Abstract
Since 1983, the Ministry of the Environment of Japan has conducted nation-wide acid deposition surveys. To investigate the effects of acid deposition on surface water, we used the nonparametric Mann–Kendall test to find temporal trends in pH, alkalinity, and electrical conductivity (EC) in more than 10 years of data collected from five lakes and their catchments (Lake Kuttara: northernmost; Lake Kamakita: near Tokyo; Lake Ijira: central; Lake Banryu: western; and Lake Unagiike: southernmost). The pH of Lake Ijira water has declined slightly since the mid-1990s, corresponding with the downward trends seen in the pH and alkalinity of the river water flowing into the lake. There were significant upward trends in the EC of both the lake and stream water; the same trends were also found for \( {\text{NO}}^{ - }_{3} \) concentrations. These trends show evidence of acidification due to atmospheric deposition, and this is the first such finding in Japan based on significant long-term trends. Lake Ijira is located about 40 km north of the Chukyo industrial area near Nagoya. The annual depositions of H+, nss-\( {\text{SO}}^{{2 - }}_{4} \), and \( {\text{NO}}^{ - }_{3} \) in Lake Ijira were among the highest of all deposition monitoring sites, suggesting that this is the main cause of the significant acidification observed in Lake Ijira. No significant trends suggesting acidification were observed in any of the other lake catchments in spite of the significant upward trends in EC. Upward trends in pH and alkalinity at Lake Banryu and upward trends in alkalinity at Lake Kamakita were detected, but no change in pH or alkalinity at Lake Kuttara and Lake Unagiike was observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In Japan, the Ministry of the Environment has been conducting nation-wide acid deposition surveys since 1983 to clarify the current conditions and the impact of acid deposition. This program, named the Japanese Acid Deposition Survey (JADS), has conducted integrated surveys at five lakes and their catchments examined in this paper for almost 20 years, carrying out long-term monitoring of wet deposition, dry deposition, soil, vegetation, and the lakes’ aquatic environments. Some of these surveys will continue as part of the acid deposition monitoring network in East Asia (EANET). International cooperative programs on the assessment and monitoring of acid deposition and on control of emissions, and the accompanying international regulations and agreements, have been in place in Europe since the Convention on Long-Range Transboundary Air Pollution (CLRTAP) was signed in 1979 (Kvaeven, Ulstein, Skjelkvale, Raddum, & Hovind, 2001). Long-term trend analyses of surface water have shown declines in the rate of acid deposition and the widespread recovery of surface water acidification in European countries (Skjelkvale, Stoddard, & Andersen, 2001; Stoddard et al., 1999). Although global sulfur emissions peaked in 1987 and declined rapidly thereafter, Asia was the largest sulfur emitter in the world in 2000 (Stern, 2005). Possible adverse impacts caused by acid deposition are of concern in some parts of China (Ye, Hao, Duan, & Zhou, 2002). In Japan, no long-term effects of acid deposition have so far been seen on surface water, because of the high acid-neutralizing capacity of the surrounding terrain (Ohte et al., 2001); although temporal decreases in pH in the surface waters were observed during rainfall (Komai, Umemoto, & Inoue, 2001). In this study, more than 10 years of data collected by JADS from five lake catchments were tested for temporal trends in pH, alkalinity, and electrical conductivity (EC) by the nonparametric seasonal Mann–Kendall test to clarify significant effects of acid deposition on surface waters in Japan.
