Abstract
A five-step sequential extraction technique, following Tessier’s protocol, has been applied to determine the chemical association of Cd, Cu, Fe, Pb, and Zn with major sedimentary phases (exchangeable, carbonate, manganese and iron oxides, organic and residual fraction) in surface sediments from 14 stations off the Libyan Mediterranean coast. This study is a first approach of chemical fractionation of these metals in one of the most economically important area of the Libyan coastline in Mediterranean Sea. The total metal content was also determined. The total concentration of metals ranged from 5–10.5 mg/kg for Cd, 9.1–22.7 mg/kg for Cu, 141.8–1056.8 mg/kg for Fe, 18.9–56.9 mg/kg for Pb, and 11.6–30.5 mg/kg for Zn. The results of the partitioning study showed that the residual form was the dominant fraction of the selected metals among most of the studied locations. The degree of surface sediment contamination was computed for risk assessment code (RAC), individual contamination factor (ICF), and Global contamination factor (GCF). Risk assessment code classification showed that the relative amounts of easily dissolved phase of trace metals in the sediments are in the order of Pb>Zn>Cd>Cu>Fe. The results of ICF and GCF showed that Sirt and Abu Kammashand had higher GCF than other sites indicating higher environmental risk. In terms of ICF value, a decrease order in environmental risk by trace metals was Pb>Zn>Cu>Cd>Fe. Therefore, Pb had highest risk to water body.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trace metals are widely dispersed in the aquatic environment and ultimately deposited in the sediment which is therefore of particular interest concerning its metal content (Gomes et al., 2010). Marine sediment serves as one of the major reservoirs for all kinds of contaminants, including metals. The properties of metals in soils and sediments depend on the physicochemical form in which they occur (Gleyzes et al., 2002). On the other hand, determination of total metals, in sediments, usually gives only a rough approach to assess the environmental impact caused by trace metals in sediment. Therefore, the assessment of the environmental risks requires the measurements not only for the total contents of trace metals in sediments but also for the amounts of trace metals in each binding form (Lin et al., 2003). Today, it is generally recognized that the particular behavior of trace metals in the environment is determined mostly by their specific physicochemical fractionation and speciation or chemical form rather than by their total concentration (Tack and Verloo, 1995). Trace metals may distribute in sediments as exchangeable, acid-soluble (bound to carbonates), reducible (bound to Fe/Mn oxides and hydroxides), oxidizable (bound to organic matter), and residual (bound to silicates and detrital materials) species. The chemical fractionation of trace metals in sediments can be investigated by carefully employing a selective extraction scheme from the several extraction schemes available in the literature (Uzairu et al., 2009). Among a range of available techniques using various extraction reagents and experimental conditions, the 5-step Tessier et al. 1979 and the 6-step extraction method, Kersten and Frostner (1986) were mostly widely used. Following these two basic schemes, some modified procedures with different sequences of reagents or experimental conditions have been developed (Ure et al., 1993; Borovec et al., 1993; Campanella et al., 1995; Gomez-Ariza et al., 2000; Zdenek, 1996; Okoro et al. 2012). The risk assessment code (RAC), the individual contamination factors (ICF), and the global contamination factor (GCF) have been used to assess environmental risks and estimate possible damage to benthic organisms caused by contaminated sediments, because as mentioned before, metals are bounded to different sediment fractions with different binding strengths leading to variable bioavailability of metals in the environment (Badri and Aston, 1983).
Libya is a large landscape country in North Africa with a total area of 1.5 M km2. The population and the industrial power are continually growing. As a result, natural water supplies are under pressure to fulfill current and future requirements of both domestic and industrial use. Libya is an oil-producing country whose economy depends on the gas industry. The oil and gas facilities are scattered all over the country, onshore and offshore, in which water demands for quality and quantity are very high (Al-Hengari, et al., 2007). The major environmental concerns in Libya are water availability and the depletion of underground water as a result of overuse in agricultural developments, causing salinity and seawater penetration into the coastal aquifers. Another significant environmental problem is water pollution on the coastal environment from the combined impact of sewage, oil byproducts, and industrial waste (El Haddad, 2012).
