. 2011; Xu et al. 2013; Fang et al. 2013). However, the national surface water acidification trend in China is still not clear. In our study, by the data from 73 national monitoring sites in ten water basins, we initially analyzed the surface water pH variations and trends from 2004 to 2014 and evaluated the possible effects of air pollutant emission control policy on the restoration of surface water acidification in China.
Materials and methods
Data source and study area
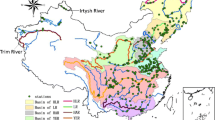
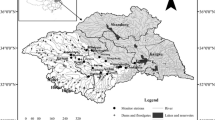
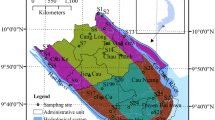
Weekly water pH data for the 73 monitoring sites in ten major river basins were obtained from the China Ministry of Environment Protection. These data are publicly accessible through the China Ministry of Environment Protection data center (available via http://datacenter.mep.gov.cn). The basins include 7 rivers (Yellow River, Liaohe River, Haihe River, Huaihe River, Yangtze River, Pearl River, Songhua River) with 62 sites and 3 lakes (Taihu Lake, Dianchi Lake, Chaohu Lake) with 11 sites (Fig. 1). For the rivers, there are 30 sites in the main stream and 32 sites in the tributary. Sixty-five sites have an 11-year period of record (2004 to 2014), 6 sites have a 10-year record (2005 to 2014), and 2 sites have an 8-year record (2007 to 2014).
Statistical analysis
Trend analysis was undertaken by the Seasonal Mann–Kendall test (SMK) (Hirsch et al. 1982; Hirsch and Slack 1984). SMK is a nonparametric method by which data are grouped within seasonal blocks and tested for monotonic trends within each block using a ranking procedure. Further, the method can be used on data that are not normally distributed, which is commonly the case with environmental data. It is also robust for extreme values and possible outliers (Helsel and Hirsch 1992).
Assuming that a time series is formed by n data (Y1, Y2, Y3, Y n ), Kendall’s S statistic is computed from each data pair:
where P is the number of Y i < Y j for all i < j and N is the number of Y i > Y j for i < j. S is asymptotically normal, with a mean of 0. The variance of S is
The SMK test accounts for seasonality by computing the Mann–Kendall test on each of m seasons separately and then combining the results. No comparisons are made across seasons. The SMK statistic S k is simply the sum of individual Kendall’s S statistics (S L ) for each of the “L = 1 to m” seasons.
When the product of number of seasons and number of years is more than about 25, the distribution of S k will be approximated quite well by a normal distribution (Hirsch et al. 1982).
The resulting statistic (Z Sk ) is evaluated against a table of the standard normal distribution.
where
and
The overall SMK trend slope for Y over time is computed as the median of all slopes between data pairs within the same season. Furthermore, the slope is unaffected by seasonality and less affected by extreme values than is a linear regression slope (Hirsch et al. 1982).
All analyses were performed using R software (“rkt” package) at a 0.05 significance level (R Core Team. R 2014).
Results
pH variations
We used annual value to reflect the variations of surface water pH in China. The annual data were calculated from the weekly pH data in all sites of each basin in 2004 and 2014. The annual pH values from the 65 stations of ten surface water basins were shown in Figs. 2 and 3. The variations of pH level ranged from 6.5 to 9.0 in 2004 and 2014. The national mean pH value was 7.70 ± 0.43 in 2004 and decreased to 7.64 ± 0.41 in 2014. The lowest pH (6.60 ± 0.16) was found at the Tongjiang station in Songhua River, and the highest pH (8.94 ± 0.37) was seen at the Xiyuan tunnel in Kunming in Dianchi Lake in 2004 (Fig. 2). While in 2014, the lowest pH (6.54 ± 0.19) was found at the Nanchang station in Jiangxi province in Yangtze River and the highest pH (8.61 ± 0.65) was seen at the Guanyin Mountain in Kunming in Dianchi Lake.