2 Materials and Methods
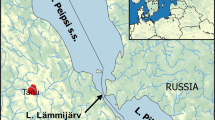
The study sites in Japan were Lake Kuttara (northernmost), Lake Kamakita (near Tokyo), Lake Ijira (central), Lake Banryu (western), and Lake Unagiike (southernmost) (Fig. 1). These lakes were selected by JADS as representative of the major types of lakes in Japan. Lake Ijira and Lake Kamakita were selected as monitoring sites to clarify the impact of two major industrial areas in Japan, the Chukyo industrial area located near Nagoya City in central Japan and the Keihin industrial area in the Tokyo Bay area, respectively. Lake Kuttara and Lake Unagiike represent Japan’s northernmost and southernmost caldera lakes, respectively. Lake Banryu is located in a region that would be affected by atmospheric deposition from continental Eurasia. Lake Ijira and Lake Banryu are EANET monitoring sites. General information on these five lakes, including the bedrock geology, type of dominant surface soil, vegetation, and the average concentrations of major ions in the surface water at the center of the lakes is summarized in Table 1. Surveys of lake water were conducted four times a year from September 1988 to October 1997 at Lake Kuttara and from October 1998 to January 1998 at Lake Unagiike. Surveys of water from Lake Kamakita and river water flowing into the west end of the lake were conducted from September 1988 to February 2002, with the exception of the period from March 1998 to June 2000 because of dredging in the lake. Surveys of lake water and river water were conducted four times a year from September 1998 to March 2003 at Lake Ijira, and surveys of water from Lake Banryu were conducted from November 1988 to December 2002.
Wet precipitation was collected monthly with a bulk precipitation collector from October 1988 to March 1998 at the five sites. Wet precipitation has also been collected weekly by a wet-only precipitation collector since June 1999 at Lake Ijira and Lake Banryu.
For detecting long-term trends in pH, alkalinity, and EC in the water of the studied sites, the seasonal Mann–Kendall test was used. The seasonal Mann–Kendall test is a non-parametric test for detection of monotonic trends that accommodates non-normal data distribution (Helsel & Hirsch, 1992).
3 Results
The variations in pH and alkalinity of the surface water at the center of each of the five lakes and of the river water flowing into Lake Kamakita and Lake Ijira are shown in Fig. 2. Table 2 shows the results of the seasonal Mann–Kendall tests for these variations in pH, alkalinity, and EC at the sites over the whole monitoring period.
Temporal variations in pH (left) and alkalinity (Alk.) (center) in surface lake water at the center of each lake and the river water flowing into Lake Kamakita and Lake Ijira. Annual rates of acid deposition (H+ (H), nss-\( {\text{SO}}^{{2 - }}_{4} \) (nssSO4), and \( {\text{NO}}^{ - }_{3} \) (NO3)) and annual amount of precipitation (precip.) at each lake site are shown on the right
In Lake Kuttara, the pH of the lake water was between 7 and 8, and there was no significant increasing or decreasing trend over the 10 years, although there was a significant increase in the trend of EC. No change in alkalinity (about 300 μeq/L) was observed in Lake Kuttara; therefore, no evidence of impact by acid deposition was seen.
Lake Kamakita had a large seasonal change in pH, but no significant increase or decrease in the trend of pH was observed over the monitoring period. Alkalinity in both lake water and river water flowing into Lake Kamakita was more than 500 μeq/L, which means that the water in Lake Kamakita had a high acid-buffering capacity. Trend analysis for Lake Kamakita water from 1988 to 1997 (Table 2) showed significant increasing trends in the alkalinity and EC. The alkalinity and EC in the river water flowing into the west end of Lake Kamakita also had significant upward trends, corresponding to significant increases in Ca2+ and \( {\text{NO}}^{ - }_{3} \) (P < 0.05) (T. Yamada, unpublished data). There was no evidence of acidification in Lake Kamakita before 1997, whereas after 2000 the pH and alkalinity in some samples of lake and river water were lower than those before 1997 (Fig. 2).
The alkalinity of the water in Lake Ijira and the rivers flowing into it was about 150 μeq/L, which was relatively low compared to the other sites. The EC in the lake and river water and the alkalinity in lake surface water showed significant increasing trends, whereas no significant trend was detected in pH in lake water and either pH or alkalinity in river water for the 10 years of data since 1988. The pH of the water in Lake Ijira has declined slightly since the mid-1990s, corresponding to the downward trends in the pH of the river water flowing into the lake (Fig. 2). The alkalinity of the river water has also shown a decreasing trend since the mid-1990s. After 1995, there were significant decreasing trends in pH in the rivers flowing into Lake Ijira and a slightly decreasing trend in alkalinity in one of the rivers (P < 0.1) (Table 2).
