Abstract
Tropical and subtropical soils are usually acidic and have high concentrations of aluminum (Al). Aluminum toxicity in plants is caused by the high affinity of the Al cation for cell walls, membranes, and metabolites. In this study, the response of the antioxidant-enzymatic system to Al was examined in two tomato genotypes: Solanum lycopersicum var. esculentum (Calabash Rouge) and Solanum lycopersicum var. cerasiforme (CNPH 0082) grown in tropical soils with varying levels of Al. Plant growth; activities of superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), guaiacol peroxidase (GPOX), and glutathione reductase (GR) enzymes; stress-indicating compounds (malondialdehyde (MDA) and hydrogen peroxide); and morphology (root length and surface area) were analyzed. Increased levels of Al in soils were correlated with reduced shoot and root biomass and with reduced root length and surface area. Calabash Rouge exhibited low Al concentrations and increased growth in soils with the highest levels of Al. Plants grown in soils with high availability of Al exhibited higher levels of stress indicators (MDA and hydrogen peroxide) and higher enzyme activity (CAT, APX, GPOX, and GR). Calabash Rouge absorbed less Al from soils than CNPH 0082, which suggests that the genotype may possess mechanisms for Al tolerance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most plants are sensitive to acidic soils (pHH2O <5) and to even micromolar concentrations of exchangeable aluminum (Al) in soils, indicating that Al toxicity can reduce agricultural productivity to a significant degree (Tamás et al. 2003). Aluminum availability increases in acidic agricultural soils (pHH2O <5.5), which accounts for approximately 40 % of arable land worldwide and for approximately 66 % of soils in Brazil (Vitorello et al. 2005).
Inhibition of root elongation is the first symptom of Al toxicity, which may be associated with interference in cell division and elongation (Sivaguru et al. 2013). However, the exact mechanisms of inhibition by Al are not yet clear (Ezaki et al. 2005). The root apex is the first region to suffer inhibition due to Al, and this region seems to play an important role in both Al toxicity and resistance to Al (Matsumoto and Motoda 2013), but the precise mechanisms of those processes have not been elucidated.
Several hypotheses exist regarding the potential mechanisms underlying Al toxicity (and resistance) in plants. These include alterations in the plasma membrane, induction of oxidative stress due to lipid peroxidation, interference in cell signaling and Al exclusion via the exudation of organic acids, and higher pH levels in the rhizosphere (Inostroza-Blancheteau et al. 2012; Kanu et al. 2013). Each of these hypotheses is supported by some indirect evidence, but there is not yet enough evidence to indicate which hypothesis is the most likely.
Aluminum can cause a redox imbalance in cells that produces a series of reactive oxygen species (ROS). ROS can be combated by defense mechanisms that involve various antioxidant enzymes, such as superoxide dismutase (SOD), ascorbate peroxidase (APX) catalase (CAT), glutathione reductase (GR), glutathione S-transferase (GST), and guaiacol peroxidase (GPOX) (Gratão et al. 2005). Non-enzymatic compounds of low molecular weight may also be involved, including ascorbic acid, reduced glutathione, flavonoids, carotenoids, and uric acid (Cuypers et al. 2010; Gill et al. 2013).
Oxidative stress due to Al toxicity was first reported by Cakmak and Horst (1991), who showed increased lipid peroxidation and small increase in the activity of antioxidant enzymes such as SOD and peroxidases as a result of Al toxicity in the tips of soybean roots. More recently, other evidence from physiological and genetic studies supports the role of oxidative stress in Al toxicity in plants because such stress is believed to inhibit cell growth (Yamamoto et al. 2001, 2002; Matsumoto and Motoda 2013). There is also evidence that the efficiency of plant antioxidant systems plays an important role, alongside the primary mechanisms of Al tolerance, in increasing plant ability to recover from stress, by reducing the effects caused by ROS (Cai et al. 2011).
Evidence suggests that Al toxicity might be mediated by oxidative stress and that the lower susceptibility of Al-tolerant maize roots after exposure to Al is, in part, due to increased activity of the antioxidant system (Giannakoula et al. 2010). Identifying the mechanisms of Al tolerance may make it possible to combine them to produce more tolerant genotypes. The efficiency of the plant antioxidant systems may thus be an important attribute for increasing tolerance of Al stress in plants, in a similar manner to what has been widely described in the literature for other heavy metals and even other abiotic stresses (Arruda and Azevedo 2009; Cia et al. 2012; Arruda et al. 2013; Boaretto et al. 2014; Bulbovas et al. 2014) and in interaction with microorganisms in the soil (Dourado et al. 2013, 2014).
The aim of this study was to quantify responses of the antioxidant enzymatic system and other changes in morphological parameters of two tomato genotypes—Solanum lycopersicum var. esculentum (Calabash Rouge, a commercial genotype chosen because it has been shown to be susceptible to Cd and possibly Al) and S. lycopersicum var. cerasiforme (the CNPH 0082 cherry tomato, a wild genotype with greater likelihood of tolerance) (Piotto 2012)—grown in tropical soils with varying concentrations of Al.
Materials and methods
Plant material, growth, and treatments
The experiment was a greenhouse study carried out with the tomato genotype Calabash Rouge (S. lycopersicum var. esculentum) and the tomato genotype CNPH 0082 (S. lycopersicum var. cerasiforme). CNPH 0082 has been previously described as Al tolerant and was selected in the region of Porte Firme, in the Brazilian state of Minas Gerais, where it was growing spontaneously and the soils have high concentrations of Al. By contrast, the Calabash Rouge genotype is native to Chiapas, Mexico, and shown to be very sensitive to other toxic elements such as cadmium (Cd), as described by Piotto (2012; see Piotto et al. 2014 for heavy metal-tolerant screening methodology).