For the pH variations in basin scale, the lowest mean pH values in 2004 (7.04 ± 0.30) and 2014 (7.25 ± 0.34) were found in Songhua River and Pearl River, respectively (Fig. 3). However, the pH value of Dianchi Lake (8.90 ± 0.05 and 8.41 ± 0.29) was the highest both in 2004 and 2014.
Long-term trends
Although no significant difference was found in the mean pH value in national scale between 2004 and 2014, the water pH for some sites showed significant increasing or decreasing trends (Fig. 4).
Examples of the three long-term surface water pH trends in China: a significant increase (the Heihe monitoring sites of Heilongjiang province in Songhua River), b significant decrease (the Yibin monitoring sites of Sichuan province in Yangtze River), and c nonsignificant (the Changchun monitoring sites of Jilin province in Songhua River)
Table 1 showed the results of the seasonal Mann–Kendall tests for long-term trends in surface water pH at the 73 points in the ten basins of China. Details of results for the SMK test were shown in Table 2. Significant decreased trends (p < 0.05) in river water pH were observed at 31 points, while we detected significant increasing trends (p < 0.05) at 22 points (Table 1 and Fig. 5). The sites which showed significant decreasing trends were mainly located in Haihe River (6/7; among a total of seven monitoring sites, six showed significant decreasing trends), Taihu Lake (4/7), and Yangtze River (10/16). The sites showing significant increasing trends belong to Songhua River (4/5) which is the northernmost basin and Pearl River (4/7) which is the southernmost basin in China (Fig. 5).
Discussion
Surface water acidification in China
Excessively low or high pH can be detrimental for the use of water. The water with a low pH (<6.5) could be acidic and contain high levels of heavy metals which are harmful to aquatic organisms. Some surface waters are in serious water acidification, for example in Scandinavia, where waters (typically inland lakes and streams) with pH below 5.0 are common (Menz and Seip 2004). In an assessment of the effect of plantation forestry on the recovery of surface waters from acidification, Malcolm et al. (2014) found that streams ranged from highly acidic (median annual pH 4.1) to circumneutral (pH 7.1) in the Loch Ard area of Central Scotland. Our analysis showed that the variations of surface water pH in China ranged from 6.5 to 9.0 in the past decade (2004–2014), which satisfied the water quality criteria in pH for protection of aquatic ecosystems in China (6.0–9.0) and USA (6.5–9.0, US-EPA 2009).
Water pH in Songhua River and Pearl River showed lower levels than in other basins. The possibility of water acidification still exists in Songhua River due to the widely distributed acidic forest soils, and the water body is less able to buffer acidity in this region (Xu et al. 2013). Some regions in Pearl River, especially those in Jiangxi and Guangdong province, are more susceptible to acidification than those in northeastern China, which attribute to their soil types and geological and acid deposition conditions (Hao et al. 2001).
Dianchi Lake showed the highest water pH, which is possibly caused by the plateau geological condition, climate condition, terrestrial heat flow, and the interaction between planktonic algae growth and change of dissolved oxygen content in the process of eutrophication (Li et al. 2007).
The trends of surface water pH and possible effects of control policy
Long-term trends of surface water pH in the world were summarized in Table 3. Acidification in surface water has been a serious environmental problem in Europe and North America. However, widespread recoveries of surface water acidification in Europe and North America have been reported since the 1990s (Garmo et al. 2014). For example, the water acidification in the UK (Battarbee et al. 2014; Murphy et al. 2014), Sweden (Wällstedt et al. 2009; Oni et al. 2013), Spain (Bouza-Deaño et al. 2008), and the USA (Kahl and Center for the Environment, 2004) have been recovering slowly following considerable emission abatement of sulfur dioxide (SO2) and nitrogen oxide (NOx) since the 1990s. In the lakes and their catchments of western Japan and Bohemia, the decline of total acid input in precipitation and regrowth of vegetation could lead to further increase of the water pH (Yamada et al. 2007; Oulehle et al. 2013). Lake Peipsi water (Estonia/Russia) has experienced a significant increase of pH due to combination of climate change and eutrophication over the last three to four decades (Minella et al. 2013). In addition, a disturbing eutrophication of the reservoir after the spraying of fertilizers and industrial wastewater had an impact on water pH of the Grouz dam in Eastern Algeria (Guerraiche et al. 2015). In contrast, a significant long-term declining trend in river water pH was found in several watersheds in Central Japan and the Maroon River in Iran. The reason for the acidification of the river waters could be the cumulative acid loading from the atmosphere to the soil due to the development of the region economy during the last several decades (Matsubara et al. 2009; Tabari et al. 2011).