The main objectives of this study were (1) to provide a better understanding of the mobility and bioavailability of Cd, Cu, Fe, Pb, and Zn in sediments of the Libyan Mediterranean coast using sequential extraction procedure and (2) to assess environmental risks and estimate possible damage to benthic organisms caused by contaminated sediments. This study supports metal pollution monitoring and control for the Libyan Mediterranean Sea. It will be a useful tool to authorities in charge of sustainable marine management.
Materials and methods
Study area
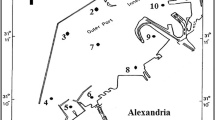
Libya has a unique position in the middle of North Africa. It is a junction between its eastern and western seashores. It embraces the southern coasts of the Mediterranean Sea, forming the heart for this great sea. The Libyan Coast extends to about 1900 km between Beir Al Ramla on the Egyptian borders to the east and Ras Gidier on the Tunisian borders to the west (Fig. 1). This distance is equal to around 37 % of the total Arab Coasts on the Mediterranean Sea. The Libyan coastline is considered as the longest African coastline on the Mediterranean Sea. In addition to its length, it is at the same time very rich in its natural resources be they in the field of fish wealth or energy and mineral raw resources (El Haddad, 2012).
Analysis of total metals concentrations
A concentrated acid digestion protocol (Oregioni and Aston, 1984) was followed and the digested solution was diluted accordingly and measured for Cd, Cu, Fe, Pb, and Zn using an atomic absorption spectrophotometer (AA6800, SHIMADZU, Kyoto, Japan). Instruments were calibrated using sets of at least five standards covering the range of concentrations encountered in the literature. All calibration curves showed good linearity (r > 0.99) and bias = 2 %. Reagent blanks, parallel replicates, and a standard reference material (IAEA-405: estuarine sediment, International Atomic Energy Agency, Vienna, Austria) were incorporated in each digestion batch for quality control and quality assurance. Accuracy and precision of the analyses were checked by analyzing ten replicate samples of the reference material. Certified values of Fe, Mn, Zn, Cr, Ni, and Pb were 37,400, 495, 279, 84, 32.5, and 74.8 mg kg−1, and their measured values were 38,334, 460, 252, 78, 31, and 77 mg kg−1, respectively. The recovery of the selected elements ranged from 90 to 104 %. Precision expressed as relative standard deviation (RSD) was always better than 7 % for all the measured elements.
Sequential extraction of selected metals
In the present study, metals were sequentially extracted following the five-step method proposed by Tessier et al. 1979 into operationally defined as exchangeable (F1), carbonate (F2), Fe/Mn hydroxide (reducible) (F3), organic (F4), and residual (F5). These fractions may be considered to decrease in availability from exchangeable to residual. After each successive extraction, samples were centrifuges at 4000 rpm for 20 min to separate the extracted from the sediments. The concentrations of Cd, Cu, Fe, Pb, and Zn in each of the leachates were determined by AAS (SHIMADZU 6800, Kyoto, Japan) in flame mode.
Results and discussion
Total metals distribution
Total metal concentrations in surface sediments of the Libyan Mediterranean coast are presented in Table 1. From the table, the measured metal contents varied greatly as follows: Fe>Pb>Zn>Cu>Cd. Among the five elements studied, concentrations of Fe were higher, whereas lower concentrations of Cd were observed in the different sampling locations.
Sequential extraction
The percentages of metals concentrations that were extracted in each step of the sequential extraction procedure used in this study were presented in Fig. 2. The trace metals associated with different fractions in the Libyan Mediterranean coastal sediments follow the order
-
Fe: residual>Fe/Mn oxides>organic>carbonate>exchangeable
-
Cu: residual>organic>Fe/Mn oxides>carbonate>exchangeable
-
Zn: residual>carbonate>organic>Fe/Mn oxides>exchangeable
-
Pb: residual>carbonate>Fe/Mn oxides>organic>exchangeable
-
Cd: residual>carbonate>organic>Fe/Mn oxides>exchangeable
Results of the sequential extraction suggest that the residual fraction dominated the Fe (82.44–96.68 %), Cd (84.24–93.59 %), Cu (82–97 %), and Zn (70–90 %) distribution in the Libyan Mediterranean Sea sediments. The exchangeable and carbonate fractions of Fe are totally geochemically insignificant (<1 % of total). The metals like Zn and Pb represent an appreciable portion in carbonate phase, as these metals have special affinity towards carbonate and may co-precipitate with its minerals (Sundaray, 2007). Further, organic bound Fe, Zn, and Cd seem to be the second dominant fraction among the non-lithogenous. The lower percentage of Fe in organic fraction probably results from the competition between Fe/Mn organic complex and hydrous Fe/Mn oxide forms (Sundaray, 2007).