Seeds of the two genotypes were treated with 50 % commercial sodium hypochlorite and immediately afterward planted in trays containing a vermiculite substrate and irrigated every 2 days with Hoagland and Arnon (1950) solution. After 40 days, seedlings were transplanted to pots containing clay soil (670 g kg−1 clay, 70 g kg−1 silt, and 260 g kg−1 sand) or sandy soil (130 g kg−1 clay, 30 g kg−1 silt, and 840 g kg−1 sand). Pots with clay soil were treated with 0, 560, and 2240 mg kg−1 soil of corrective (applied as CaO), and those with sandy soil were treated with 0, 280, and 1120 mg kg−1 soil. These lime rates were determined using neutralization curves that were performed previously for each soil, with the goal of yielding high, medium, and almost zero levels of Al in the clay and sandy soils. Soils were collected from the 0–0.2-m layer from areas with little anthropogenic disturbance (forest fragments) and had initial bioavailable Al concentrations of 14 mmolc kg−1 in clay soil and 12 in sandy soil. Bioavailable Al concentrations were extracted in 1 mol L−1 KCl solution and determined by titration in a 0.025 mol L−1 NaOH solution (Halonen et al. 1983).
Al concentration
Plants were collected 18 days after being transplanted onto soils with varying levels of Al. The plant material was separated into shoots and roots and dried in a forced-air oven at 65 °C for 72 h and then ground in a Wiley mill. Nitric-perchloric digestion was performed on the samples following Malavolta et al. (1997) and Al concentrations determined by ICP-OES.
Root length and surface area
After separating roots from the soil, subsamples (~20 % of the total fresh weight) were collected following Rossielo et al. (1995), stained with 50 mg L−1 gentian violet, and digitally photographed for subsequent measurement of surface area and total root system length using the Integrated System for the Analysis of Roots and Soil Cover software ver. 3.0 (Crestana et al. 1994).
Lipid peroxidation and H2O2 content
2-Thiobarbituric acid (TBA) test to evaluate lipid peroxidation was used. Thiobarbituric acid reactive substance (TBARS) content was measured as the final product of lipid peroxidation, with readings at 535 and 600 nm (Gratão et al. 2012). Malondialdehyde (MDA) content was estimated by the specific equation for this reaction (Mihara et al. 1980). H2O2 content was determined following Gay et al. (1999). The H2O2 in the samples donates electrons to Fe, which then bonds to xylenol during the 30-min incubation time. Readings were taken with a spectrophotometer at 390 nm.
Root and shoot protein extraction and measurement
Plant samples used in enzyme analysis were collected and immediately frozen in liquid nitrogen and maintained in a freezer at −80 °C for further analysis. Proteins were extracted from 1-g tissue samples macerated using a mortar and pestle with liquid nitrogen. Samples were homogenized in a 100 mM, pH 7.5 potassium phosphate buffer with 1 mM ethylenediaminetetraacetic acid (EDTA), 3 mM ditiothreitol (DTT), and 40 mg L−1 (p/v) PVPP (polyvinylpolypyrrolidone) following Monteiro et al. (2011). The homogenate was centrifuged at 10,000 g for 30 min at 4 °C and the supernatant measured and stored in a freezer at −80 °C until analysis. Total protein concentration was determined following Bradford (1976) using bovine serum albumin (BSA) as a buffer.
Antioxidant enzymes
CAT
CAT was measured by the reaction of a mixture containing 1-mL potassium phosphate buffer (100 mM, pH 7.5) in 2.5 μL 30 % H2O2, to which 15 μL of the protein extract was added. Activity was determined by the decomposition of H2O2 for 1 min, read at 240 nm at 25 °C in a spectrophotometer (Monteiro et al. 2011).
APX
APX activity was measured by spectrophotometry at 290 nm. The reaction mixture consisted of a solution containing 650-μL potassium phosphate buffer (80 mM, pH 7.0), 100 μL ascorbate 5 mM, 100 μL EDTA 1 mM, 100 μL H2O2 1 mM, and 50 μL of extract in a 30 °C water bath. H2O2 was added at the time of the reading after 1 min in a quartz cuvette (Cakmak and Horst 1991). APX activity (expressed as nmol ascorbate min−1 mg−1 protein) was calculated using the extinction coefficient 2.8 mM−1 cm−1 of ascorbate.
GR
GR activity was measured by spectrophotometry at 412 nm at 30 °C. Fifty microliters of extract was added to the reaction mixture containing 3-mL potassium phosphate buffer 100 mM pH 7.5, 1.5 mL 5.5′-dithio-bis (2-nitrobenzoic acid, NBT), and 1 mM oxidized glutathione and 0.1 mM NADPH. GR activity was estimated by the reduction of oxidated glutathione (Gratão et al. 2008) and expressed as μmol min−1 mg−1 protein.
GPOX
GPOX activity was measured following Matsuno and Uritani (1972). The reaction medium consisted of a phosphate-citrate buffer at pH 5, guaiacol 0.5 %, and the protein extract, heated at 30 °C for 15 min. An ice bath and the addition of 2 % sodium metabisulfite were used to stop the reaction, and readings were taken at 450 nm.
Superoxide dismutase (SOD)
SOD activity was detected on a 9 % non-denaturing polyacrylamide gel (PAGE). SOD activity staining was carried out as described by Azevedo et al. (1998). After non-denaturing-PAGE separation, the gel was rinsed in distilled-deionized water and incubated in the dark in 50-mM potassium phosphate buffer (pH 7.8) containing 1 mM EDTA, 0.1 mM nitroblue tetrazolium, 0.05 mM riboflavin, and 0.3 g L−1 N,N,N0,N0-tetramethylethyllenediamine. The gels were then rinsed with distilled-deionized water and illuminated in water until the achromatic bands of SOD activity were visible on a purple-stained gel. Bovine SOD buffer was used as a standard.