Acid deposition caused by sulfur dioxide (SO2) and nitrogen oxide (NOx) emissions has become a major concern in China (Tang 2006). Although we did not find water acidification in our analysis (Fig. 3), the pH in many monitoring sites showed significant decreasing trends, especially in Haihe River, Taihu Lake, and Yangtze River (Fig. 5). To protect the aquatic ecosystems and improve the air quality further, the Chinese government has established compulsory targets to reduce SO2 emissions by 10 % during 2005–2010 and a further 8 % by 2015 and reduce NOx emissions by 10 % during 2010–2015. The SO2 emissions generally showed decreasing trends resulting from the compulsory policy (Fig. 6a). However, the NOx emissions showed different trends in different regions. Decreasing trends of NOx emissions were found in Guangdong (Pearl River) and Liaoning province (Songhua River), while increasing trends of NOx emissions were found in other three basins (Fig. 6b).
Water pH trends are usually related to anthropogenic pollutions, such as acid rain which comes from the reaction of water with nitrogen oxides, sulfur oxides, and other acidic compounds. Direct correlations were found between sulfur emissions and sulfur deposition, including sulfur dioxide and nitrogen oxides (Lynch et al. 2000). Our analysis suggested that the increasing trends of water pH in Songhua River and Pearl River are possibly caused by the reductions of SO2 and NOx emissions in the two basins. However, the increased NOx emissions in Haihe River, Taihu Lake, and Yangtze River may counteract the reductions of the SO2 emissions, which caused the decreased water pH in these three basins. It seems that the increase of N deposition would delay the recovery of stream water from acidification due to control of S deposition in these catchments in China in recent years. Therefore, to prevent the potential acidification of susceptible water bodies, the NOx emissions should be also controlled at the basin scale. In addition to the atmospheric deposition, the source pollutant emissions which come from agricultural runoff, wastewater discharge, or industrial runoff could also affect the water pH (Matsubara et al. 2009). Our analysis suggested that China is also facing an increased potential acidification of susceptible water bodies. Besides the control policy of sulfur dioxide (SO2) emission, the emission of nitrous oxides (NOx) and other factors like point source pollution should also be reduced to protect the aquatic systems in China.
Other factors could also have an impact on pH changes in surface water, e.g., changes in hydrological regime, land use, or wastewater emissions. The agricultural nonpoint source pollution caused by land use change in the upper reaches of Yellow River is becoming the main pollution source of Yellow River, which threatens the water quality including pH in the lower reaches of the Yellow River basin (Hu et al. 2013). Nutrients from farmland fertilizers and urban wastewater discharged into rivers contribute main pollutants to the surface water in Chaohu and Dianchi Lake, thereby tending to induce serious ecological problems such as eutrophication, which interrelates with the water pH (Zhang et al. 2012; Tanaka et al. 2013; Jiang et al. 2014). The water in the Liaohe River Estuary area has been seriously polluted by discharges of wastewater containing petroleum pollutants and nutrients (Ye et al. 2013). In recent years, many industries developed rapidly along the main and branch streams of Huaihe River, which have discharged a number of wastewater and led to the deterioration of water quality (Dou et al. 2016).