The water of Lake Banryu was affected by sea salt, and the concentrations of Na+ and Cl− in the lake water were higher than those in the other lakes (Table 1). The pH and alkalinity of surface water in the center of the lake were relatively low, ranging from 6.5 to 7.6 and from 100 to 250 μeq/L, respectively. Lake Banryu had increasing trends in pH, alkalinity, and EC over the study period. These upward trends in pH and alkalinity indicate the alkalization of Lake Banryu water.
In Lake Unagiike, there was some seasonal fluctuation in pH, but the long-term trend analysis detected no change. Alkalinity in the lake was high, at about 600 μeq/L, with little seasonal fluctuation. Lake Unagiike also had increasing trends in EC over the study period.
4 Discussion
The rates of acid deposition (as H+, nss-\( {\text{SO}}^{{2 - }}_{4} \), and \( {\text{NO}}^{ - }_{3} \)) at the five monitoring sites in this paper were among the highest of all sites included in the JADS surveys on acid deposition (Committee for Acid Deposition Measures, 2004). Nevertheless, most of the sites showed no significant trends revealing acidification of the water, such as pH decline (Fig. 3). Lake Kuttara and Lake Unagiike are both caldera lakes with no inflowing rivers, and their catchments are covered with volcanic rocks with high acid-buffering capacity, which is the reason there is no trend toward acidification. That there was no evidence for acidification in Lake Kamakita water over the monitoring period was also due to the geology of the Lake Kamakita catchment, which includes bedrock with high acid-buffering capacity, such as limestone (Table 1). The rate of \( {\text{NO}}^{ - }_{3} \) deposition in Lake Kamakita, located near the Keihin industrial area, was among the highest in Japan (Fig. 3) suggesting that the significant trend of increasing \( {\text{NO}}^{ - }_{3} \) in Lake Kamakita is due to acid deposition. The concentrations of \( {\text{NO}}^{ - }_{3} \) in precipitation have increased in the Tokyo metropolitan area since 1990 (Okuda et al., 2004) and continuous monitoring of Lake Kamakita is needed in the future.
Ogive of the rate of acid deposition in the five study sites and the other JADS monitoring sites in Japan (Committee for Acid Deposition Measures, 2004)
Acidification in the inflowing river water and in the lake water was detected by the long-term monitoring only in the Lake Ijira catchment. The Lake Ijira catchment is unpopulated and there are no natural acidification factors, such as volcanoes, or artificial sources of pollution in the catchment. The alkalinity of both the river water and the water in Lake Ijira was low among the lakes studied. The bedrock of the Lake Ijira catchment is mainly chert with low acid-buffering capacity and brown forest soils with low acid-buffering capacity are dominant in the catchment. This indicates that the aquatic environment of Lake Ijira is more sensitive to acid deposition than the aquatic environments of the other catchments. The rate of acid deposition in Lake Ijira was among the highest of all the monitoring sites in Japan (Fig. 3). Lake Ijira is located about 40 km north of the Chukyo industrial area near Nagoya, one of the biggest industrial areas in Japan. Air pollution from this industrial area is transported to the mountainous areas that include the Lake Ijira catchment (Kitada, Okamura, Nakanishi, & Mori, 2000). In the Lake Ijira catchment, the change in estimated proton loads due to acid deposition from 1989 to 1997 was consistent with the change in pH in surface water; the estimated proton load due to atmospheric deposition was larger than the rate of carbonic acid weathering (O. Nakahara, unpublished data), leading to the conclusion that the significant decline in pH and alkalinity in Lake Ijira is because of acid deposition acidifying inland waters. This is the first time in Japan that the results of long-term monitoring have pointed to acidification of surface water by acid deposition.