Statistical analysis
The results were expressed as the means and standard deviations of the means (±SD) of three independent replications of root and shoot dry mass production; Al concentration; root length and surface area; and the enzymatic assays for each extraction of TBARS and H2O2 content and CAT, GR, GPOX, APX, and SOD activity.
Results
Al content in the soil
Al availability varied of the equivalent of 0, 560, and 2240 mg kg−1 of lime in clay soil and 0, 280, and 1120 mg kg−1 in sandy soil. These treatments resulted in high, medium, and low Al availability for the plants. Mean exchangeable Al concentrations in clay soils with each treatment were 12, 5.2, and 0.3 mmolc kg−1, respectively, and in sandy soils 11.8, 5.0, and 0.0 mmolc kg−1, respectively. A high amount of lime materials was applied to the clay soil because it had higher buffer than the sandy soil, in order to reach about the same exchangeable Al concentrations in both soils. The amount of the cationic Al species depends on the soil type, and clay soils are usually richer in Al components than sandy soils (Mengel and Kirkby 2001).
Growth analysis
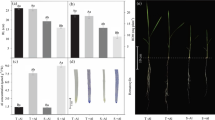
Although shoot growth was lowered as the concentrations of available Al was increased, Calabash Rouge genotype exhibited growth increase in shoot more pronounced than CNPH 0082 at all Al available concentrations in both soil types (Fig. 1a and b).
At the highest concentration of available Al (i.e., no lime), the Calabash Rouge genotype exhibited shoot dry weight 2.4 (in clay soil) and 6.2-fold (in sandy soil) more pronounced than those of CNPH 0082 (Fig. 1a and b), whereas shoot dry weight of Calabash Rouge genotype was 11.4 and 2.5-fold reduced at the highest concentrations of available Al (no lime) than at the lowest (2240 and 1120 mg kg−1 lime rate) in clay and sandy soils, respectively. The values observed for CNPH 0082 were 14.4 and 13-fold, respectively.
Root growth also decreased with increasing levels of available Al for both genotypes (Fig. 1c and d). In clay soil, root dry weight of Calabash Rouge was 2.2-fold greater than that of CNPH 0082 when grown at the highest Al concentration (without lime). In sandy soil, the comparable value was 2-fold. Root dry weight of Calabash Rouge was 1.9 and 4.3-fold lower at the highest Al concentration than at the lowest concentration, in clay and sandy soils, respectively. The comparable values for CNPH 0082 were 5 and 3.3-fold, respectively.
Al concentration
Treatments with lime reduced the amount of Al taken up by both tomato genotypes used (Fig. 2a and b). Al absorption was similar in both genotypes when grown in clay soil. In sandy soils with the highest Al concentrations (no lime), CNPH 0082 exhibited 4.4-fold more Al in shoots than Calabash Rouge.
An increase in Al accumulation was more pronounced in roots than in shoot tissues in both genotypes and in both soils. Al root concentrations decreased with increasing amounts of lime (Fig. 2c and d). At the highest level of available Al in clay soils (no lime), Al root concentration was 1.6-fold higher in CNPH 0082 than in Calabash Rouge (Fig. 2c and d).
Root length and surface area
The Calabash Rouge genotype exhibited longer roots (Fig. 3a and b) and greater root surface area (Fig. 3c and d) than CNPH 0082, but both genotypes exhibited longer roots and greater root surface area in sandy soils at the same time that they also exhibited increased root length and surface area with increased rates of lime in both soils (Fig. 3).
Lipid peroxidation and H2O2 content
Lipid peroxidation (expressed as MDA content) was induced by the availability of Al in the soils. Calabash Rouge exhibited the highest values for both shoot and roots tissues in both soil types at the highest availability of Al (no lime, Fig. 4). The shoot tissue exhibited higher peroxidation rates (Fig. 4a and b) when compared to the root tissue in clay soil (Fig. 4c).
Lipid peroxidation measured as malondialdehyde (MDA) concentration in shoots (a, b) and roots (c, d) of Calabash Rouge and CNPH 0082 genotypes, as related to lime rates in two soil types. Values are the means of three replications ± SD. Different letters within each genotype indicate significant differences among means (Tukey, 5 %)
Plants subjected to stress caused by high Al availability exhibited increased H2O2 concentrations in both shoots (Fig. 5a and b) and roots (Fig. 5c and d). Calabash Rouge exhibited higher H2O2 concentrations than CNPH 0082 at all concentrations of available Al. Calabash Rouge produced more H2O2 in sandy soils than in clay soils.
Hydrogen peroxide (H2O2) concentration in shoots (a, b) and roots (c, d) of Calabash Rouge and CNPH 0082 genotypes, as related to lime rates in two soil types. Values are the means of three replications ± SD. Different letters within each genotype indicate significant differences among means (Tukey, 5 %)
Antioxidant enzymes
SOD activity in extracts of plants was determined based on the separation of isoenzymes by non-denaturing PAGE (Fig. 6). Three distinct SOD isoenzymes were detected in shoot and roots (bands I, II, and III). Although a densitometric analysis was not carried out, it was observed that roots of plants grown in clay soil exhibited a continuous increase of SOD band I, II, and III intensities for both genotypes with decreased levels of available Al, while roots of plants grown in sandy soil exhibited very similar levels of activity for treatments and genotypes (Fig. 6c and d). In shoots, increases in SOD band II and III intensities were observed for the CNPH 0082 genotype for both soils with decreased levels of available Al (Fig. 6a and b), being more pronounced for SOD band III.