Conclusion
In this study, with the nonparametric Seasonal Kendall test method, the surface water pH trends were analyzed in 73 sites from ten river basins in China from 2004 to 2014. The variations of surface water pH in China ranged from 6.5 to 9.0 in the past decade (2004–2014), with relative lower levels in Songhua River and Pearl River but higher levels in Dianchi Lake. Significantly decreasing trends in surface water pH were found in 31 monitoring sites, which were mainly located in Haihe River, Taihu River, and Yangtze River. In contrast, the pH value showed significantly increasing trends in 22 sites, which were mainly located in Songhua River and Pearl River. Decreasing trends of NOx emissions were found in Guangdong (Pearl River) and Liaoning province (Songhua River), while increasing trends of NOx emissions were found in other three basins. Our results suggested that the increasing trends of water pH in Songhua River and Pearl River are possibly caused by the reductions of SO2 and NOx emissions in these two basins. However, the increased NOx emissions in Haihe River, Taihu Lake, and Yangtze River may counteract the reductions of the SO2 emissions. To effectively protect the aquatic systems, the emission of nitrous oxides (NOx) should also be controlled in China.
References
Battarbee, R. W., Shilland, E. M., Kernan, M., Monteith, D. T., & Curtis, C. J. (2014). Recovery of acidified surface waters from acidification in the United Kingdom after twenty years of chemical and biological monitoring (1988–2008). Ecological Indicators, 37, 267–273. doi:10.1016/j.ecolind.2013.10.011.
Bouza-Deaño, R., Ternero-Rodríguez, M., & Fernández-Espinosa, A. J. (2008). Trend study and assessment of surface water quality in the Ebro River (Spain). Journal of Hydrology, 361(3–4), 227–239. doi:10.1016/j.jhydrol.2008.07.048.
Burns, D. A., McHale, M. R., Driscoll, C. T., & Roy, K. M. (2006). Response of surface water chemistry to reduced levels of acid precipitation: comparison of trends in two regions of New York, USA. Hydrological Processes, 20(7), 1611–1627. doi:10.1002/hyp.5961.
Charles, D. F., Whitehead, D. R., Engstrom, D. R., Fry, B. D., Hites, R. A., Norton, S. A., et al. (1987). Paleolimnological evidence for recent acidification of Big Moose Lake, Adirondack Mountains, N.Y. (USA). Biogeochemistry, 3(1–3), 267–296. doi:10.1007/BF02185196.
Driscoll, C. T. L. G. B. B. A. J. B. T. J. C. C. S. E. C. K. F. L. G. E. S. J. L. W. K. (2001). Acidic deposition in the northeastern United States: sources and inputs, ecosystem effects, and management strategies: the effects of acidic deposition in the northeastern United States include the acidification of soil and water, which stresses terrestrial and aquatic biota. BioScience, 51(3).
Dou, M., Zuo, Q., Ma, J., & Li, G. (2016). Simulation and control of the linked systems of water quantity–water quality–socio-economics in the Huaihe River basin. Hydrological Sciences Journal, 61(4), 763–774. doi:10.1080/02626667.2014.959953.
Duan, L., Ma, X., Larssen, T., Mulder, J., & Hao, J. (2011). Response of surface water acidification in upper Yangtze River to SO2 emissions abatement in China. Environmental Science and Technology, 45(8), 3275–3281. doi:10.1021/es1038672.
EPA. (2012). pH. In Water: Monitoring and Assessment. United States Environmental Protection Agency (USEPA). http://water.epa.gov/type/rsl/monitoring/vms54.cfm
Evans, C. D., Chadwick, T., Norris, D., Rowe, E. C., Heaton, T. H. E., Brown, P., et al. (2014). Persistent surface water acidification in an organic soil-dominated upland region subject to high atmospheric deposition: the North York Moors, UK. Ecological Indicators, 37, 304–316. doi:10.1016/j.ecolind.2012.02.018.