Contamination assessment
Risk assessment code
The distribution of metals in different fractions obtained by sequential extraction procedure offers an indication of their availability, which, in turn, allows the assessment of the risk of their presence in the aquatic environment (Jain, 2004). Risk assessment code (RAC) as proposed by Perin et al. 1985, mainly applies to the sum of exchangeable and carbonate-bound fractions for assessing the availability of metals. If a sediment sample can release in these fraction, less than 1 % of the total metal will be considered safe for the environment. On the contrary, sediment releasing in the same fractions more than 50 % of the total metal has to be considered highly dangerous and can easily enter the food chain (Zakir and Shikazono, 2011). This classification is tabulated in Table 2.
RAC applied to the present study revealed that on average, 41.7 % of total Pb of the study sites was either adsorbed, exchangeable, or carbonate bound and, therefore, comes under the high risk category to local environment and can easily enter into the food chain (Table 2). On the other hand, only 0.98–4.09 % of total Fe was found in the exchangeable and carbonate-bound fraction with an average value of 2.14 % and, therefore, comes under the low risk category indicating lower availability from which Fe cannot be easily leached out for the aquatic environment. Furthermore, on an average, 4.1, 3.25, and 14.8 % of total Cd, Cu, and Zn, respectively, of the study sites either is exchangeable or carbonate bound. The relative amounts of easily dissolved phase of trace metals in the sediments are in the order of Pb>Zn>Cd>Cu>Fe.
The individual and global contamination factor
The determination of contamination factor of heavy metals is an important aspect that indicates the degree of risk of trace metals to environment in relation with its retention time (Nemati et al., 2009). Table 3 shows the individual contamination factor (ICF) and the global contamination factor (GCF) for Cd, Cu, Fe, Pb, and Zn in the Libyan Mediterranean Sea sediments. The ICF are defined as the sum of trace metal concentration in non-residual phases of sample divided by that in residual phase. The GCF for each site was calculated by summing up the ICF for all toxic elements obtained from a site (Barona et al., 1999). The applicability of many toxic metals in calculating GCF is significant, which reflects the overall potential risks posed by the toxic metals to the environment and biota. These methods of assessment based on contamination factors had been used by other authors (IKem et al., 2003; Margui et al., 2004). On the basis of the Ikem et al. (2003), ICF reflects the risk of contamination of a water body by a pollutant. In terms of ICF value, a decrease order in environmental risk by trace metals was Pb>Zn>Cu>Cd>Fe. Therefore, Pb had the highest risk to water body. However, the bioavailability of metals from sediment into the water column will be influenced by factors such as pH, chemical forms of the heavy metals, and the physicochemical characteristics of the water column (Ikem et al., 2003). The global contamination factor analyzed from ICF values showed that Sirt and Abu Kammashand had a higher GCF than other sites indicating a higher environmental risk.
Relation between organic fraction and total organic matter
Organic compound in sediment, frequently existing in considerable amounts in particle form, plays an important role in trace metal transformation. In sediment, the solubility of organic matters usually directly determines the mobility of trace metals. Normally, the complexation of metal ions with insoluble organic compounds can strongly lower their mobility, whereas the formation of soluble metal complexes with dissolved organic compounds would enhance their mobility (Sekaly et al., 1999). The relationship between concentration of Cd, Cu, Fe, Pb, and Zn at the organic bound fraction with OM in surface sediment of The Libyan Mediterranean coast was identified. The results showed that there was a negative linear correlation between Fe (r = −0.127) and Zn (r = −0.408) with OM. On the other hand, there was a non-significant positive linear correlation between Cd (r = 0.201) and Pb (r = 0.023) with OM, while Cu showed a strong positive correlation between Cu (r = 0.683, p = 0.05) with OM. OM had a positive function to complex with Cu in surface sediments from The Libyan Mediterranean coast.