Activity staining for superoxide dismutase (SOD) following non-denaturing polyacrylamide gel electrophoresis of extracts of shoots (a, b) and roots (c, d) of Calabash Rouge and CNPH 0082 plants grown over an 18-day period in the presence of treatments. The lanes listed in clay soil are 1 bovine SOD standard, 2 control, 3 560 mg kg−1 soil, and 4 2240 mg kg−1 soil. The lanes listed in sandy soil are 1 bovine SOD standard, 2 control, 3 280 mg kg−1 soil, and 4 1120 mg kg−1 soil
In clay soils, CAT activity was much more pronounced in CNPH 0082 than in Calabash Rouge, while in sandy soils, CAT activity was much pronounced in Calabash Rouge (Fig. 7a and b). CAT activity in roots was more pronounced in CNPH 0082 in both soils (Fig. 7c and d). CAT activity in roots was 1.5 and 4.0-fold higher than CAT activity in shoots of both genotypes in clay soil with the highest levels of available Al (no lime). The comparable numbers for sandy soil were 1.1 and 6.1-fold greater.
APX activity increased with increasing Al availability on both soils in both genotypes (Fig. 8). APX activity was less pronounced in shoots (Fig. 8a and b) than in roots (Fig. 8c and d). APX activity was very low at the lowest level of available Al in clay and sandy soils (2240 and 1120 mg kg−1 of lime, respectively). APX activity was more pronounced in Calabash Rouge than in CNPH 0082 only in the shoots of plants grown in clay soils (Fig. 8a), when the plants were grown without lime and with 560 mg kg−1 of soil corrective.
Ascorbate peroxidase (APX) activity in shoots (a, b) and roots (c, d) of Calabash Rouge and CNPH 0082 genotypes, as related to lime rates in two soil types. Values are the means of three replications ± SD. Different letters within each genotype indicate significant differences among means (Tukey, 5 %)
GR activity increased with increasing levels of available Al in soils and peaked at the highest level of available Al (no lime). In clay soils, GR activity was much more pronounced in CNPH 0082 than in Calabash Rouge for both shoots and roots (Fig. 9a and c) and was 7-fold higher in roots than in shoots. In sandy soils, by contrast, GR activity was similar in both tomato genotypes (Fig. 9b and d).
Glutathione reductase (GR) activity in shoots (a, b) and roots (c, d) of Calabash Rouge and CNPH 0082 genotypes, as related to lime rates in two soil types. Values are the means of three replications ± SD. Different letters within each genotype indicate significant differences among means (Tukey, 5 %)
GPOX activity was higher in roots (Fig. 10c and d) of CNPH 0082 than in those of the Calabash Rouge genotype. GPOX activity in shoots at the highest levels of available Al (no lime) was similar in both genotypes in both soils (Fig. 10a and b).
Guaiacol peroxidase (GPOX) activity in shoots (a, b) and roots (c, d) of Calabash Rouge and CNPH 0082 genotypes, as related to lime rates in two soil types. Values are the means of three replications ± SD. Different letters within each genotype indicate significant differences among means (Tukey, 5 %)
The difference found in the activities of enzymes related to the antioxidant system was evident between the genotypes and between plants grown in both soils. Generally, the enzyme activity was higher in roots than in shoots.
Discussion
Exposure to biotic or abiotic stress factors can alter the redox metabolism, culminating in an imbalance between the pathways that produce ROS and the pathways that detoxify those species. The result of this process is an increase in ROS concentrations and consequent damage to cell structure (Gratão et al. 2005).
The aim of this study was to assess and understand the effects of Al on the antioxidant enzymatic system of two tomato genotypes, which were previously shown to display different tolerance levels to Al (Piotto 2012). Al concentrations in soil varied using soil lime rates that neutralized the phytotoxic effect of the metal to varying degrees in clay and sandy soils, yielding high, medium, and low levels of Al in soils that have naturally high Al concentrations.
Some growth parameters, such as root and shoot dry mass and root length and surface area, declined with increasing levels of available Al in soils. This was expected, given the well-known toxic effects of Al on roots. Within a number of known Al effects, the binding to cell walls interfering with root elongation (Rangel et al. 2009), the interaction with cell membranes, and the alteration of the uptake of nutrients and water (Purcino et al. 2003) must be highlighted. Inhibited growth caused by Al-induced stress has been reported for hops (Guo et al. 2004), rice (Ma et al. 2007), sunflower (Gallego et al. 2002), and mustard (Pandey et al. 2005). Yet and despite the declines mentioned, Calabash Rouge, an Al-sensitive genotype (Piotto 2012), was more tolerant to Al than CNPH 0082 at almost every level of available Al and in both soils (Figs. 1 and 3). Calabash Rouge appears to have internal defense mechanisms, such as the complexation of Al by organic acids or low-molecular-weight proteins, that are more efficient than those in CNPH 0082, since Calabash Rouge exhibited more pronounced growth, longer roots, and more root surface area than CNPH 0082 at lower or similar Al concentrations in the plant. Most plants that are Al tolerant minimize toxic effects of Al in Al-rich soils by releasing organic acids such as exudates of malate, citrate, and oxalate through the roots (Kochian et al. 2004). For some plant species, such as sorghum and wheat, the complex formed by organic acid and Al prevents Al from entering cells (Magalhães et al. 2007), which reduces the potentially toxic concentration in the roots (Ma et al. 2001). However, it is also important to take into consideration the amount of time that plants are exposed to the metal, and experiments that incorporate both varying levels of Al in soils and varying exposure times are needed to test this hypothesis.
The stress indicators MDA and H2O2 exhibited higher values for Calabash Rouge at almost all Al concentrations and in both soils (Figs. 4 and 5). H2O2 is known to play a role in cellular expansion by facilitating the expanding abilities of the cell wall, and for that reason, growth can restrict a greater impact of peroxide, as shown by the more pronounced growth of Calabash Rouge. The drastic increase in the production of H2O2 was a function of Al availability, especially in sandy soil, where Al is less tightly retained by the cationic exchange complex of the soil (Fig. 5), and applying lime thus had a very clear influence, reducing stress and H2O2 production. Calabash Rouge absorbed less Al than CNPH 0082 but was more sensitive in terms of producing H2O2 with lower quantities of absorbed Al. Similar increases in H2O2 production in Al-exposed plants have been found in rice (Ma et al. 2007), hops (Tamás et al. 2004), and tobacco leaves (Zhang et al. 2009).