Evans, C. D., Cullen, J. M., Alewell, C., Kopacek, J., Marchetto, A., Moldan, F., et al. (2001). Recovery from acidification in European surface waters. Hydrology and Earth System Sciences, 5(3), 16.
Fang, Y. T., Wang, X. M., Zhu, F. F., Wu, Z. Y., Li, J., Zhong, L. J., et al. (2013). Three-decade changes in chemical composition of precipitation in Guangzhou city, southern China: has precipitation recovered from acidification following sulphur dioxide emission control? [Article]. Tellus Series B-Chemical and Physical Meteorology, 65, 15. doi:10.3402/tellusb.v65i0.20213.
Fischer, R. M. V. E. G. M. (2007). Monitoring of atmospheric deposition in European forests and an overview on its implication on forest condition. Applied Geochemistry, 22(6), 11.
Garmo, Ø., Skjelkvåle, B., de Wit, H., Colombo, L., Curtis, C., Fölster, J., et al. (2014). Trends in surface water chemistry in acidified areas in Europe and North America from 1990 to 2008. Water, Air, & Soil Pollution, 225(3), 1–14. doi:10.1007/s11270-014-1880-6.
Guerraiche, Z., Boudoukha, A., & Benkadja, R. (2015). Variation of the chemical composition of Grouz dam waters, Eastern Algeria. Desalination and Water Treatment, 57(11), 4878–4887. doi:10.1080/19443994.2014.995131.
Haines, T., & Baker, J. (1986). Evidence of fish population responses to acidification in the Eastern United States. Water, air, and soil pollution, 31(3–4), 605–629. doi:10.1007/BF00284216.
Hao, J., Ye, X., Duan, L., & Zhou, Z. (2001). Calculating critical loads of sulfur deposition for 100 surface waters in China using the magic model. [Article]. Water Air and Soil Pollution, 131(1–4 Part 3), 1157–1162.
Helsel, D. R & Hirsch, R.M. (1992). Statistical Methods in Water Resources: U.S. Geological Survey Techniques of Water Resources Investigations Book 4, Chapter A3. http://water.usgs.gov/pubs/twri/twri4a3/
Hirsch, R. M., & Slack, J. R. (1984). A nonparametric trend test for seasonal data with serial dependence. Water Resources Research, 20(6), 727–732. doi:10.1029/WR020i006p00727.
Hirsch, R. M., Slack, J. R., & Smith, R. A. (1982). Techniques of trend analysis for monthly water quality data. Water Resources Research, 18(1), 107–121. doi:10.1029/WR018i001p00107.
Hu, Y., Wu, P., Zhao, Y., Zhang, Z., & Xu, B. (2013). Water quality of cropland drainage ditches in the Yellow River irrigation regions of Ningxia and Inner Mongolia, China. [Article]. Shengtaixue Zazh, 32(7), 1730–1738.
Jiang, Y.-J., He, W., Liu, W.-X., Qin, N., Ouyang, H.-L., Wang, Q.-M., et al. (2014). The seasonal and spatial variations of phytoplankton community and their correlation with environmental factors in a large eutrophic Chinese lake (Lake Chaohu). Ecological Indicators, 40, 58–67. doi:10.1016/j.ecolind.2014.01.006.
Kahl, J. S., & Center for the Environment, P. S. U. U. S. A. S. J. R. S. R. F. J. D. W. C. A. J. P. K. K. N. S. (2004). Have U.S. surface waters responded to the 1990 Clean Air Act amendments? Environmental Science & Technology, 38(24), 484A–490A.
Kvaeven, B., Ulstein, M., Skjelkvåle, B., Raddum, G., & Hovind, H. (2001). ICP Waters—an international programme for surface water monitoring. Water, air, and soil pollution, 130(1–4), 775–780. doi:10.1023/A:1013802122401.
Li, H. B., Hou, G. X., Dakui, F., Xiao, B. D., Song, L. R., & Liu, Y. D. (2007). Prediction and elucidation of the population dynamics of Microcystis spp. in Lake Dianchi (China) by means of artificial neural networks. [Article]. Ecological Informatics, 2(2), 184–192. doi:10.1016/j.ecoinf.2007.03.007.