Conclusion
Distribution and partitioning phases of trace metals is of a major environmental concern in relation to chemical fractionation and eco-toxicological aspects. It can also be used as a tool to provide information on the bioavailability and mobility of heavy metals in the sediments. The chemical fractionation of metals in the majority of stations collected along the Mediterranean coast of Libya was in the order of residual>carbonate>organic>Fe/Mn oxides>exchangeable for Cd, residual>organic>Fe/Mn oxides>carbonate>exchangeable for Cu, residual>Fe/Mn oxides>organic>carbonate>exchangeable for Fe, residual>carbonate>Fe/Mn oxides>organic>exchangeable for Pb, and residual>carbonate>organic>Fe/Mn oxides>exchangeable for Zn. The relative amounts of easily dissolved phase of trace metals in the sediments are in the order of Pb>Zn>Cd>Cu>Fe. Risk assessment code values were reflective of average risks to the environment for these metals in most samples. On average, 41.7 % of total Pb of the study sites was either adsorbed, exchangeable, or carbonate bound and, therefore, comes under the high risk category to local environment and can easily enter into the food chain. Sirt and Abu Kammash showed higher GCF than other sites indicating higher environmental risk. Information of this study constitute a baseline of metal fractionation in sediments along the Libyan Mediterranean coast and should be used as a reference for future studies on the changes of labile and residual metal fractions over time.
Compliance with ethical standards
This paper complies with the ethical standards.
References
Al-Hengari, S., El-Bousiffi, M., & El-Moudir, W. (2007). Libyan Petroleum Institute experience in evaluation of desalination plants in the Libyan oil sector. Desalination, 206, 633–752.
Badri, M. A., & Aston, S. R. (1983). Observation on heavy metal geochemical associations in polluted and nonpolluted estuarine sediments. Environmental Pollution (Series B), 6, 181.
Barona, A., Aranguiz, I., & Elias, A. (1999). Assessment of metal extraction, distribution and contamination in surface soils by a 3-step sequential extraction procedure. Chemosphere, 39(1), 1 911–1 922.
Borovec, Z., Tolar, V., & Mraz, L. (1993). Distribution of some metals in sediments of the central part of the Labe (Eibe) River: Czech Republic. Ambio, 22, 200–205.
Campanella, L., Dorazio, D., Petronio, B. M., & Pietrantonio, E. (1995). Proposal for a metal speciation study in sediments. Analytica Chimica Acta, 309, 387–393.
El Haddad, H. S. (2012). Assessment of heavy metals and petroleum hydrocarbons pollution in bottom sediment along the Libyan Coast (Tobruk–Ras Gidier). Ph.D. Thesis, Alexandria University, Egypt.
Gleyzes, C., Tellier, S., & Astruc, M. (2002). Fractionation studies of trace elements in contaminated soils and sediments: a review of sequential extraction procedures. Trends in Analytical Chemistry, 21(6-7), 451–467.
Gomes, M. V. T., Costa, A. S., Garcia, C. A. B., Passos, E. A., & Alves, J. P. H. (2010). Concentrations and geochemical associations of Pb and Zn in sediments of the river São Francisco impacted by wastes from industrial zinc production. Quimica Nova, 33(10), 2088–2092.
Gomez-Ariza, J. L., Giraldez, I., Sanchez-Rodas, D. E., & Morales, E. (2000). Metal sequential extraction procedure optimized for heavy metal polluted and iron-oxide rich sediments. Analytica Chimica Acta, 414, 151–164.
Ikem, A., Egiebor, N. O., & Nyavor, K. (2003). Trace elements in water, fish and sediment from Tuskegee Lake, Southeastern USA. Water, Air, and Soil Pollution, 149(1-4), 51–75.