Calabash Rouge may also have mechanisms to inhibit uptake of Al in the roots. Some plant species are known to have organic acid exudates that detoxify Al in the rhizosphere through chelation (Ma et al. 2001). Organic acid transport is mediated by ionic channels in the plasma membrane that are activated by Al, as found in wheat and corn (Ma et al. 2001). It is thus possible that Calabash Rouge produces organic acids that either minimize uptake by the roots or form organic acid metal complexes that precipitate Al into non-soluble complexes that make it impossible to detect. Such hypotheses are currently being investigated in an ongoing project in our laboratory.
The redox status of plants depends on the trade-off between production and consumption of ROS over time (Gratão et al. 2005; Sharma and Dubey 2007). Under stressful conditions, including metal toxicity, this trade-off depends on enzyme activity of the plant antioxidant system (Gratão et al. 2005; Cia et al. 2012; Boaretto et al. 2014). In this study, it was attempted to understand how the antioxidant systems of two tomato genotypes respond to Al exposure and what relationships exist among toxicity, oxidative stress, and plant growth in two tropical soils. In general, the results support the idea that the oxidative state of the two genotypes was affected differently by Al availability in the two tropical soils studied. The physiological response to stress can be divided into two parts; the first consists of enzymes that fight ROS directly, such as SOD and peroxidases such as CAT, APX, GPOX, and glutathione peroxidase (GSH-Px) (Gratão et al. 2012), while the second and just as important part is a redox system in the cellular metabolism that encompass the enzymes GR and GST and non-enzymatic compounds, such as reduced glutathione (GSH) (Ghelfi et al. 2011). Increases in the production of MDA and H2O2 and total activity of the SOD, APX, CAT, and GR enzymes have been clearly documented in plants subjected to Al-induced stress, including potato (Tabaldi et al. 2009), tobacco (Yin et al. 2010), rice (Ma et al. 2007; Wang and Kao 2007), and hops (Guo et al. 2004; Simonovicova et al. 2004).
When antioxidant enzymes are concerned, SOD is involved in the dismutation of O2− into H2O2 and is located in different cell organelles (Azevedo et al. 1998). The increase in SOD III activity observed in shoots of CNPH 0082 might play an important role in the cellular protection against Al toxicity, whereas in the roots, a continuous increase in SOD activity in clay soil can indicate different responses depending on the soil types used.
When the activity of enzymes that directly combat ROS is concerned, CAT activity was shown to respond differently in different plant parts and soil types. The highest CAT activity observed in roots of the tomato genotypes is likely to be a response to the higher concentrations of Al found in roots than in shoots (Fig. 7). With the increasing amounts of lime applied, CAT activity declined reflecting the reduced availability of Al in the soil and consequently to the tomato plants. This increase in CAT activity can be considered a plant defense mechanism against H2O2 (Darko et al. 2004), and greater CAT activity occurred at the Al concentration when H2O2 was also highest. For instance, tea (Ghanati et al. 2005), wheat (Darko et al. 2004), and potato (Tabaldi et al. 2009) plants have also shown increased CAT activity in response to Al exposure.
APX activity was shown to be much more pronounced in the roots and shoots of the two tomato genotypes when the plants were grown without lime (i.e., when available Al level was greatest, Fig. 8). Increases in APX activity have been shown to be associated with high level of ascorbic acid and reduced level of H2O2 (Dipierro et al. 2005), both aimed at eliminating ROS. Moreover, increases in APX activity under Al-induced stressful conditions have also been observed in rice (Sharma and Dubey 2007) and rye (Silva et al. 2013) following 2–3 weeks of exposure to the metal. In soybean roots, APX activity increased linearly with Al concentration and exposure time (24, 36, and 48 h; Du et al. 2010). Such findings support the suggestion that APX plays an important role in detoxifying H2O2 under stressful conditions, as those used in this study.
GR was the enzyme that exhibited the greatest differences in tomato plants grown in soils of differing textures (Fig. 9). GR activity was similar in shoots and roots for the two tomato genotypes within a single soil type. In clay soil, both genotypes exhibited higher GR activity in the roots than in the shoots, and a similar pattern was found in sandy soil. Aluminum is more available for plants to absorb in sandy soils, given that in clay soils, Al is retained in a cationic exchange complex (Mengel and Kirkby 2001). GR activity followed the same pattern as Al absorption by the genotypes, resulting in increased activity at the highest levels of Al absorption by plants. The literature shows that increases in GR activity have also been observed in hops (Tamás et al. 2006), rice (Sharma and Dubey 2007), and rye (Silva et al. 2013) plants when subjected to Al exposure. Therefore, maintaining GSH at adequate levels appears to be important for roots as well as for shoots when plants are exposed to Al for long periods of time.
GPOX activity was also high in roots, especially in CNPH 0082, and is another important enzyme involved in H2O2 detoxification. GPOX activity levels were similar in the two tomato genotypes grown in clay and sandy soils (Fig. 10). In shoots, the activity was similar between the genotypes at the highest levels of available Al (no lime), while in the roots GPOX, activity was greater in CNPH 0082. These results clearly indicate that GPOX was the enzyme least influenced by soil type, showing equal responses in sandy and clay soils. Other studies with Al have reported increases in GPOX activity when plants are exposed for 72 h to Al in roots (Hossain et al. 2005; Meriga et al. 2004; Simonovicova et al. 2004) and for 4 h in shoots (Panda and Matsumoto 2010).