Lynch, J. A., Bowersox, V. C., & Grimm, J. W. (2000). Acid rain reduced in Eastern United States. Environmental Science & Technology, 34(6), 940–949. doi:10.1021/es9901258.
Malcolm, I. A., Bacon, P. J., Middlemas, S. J., Fryer, R. J., Shilland, E. M., & Collen, P. (2014). Relationships between hydrochemistry and the presence of juvenile brown trout (Salmo trutta) in headwater streams recovering from acidification. Ecological Indicators, 37(Part B), 351–364. doi:10.1016/j.ecolind.2012.02.029.
Matsubara, H., Morimoto, S., Sase, H., Ohizumi, T., Sumida, H., Nakata, M., et al. (2009). Long-term declining trends in river water pH in Central Japan. Water, air, and soil pollution, 200(1–4), 253–265. doi:10.1007/s11270-008-9909-3.
Matzner, E. M. D. (1995). Soil changes induced by air pollutant deposition and their implication for forests in Central Europe. Water, air, and soil pollution, 85(1), 63–76.
Menz, F. C., & Seip, H. M. (2004). Acid rain in Europe and the United States: an update. Environmental Science & Policy, 7(4), 253–265. doi:10.1016/j.envsci.2004.05.005.
Minella, M., De Laurentiis, E., Buhvestova, O., Haldna, M., Kangur, K., Maurino, V., et al. (2013). Modelling lake-water photochemistry: three-decade assessment of the steady-state concentration of photoreactive transients (.OH, CO3(−.) and (3)CDOM( *)) in the surface water of polymictic Lake Peipsi (Estonia/Russia). Chemosphere, 90(10), 2589–2596. doi:10.1016/j.chemosphere.2012.10.103.
Murphy, J. F., Winterbottom, J. H., Orton, S., Simpson, G. L., Shilland, E. M., & Hildrew, A. G. (2014). Evidence of recovery from acidification in the macroinvertebrate assemblages of UK fresh waters: a 20-year time series. Ecological Indicators, 37, 330–340. doi:10.1016/j.ecolind.2012.07.009.
Nordberg, G. (2003). Cadmium and human health: a perspective based on recent studies in China. The Journal of Trace Elements in Experimental Medicine, 16(4), 307–319. doi:10.1002/jtra.10039.
Oni, S. K., Futter, M. N., Bishop, K., Köhler, S. J., Ottosson-Löfvenius, M., & Laudon, H. (2013). Long-term patterns in dissolved organic carbon, major elements and trace metals in boreal headwater catchments: trends, mechanisms and heterogeneity. Biogeosciences, 10(4), 2315–2330. doi:10.5194/bg-10-2315–2013.
Oulehle, F., Chuman, T., Majer, V., & Hruška, J. (2013). Chemical recovery of acidified Bohemian lakes between 1984 and 2012: the role of acid deposition and bark beetle induced forest disturbance. Biogeochemistry, 116(1–3), 83–101. doi:10.1007/s10533-013-9865-x.
Ouyang, X.-J., Zhou, G.-Y., Huang, Z.-L., Liu, J.-X., Zhang, D.-Q., & Li, J. (2008). Effect of simulated acid rain on potential carbon and nitrogen mineralization in forest soils1. Pedosphere, 18(4), 503–514. doi:10.1016/S1002-0160(08)60041-7.
R Core Team. R. (2014). A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. http://www.R-project.org/.
Skjelkvale, B. L., Wright, R. F., & Henriksen, A. (1998). Norwegian lakes show widespread recovery from acidification; results from national surveys of lakewater chemistry 1986–1997. Hydrology and Earth System Sciences, 2(4), 555–562.
Stoddard, J. L., Jeffries, D. S., Lukewille, A., Clair, T. A., Dillon, P. J., Driscoll, C. T., et al. (1999). Regional trends in aquatic recovery from acidification in North America and Europe. Nature, 401(6753), 575–578. doi:10.1038/44114.