Because of the high acid-neutralizing capacity of the geology, acid deposition has so far had no obvious effect on acidification of surface waters in Japan (Ohte et al., 2001). Most of the lakes in this study also showed no long-term trend toward acidification. However, the estimated accumulated emission of sulfur in Japan since 1850 is almost the same now as that in Europe in the 1960s (Fig. 4; Lefohn, J. D. Husar, & R. B. Husar, 1999), suggesting that the impact of acid deposition might soon become obvious in Japan in regions, such as Lake Ijira, where the catchment is relatively sensitive to acidification and where there are high loads of acid deposition.
Estimated accumulated emissions of sulfur in Japan and Europe since 1850 (Lefohn et al., 1999)
5 Conclusions
Analysis of the data collected during the long-term monitoring of five representative lakes showed acidification due to acid deposition in the Lake Ijira catchment since the mid-1990s. Changes such as the alkalization of lake water and increases in EC were also detected in the other sites in this study, but we observed no evidence of acidification in sites other than Lake Ijira. The acidification in Lake Ijira was related to the geology of the catchment and the rate of acid deposition loading.
References
Committee for Acid Deposition Measures. (2004). Comprehensive Report on Acid Deposition Survey (in Japanese), the Ministry of the Environment, Japan, p. 432.
Helsel, D., & Hirsch, R. (1992). Statistical methods in water resources: Studies in environmental science 49 (pp. 323–355). New York: Elsevier.
Kitada, T., Okamura, K., Nakanishi, H., & Mori, H. (2000). Production and transport of ozone in local flows over central Japan – Comparison of numerical calculation with airborne observation. In Air pollution modeling and its application XIII (pp. 95–106). New York: Plenum.
Komai, Y., Umemoto, S., & Inoue, T. (2001). Influence of acid deposition on inland water chemistry – A case study from Hyogo prefecture, Japan. Water, Air, and Soil Pollution, 130, 1535–1540.
Kvaeven, B., Ulstein, M. J., Skjelkvale, B. L., Raddum, G. G., & Hovind, H. (2001). ICP waters – An international programme for surface water monitoring. Water, Air, and Soil Pollution, 130, 775–780.
Lefohn, A. S., Husar, J. D., & Husar, R. B. (1999). Estimating historical anthropogenic global sulfur emission patterns for the period 1850–1990. Atmospheric Environment, 33(21), 3435–3444.
Ohte, N., Tokuchi, N., Shibata, H., Tsujimura, M., Tanaka, T., & Mitchell, M. J. (2001). Hydrobiogeochemistry of forest ecosystems in Japan: Major themes and research issues. Hydrological Processes, 15, 1771–1789.
Okuda, T., Iwase, T., Ueda, H., Suda, Y., Tanaka, S., Dokiya, Y., et al. (2004). Long-term trend of chemical constituents in precipitation in Tokyo metropolitan area, Japan, from 1990 to 2002. Science of the Total Environment, 339, 127–141.
Skjelkvale, B. L., Stoddard, J. L., & Andersen, T. (2001). Trends in surface water acidification in Europe and North America (1989–1998). Water, Air, and Soil Pollution, 130, 787–792.
Stern, D. I. (2005). Global sulfur emissions from 1850 to 2000. Chemosphere, 58, 163–175.
Stoddard, J. L., Jeffries, D. S., Lukewille, A., Clair, T. A., Dillon, P. J., Driscoll, C. T., et al. (1999). Regional trends in aquatic recovery from acidification in North America and Europe. Nature, 401, 575–578.
Ye, X., Hao, J., Duan, L., & Zhou, Z. (2002). Acidification sensitivity and critical loads of acid deposition for surface water in China. Science of the Total Environment, 289, 189–203.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamada, T., Inoue, T., Fukuhara, H. et al. Long-term Trends in Surface Water Quality of Five Lakes in Japan. Water Air Soil Pollut: Focus 7, 259–266 (2007). https://doi.org/10.1007/s11267-006-9076-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11267-006-9076-8