Metal affinity to the soil colloids is greatly important for the availability of such elements to plants. By growing soybean with added toxic elements (Ba and Cd) to soil types in a greenhouse experiment, Melo et al. (2011) found that soil type was the main factor affecting plant responses to metal availability. They also reported that the changes in enzyme activities were more dependent on soil type and period of exposure than on the metal concentrations in the soil. Some enzymes that act in the antioxidant system only exhibited increased activities after a long period of exposure to the toxic element, which confirms the importance of long-time experiments involving soil, metals, and antioxidant system studies in plants, as well as the use of chronic and acute treatments (Gratão et al. 2008).
In this study, it is demonstrated that the two tomato contrasting genotypes for Al tolerance/sensibility varied in their responses to Al toxicity when grown in two soil types. This highlights the importance of studying responses of the antioxidant systems of plants grown in soils of varying both attributes and concentrations of metals. It is important to mention that the large majority of reports have consistently used hydroponic systems to investigate the responses of plants to metals, whereas in this experiment, plant genetic diversity, Al concentration, and soil type were combined. Additionally, the better understanding of tomato plant tolerance to metal-induced stress and the strategies involved to minimize the stress established are key important sources of information that can be applied to agricultural practices with other crop plants in similar situations. This study demonstrates the need for experiments on plant responses to long-term exposure to Al, which should better reflect responses under real-world conditions where plants are grown in naturally Al-rich soils. Future research should focus also in exposure period and on microorganisms which can influence on the availability and absorption of the metal ion (Andrade et al. 2009; Li et al. 2009).
In conclusion, despite the fact that tropical soils are naturally rich in Al, the toxic effect of the element can easily be remediated by liming the soil to increase soil pH and reduce Al availability to plants. The magnitude of the toxic effects of Al may also vary with soil type and the metabolic defense mechanisms of the tomato genotypes. Future studies with longer exposure of the plant to the metal are needed to elucidate the mechanisms of tolerance of crops to phytotoxic effects of Al when grown in soils rich in this element.
References
Andrade, S. A. L., Gratão, P. L., Schiavinato, M. A., Silveira, A. P. D., Azevedo, R. A., & Mazzafera, P. (2009). Zn uptake, physiological response and stress attenuation in mycorrhizal jack bean growing in soil with increasing Zn concentrations. Chemosphere, 75, 1363–1370. doi:10.1016/j.chemosphere.2009.02.008.
Arruda, M. A. Z., & Azevedo, R. A. (2009). Metallomics and chemical speciation: towards a better understanding of metal-induced stress in plants. Annals of Applied Biology, 155, 301–307. doi:10.1111/j.1744-7348.2009.00371.×.
Arruda, S. C. C., Barbosa, H. S., Azevedo, R. A., & Arruda, M. A. Z. (2013). Comparative studies focusing on transgenic through cp4EPSPS gene and non-transgenic soybean plants: an analysis of protein species and enzymes. Journal of Proteomics, 93, 107–116. doi:10.1016/j.jprot.2013.05.039.
Azevedo, R. A., Alas, R. M., Smith, R. J., & Lea, P. J. (1998). Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild-type and a catalase-deficient mutant of barley. Physiologia Plantarum, 104, 280–292. doi:10.1034/j.1399-3054.1998.1040217.×.
Boaretto, L. F., Carvalho, G., Borgo, L., Creste, S., Landell, M. G. A., Mazzafera, P., et al. (2014). Water stress reveals differential antioxidant responses of tolerant and non-tolerant sugarcane genotypes. Plant Physiology and Biochemistry, 74, 165–175. doi:10.1016/j.plaphy.2013.11.016.
Bradford, M. M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principles of protein-dye binding. Analytical Biochemistry, 71, 248–254. doi:10.1016/0003-2697(76)90527-3.
Bulbovas, P., Souza, S. R., Esposito, J. B. N., Moraes, R. M., Alves, E. S., Domingos, M., et al. (2014). Assessment of the ozone tolerance of two soybean cultivars (Glycine max cv. Sambaiba and Tracaja) cultivated in Amazonian areas. Environmental Science and Pollution Research, 21, 10514–10524. doi:10.1007/s11356-014-2934-4.
Cai, M. Z., Wang, F. M., Li, R. F., Zhang, S. N., Wang, N., & Xu, G. D. (2011). Response and tolerance of root border cells to aluminum toxicity in soybean seedlings. Journal of Inorganic Biochemistry, 105, 966–971. doi:10.1016/j.jinorgbio.2011.04.004.
Cakmak, I., & Horst, W. J. (1991). Effect of aluminium on lipid peroxidation, superoxide dismutase catalase and peroxidase activities in root tips of soybean (Glycine max). Physiologia Plantarum, 83, 463–468. doi:10.1111/j.1399-3054.1991.tb00121.×.
Cia, M. C., Guimarães, A. C. R., Medici, L. O., Chabregas, S. M., & Azevedo, R. A. (2012). Antioxidant responses to water deficit by drought-tolerant and -sensitive sugarcane varieties. Annals of Applied Biology, 161, 313–324. doi:10.1111/j.1744-7348.2012.00575.×.
Crestana, S., Guimarães, M. F., Jorge, L. A. C., Ralisch, R., Tozzi, C. L., Torre, A., et al. (1994). Avaliação da distribuição de raízes no solo auxiliada por processamento de imagens digitais. Revista Brasileira de Ciência do Solo, 18, 365–371.
Cuypers, A., Plusquin, M., Remans, T., Jozefczak, M., Keunen, E., Gielen, H., et al. (2010). Cadmium stress: an oxidative challenge. Biometals, 23, 927–940. doi:10.1007/s10534-010-9329-×.