Jain, C. K. (2004). Metal fractionation study on bed sediments of River Yamuna, India. Water Research, 38, 569–578.
Kersten, M., & Frostner, U. (1986). Chemical fractionation of heavy metals in anoxic estuaries and coastal sediments. Water Science and Technology, 18, 121–130.
Lin, J. G., Chen, S. Y., & Su, C. R. (2003). Assessment of sediment toxicity by metal speciation in different particle size fractions of river sediment. Water Science and Technology, 47(7-8), 233–241.
Margui, E., Salvado, V., Queralt, I., & Hidalgo, M. (2004). Comparison of three-stage sequential extraction and toxicity characteristic leaching tests to evaluate metal mobility in mining wastes. Analytica Chimica Acta, 524(1-2), 151–159.
Nemati, K., Abu Bakar, N. K., & Abas, M. R. (2009). Investigation of heavy metals mobility in shrimp aquaculture sludge—comparison of two sequential extraction procedures. Microchemical Journal, 91, 227.
Okoro, H.K., Fatoki, O.S., Adekola, F.A., Ximba, B.J., Snyman, R.G. (2012). A review of sequential extraction procedures for heavy metals speciation in soil and sediments. 1: 181. doi:10.4172/scientificreports.181
Oregioni, B., Aston, S.R. (1984). Determination of selected trace metals in marine sediments by flame/flameless atomic absorption spectrophotometer. IAEA Monaco Laboratory Internal Report. Now cited in reference method in pollution studies No. 38, UNEP, 1986.
Perin, G. Craboledda, L., Lucchese, M., Cirillo,R. Dotta,L., Zanette, M., Orio,A. (1985). Heavy metals speciation in the sediments of Northern Adriatic Sea. A new approach for environmental toxicity determination. In: Lekkas, T.D. (E.d.), Heavy metals in the environment, vol. 21, pp.454-456.
Sekaly, A. L. R., Mandal, R., Nouri, M. H., Murimboh, J., Chakrabart, C. L., Back, M. H., Gregoire, D. C., & Schroeder, W. H. (1999). Effect of metal/fulvic acid mole ratios on the binding of Ni (II), Pb (II), Cu (II), Cd (II), and Al (III) by two well characterized fulvic acids in aqueous model solutions. Analytica Chimica Acta, 402, 211–221.
Sundaray, S.K. (2007). Water quality assessment of Mahanadi River, Orissa, India using multivariate statistical approach. Ph.D. Thesis, Utkal University, Bhubaneswar, India.
Tack, F. M., & Verloo, M. G. (1995). Chemical speciation and fractionation in soil and sediment heavy metal analysis: a review. International Journal of Environmental Analytical Chemistry., 59(2-4), 225–238.
Tessier, A., Campbell, P. G. C., & Biason, M. (1979). Sequential extraction procedure for speciation of particulate trace metals. Journal of Analytical Chemistry, 51(7), 844–851.
Ure, A. M., Quevauviller, V., Muntau, H., & Griepink, B. (1993). Speciation of heavy metals in solids and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. International Journal of Environmental Analytical Chemistry, 51, 135.
Uzairu, A., Harrison, G. F. S., Balarabe, M. L., & Nnaji, J. C. (2009). Concentration levels of trace metals in fish and sediment from Kubanni river, northern Nigeria. Bulletin of the Chemical Society of Ethiopia, 23(1), 9–17.
Zakir, H. M., & Shikazono, N. (2011). Environmental mobility and geochemical partitioning of Fe, Mn, Co, Ni and Mo in sediments of an urban river. Journal of Environmental Chemistry and Ecotoxicology, 3, 116–126.
Zdenek, B. (1996). Evaluation of the concentration of trace elements in stream sediments by factor and analysis and the sequential extraction procedure. Science of the Total Environment, 177, 237–250.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nasr, S.M., Okbah, M.A., El Haddad, H.S. et al. Fractionation profile and mobility pattern of metals in sediments from the Mediterranean Coast, Libya. Environ Monit Assess 187, 430 (2015). https://doi.org/10.1007/s10661-015-4668-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4668-2