Tabari, H., Marofi, S., & Ahmadi, M. (2011). Long-term variations of water quality parameters in the Maroon River, Iran. Environmental Monitoring and Assessment, 177(1–4), 273–287. doi:10.1007/s10661-010-1633-y.
Tanaka, T., Sato, T., Watanabe, K., Wang, Y., Yang, D., Inoue, H., et al. (2013). Irrigation system and land use effect on surface water quality in river, at lake Dianchi, Yunnan, China. Journal of Environmental Sciences, 25(6), 1107–1116. doi:10.1016/s1001-0742(12)60206-x.
Tang, D. (2006). Acid rain in China. Rapid industrialization has put citizens and ecosystems at risk. Environmental Science and Technology, 40(2), 418–425.
US-EPA [United States Environmental Protection Agency] (2009). National Recommend Water Quality Criteria. Office of Water, Office of Science and Technology. Available via: http://water.epa.gov/scitech/ swguidance/ standards/current/upload/nrwqc-2009.pdf
Wällstedt, T., Edberg, F., & Borg, H. (2009). Long-term water chemical trends in two Swedish lakes after termination of liming. Science of the Total Environment, 407(11), 3554–3562. doi:10.1016/j.scitotenv.2009.01.031.
Watt, W. D., Scott, C. D., & White, W. J. (1983). Evidence of acidification of some Nova Scotian Rivers and its impact on Atlantic Salmon, Salmo solar. Canadian Journal of Fisheries and Aquatic Sciences, 40(4), 462–473. doi:10.1139/f83-065.
Wright, R., Dale, T., Gjessing, E., Hendrey, G., Henriksen, A., Johannessen, M., et al. (1976). Impact of acid precipitation on freshwater ecosystems in Norway. Water, air, and soil pollution, 6(2–4), 483–499. doi:10.1007/BF00182887.
Xu, G., Kang, R., Luo, Y., & Duan, L. (2013). Current status of surface water acidification in Northeast China. [Article]. Huanjing Kexue, 34(5), 1695–1699.
Yamada, T., Inoue, T., Fukuhara, H., Nakahara, O., Izuta, T., Suda, R., et al. (2007). Long-term trends in surface water quality of five lakes in Japan. Water, Air, & Soil Pollution: Focus, 7(1), 259–266. doi:10.1007/s11267-006-9076-8.
Ye, H. F., Guo, S. H., Li, F. M., & Li, G. (2013). Water quality evaluation in Tidal River reaches of Liaohe River Estuary, China using a revised QUAL2K model. [Article]. Chinese Geographical Science, 23(3), 301–311. doi:10.1007/s11769-013-0586-9.
Zhang, J.-E., Ouyang, Y., & Ling, D.-J. (2007). Impacts of simulated acid rain on cation leaching from the Latosol in south China. Chemosphere, 67(11), 2131–2137. doi:10.1016/j.chemosphere.2006.12.095.
Zhang, X. M., Chai, F. H., Wang, S. L., Sun, X. Z., & Han, M. (2010). Acid rain research status in China. Research of Environmental Sciences, 23(5), 527–532. doi:10.13198/j.res.2010.05.3.zhangxm.005.
Zhang, Y., Gao, X., & Zhang, H. (2012). Association study between water quality of Chaohu Lake and resources input in agriculture of basin. [Article]. Huanjing Kexue, 33(9), 3009–3013.
Acknowledgments
We thank the editor and two anonymous referees whose comments and suggestions greatly improved the quality of the article. This research was funded by the National Water Pollution Control and Treatment Science and Technology Major Project (2012ZX07501003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiao, Y., Feng, J., Liu, X. et al. Surface water pH variations and trends in China from 2004 to 2014. Environ Monit Assess 188, 443 (2016). https://doi.org/10.1007/s10661-016-5454-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5454-5