Darko, E., Ambrus, H., Stefanovits, E., Anyai, B., Fodor, J., Bakos, F., et al. (2004). Aluminium toxicity. Al tolerance and oxidative stress in an Al-sensitive wheat genotype and in Al-tolerant lines developed by in vitro microspore selection. Plant Science, 166, 583–591. doi:10.1016/j.plantsci.2003.10.023.
Dipierro, N., Mondelli, D., Paciolla, C., Brunetti, G., & Dipierro, S. (2005). Changes in the ascorbate system response of pumpkin (Curcubita pepo L.) roots to aluminum stress. Journal of Plant Physiology, 162, 529–536. doi:10.1016/j.jplph.2004.06.008.
Dourado, M. N., Martins, P. F., Quecine, M. C., Piotto, F. A., Souza, L. A., Franco, M. R., et al. (2013). Burkholderia sp SCMS54 reduces cadmium toxicity and promotes growth in tomato. Annals of Applied Biology, 163, 494–507. doi:10.1111/aab.12066.
Dourado, M. N., Souza, L. A., Martins, P. F., Peters, L. P., Piotto, F. A., & Azevedo, R. A. (2014). Burkholderia sp SCMS54 triggers a global stress defense in tomato enhancing cadmium tolerance. Water, Air and Soil Pollution, 225, 2159. doi:10.1007/s11270-014-2159-7.
Du, B., Nian, H., Zhang, Z., & Yang, C. (2010). Effects of aluminum on superoxide dismutase and peroxidase activities, and lipid peroxidation in roots and calluses of soybeans differing in aluminum tolerance. Acta Physiologiae Plantatum, 32, 883–890. doi:10.1007/s11738-010-0476-z.
Ezaki, B., Sasaki, K., Matsumoto, H., & Nakashima, S. (2005). Functions of two genes in aluminum stress resistance: repression of oxidative damage by the AtBCB gene and promotion of efflux of Al ions by the NtGDI1 gene. Journal of Experimental Botany, 56, 2661–2671. doi:10.1093/jxb/eri259.
Gallego, S., Benavides, M., & Tomaro, M. (2002). Involvement of an antioxidant defence system in the adaptive response to heavy metal ions in Helianthus annuus L. cells. Plant Growth Regulation, 36, 267–273. doi:10.1023/A:1016536319908.
Gay, C., Collins, J., & Gebicki, J. M. (1999). Hydroperoxide assay with the ferric-xylenol orange complex. Analytical Biochemistry, 273, 149–155. doi:10.1006/abio.1999.4208.
Ghanati, F., Morita, A., & Yokota, H. (2005). Effects of aluminum on the growth tea plants and activation of antioxidant system. Plant and Soil, 276, 133–141. doi:10.1007/s11104-005-3697-y.
Ghelfi, A., Gaziola, S. A., Cia, M. C., Chabregas, S. M., Falco, M. C., Kuser-Falcão, P. R., et al. (2011). Cloning, expression, molecular modelling and docking analysis of glutathione transferase from Saccharum officinarum. Annals of Applied Biology, 159, 267–280. doi:10.1111/j.1744-7348.2011.00491.×.
Giannakoula, A., Moustakas, M., Syros, T., & Yupsanis, T. (2010). Aluminum stress induces up-regulation of an efficient antioxidant system in the Al-tolerant maize line but not in the Al-sensitive line. Environmental and Experimental Botany, 67, 487–494. doi:10.1016/j.envexpbot.2009.07.010.
Gill, S. S., Hasanuzzaman, M., Nahar, K., Macovei, A., & Tuteja, N. (2013). Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiology and Biochemistry, 63, 254–261. doi:10.1016/j.plaphy.2012.12.001.
Gratão, P. L., Polle, A., Lea, P. J., & Azevedo, R. A. (2005). Making the life of heavy metal-stressed plants a little easier. Functional Plant Biology, 32, 481–494. doi:10.1071/FP05016.
Gratão, P. L., Monteiro, C. C., Antunes, A. M., Peres, L. E. P., & Azevedo, R. A. (2008). Acquired tolerance of tomato (Lycopersicon esculentum cv. Micro-Tom) plants to cadmium induced stress. Annals of Applied Biology, 153, 321–333. doi:10.1111/j.1744-7348.2008.00299.×.
Gratão, P. L., Monteiro, C. C., Carvalho, R. F., Tezotto, T., Piotto, F. A., Peres, L. E. P., et al. (2012). Biochemical dissection of diageotropica and Never ripe tomato mutants to Cd-stressful conditions. Plant Physiology and Biochemistry, 56, 79–96. doi:10.1016/j.plaphy.2012.04.009.
Guo, T. R., Zhang, G. P., Zhou, M. X., Wu, F. B., & Chen, J. X. (2004). Effect of aluminum and cadmium toxicity on growth and antioxidant enzyme activities of two barley genotypes with different Al tolerance. Plant and Soil, 258, 241–248. doi:10.1023/B:PLSO.0000016554.87519.d6.
Halonen, O., Tulkki, H., & Derome, J. (1983). Nutrient analysis methods. Metsäntutkimuslaitoksen tiedonantoja, 121, 1–28.
Hoagland, D., & Arnon, D. I. (1950). The water culture method for growing plants without soil (p. 32p). Berkeley: California Agricultural Experiment Station.
Hossain, M. A., Hossain, A. K. M. Z., Kihara, T., Koyama, H., & Hara, T. (2005). Aluminum-induced lipid peroxidation and lignin deposition are associated with an increase in H2O2 generation in wheat seedlings. Soil Science and Plant Nutrition, 51, 223–230. doi:10.1111/j.1747-0765.2005.tb00026.x.
Inostroza-Blancheteau, C., Rengel, Z., Alberdi, M., Mora, M. L., Aquea, F., Arce-Johnson, P., et al. (2012). Molecular and physiological strategies to increase aluminum resistance in plants. Molecular Biology Reports, 39, 2069–2079. doi:10.1007/s11033-011-0954-4.
Kanu, S. A., Okonkwo, J. O., & Dakora, F. D. (2013). Aspalathus linearis (Rooibos tea) as potential phytoremediation agent: a review on tolerance mechanisms for aluminum uptake. Environmental Reviews, 21, 85–92. doi:10.1139/er-2012-0055.
Kochian, L. V., Hoekenga, O. A., & Piñeros, M. A. (2004). How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorus efficiency. Annual Review of Plant Biology, 55, 459–493. doi:10.1146/annurev.arplant.55.031903.141655.
Li, H. F., Gray, C., Mico, C., Zhao, F. J., & McGrath, S. P. (2009). Phytotoxicity and bioavailability of cobalt to plants in a range of soils. Chemosphere, 75, 979–986. doi:10.1016/j.chemosphere.2008.12.068.
Ma, J. F., Ryan, P. R., & Delhaize, E. (2001). Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Science, 6, 273–278. doi:10.1016/S1360-1385(01)01961-6.
Ma, B., Wan, J., & Shen, Z. (2007). H2O2 production and antioxidant responses in seeds and early seedlings of two different rice varieties exposed to aluminum. Plant Growth Regulation, 52, 91–100. doi:10.1007/s10725-007-9183-1.
Magalhães, J. V., Liu, J., Guimarães, C. T., Lana, U. G., Alves, V. M., Wang, Y. H., et al. (2007). A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nature Genetics, 39, 1156–1161. doi:10.1038/ng2074.
Malavolta, E., Vitti, G. C., & Oliveira, S. A. (1997). Assessment of nutritional status of plants: principles and applications (2nd ed., p. 319). Piracicaba: Associação Brasileira para Pesquisa da Potassa e do Fosfato. in Portuguese.
Matsumoto, H., & Motoda, H. (2013). Oxidative stress is associated with aluminum toxicity recovery in apex of pea root. Plant and Soil, 36, 399–410. doi:10.1007/s11104-012-1396-z.
Matsuno, H., & Uritani, I. (1972). Physiological behavior of peroxidase isozymes in sweet potato root tissue injured by cutting or with black rot. Plant and Cell Physiology, 13, 1091–1101.
Melo, L. C. A., Alleoni, L. R. F., Carvalho, G., & Azevedo, R. A. (2011). Cadmium and barium toxicity effects on growth and antioxidant capacity of soybean (Glycine max L.) plants, grown in two soil types with different physicochemical properties. Journal of Plant Nutrition and Soil Science, 174, 847–859. doi:10.1002/jpln.201000250.
Mengel, K., & Kirkby, E. (2001). Principles of plant nutrition (5th ed., p. 849p). Dordrecht/Boston/London: Kluwer Academic Publishers.
Meriga, G., Reddy, B. K., Rao, K. R., & Kishor, P. B. K. (2004). Aluminum-induced production of oxygen radicals, lipid peroxidation and DNA damage in seedlings of rice (Oryza sativa). Journal of Plant Physiology, 161, 63–68. doi:10.1078/0176-1617-01156.
Mihara, M., Uchiyama, M., & Fukuzawa, K. (1980). Thiobarbituric acid value on fresh homogenate of rat as a parameter of lipid peroxidation in aging, CCl4 intoxication, and vitamin E deficiency. Biochemical Medicine, 23, 302–311. doi:10.1016/0006-2944(80)90040-×.
Monteiro, C. C., Carvalho, R. F., Gratão, P. L., Carvalho, G., Tezotto, T., Medici, L. O., et al. (2011). Biochemical responses of the ethylene-insensitive Never ripe tomato mutant subjected to cadmium and sodium stresses. Environmental and Experimental Botany, 71, 306–320. doi:10.1016/j.envexpbot.2010.12.020.
Panda, S. K., & Matsumoto, H. (2010). Changes in antioxidant gene expression and induction of oxidative stress in pea (Pisum sativum L.) under Al stress. Biometals, 23, 753–762. doi:10.1007/s10534-010-9342-0.
Pandey, V., Dixit, V., & Shyam, R. (2005). Antioxidative responses in relation to growth of mustard (Brassica juncea cv. Pusa Jaikisan) plants exposed to hexavalent chromium. Chemosphere, 61, 40–47. doi:10.1016/j.chemosphere.2005.03.026.
Piotto, F. A. (2012). Avaliação de tolerância ao Cádmio em tomateiro (Solanum lycopersicum L.). Resource document. Escola Superior de Agricultura Luiz de Queiroz, Universidade de São Paulo, Ph.D. Thesis (in Portuguese). http://www.teses.usp.br/teses/disponiveis/11/11137/tde-18092012-134028/publico/Fernando_Angelo_Piotto.pdf. Accessed 4 Jan 2015.
Piotto, F. A., Tulmann Neto, A., Franco, M. R., Boaretto, L. F., & Azevedo, R. A. (2014). Rapid screening for selection heavy metals tolerant plants. Crop Breeding and Applied Biotechnology, 14, 1–7.
Purcino, A. A. C., Alves, V. M. C., Parentoni, S. N., Belele, C. L., & Loguercio, L. L. (2003). Aluminum effects on nitrogen uptake and nitrogen assimilating enzymes in maize genotypes with contrasting tolerance to aluminum toxicity. Journal of Plant Nutrition, 26, 31–61. doi:10.1081/PLN-120016496.
Rangel, A. F., Rao, I. M., & Horst, W. J. (2009). Intracellular distribution and binding state of aluminum in root apices of two common bean (Phaseolus vulgaris) genotypes in relation to Al toxicity. Physiologia Plantarum, 135, 162–173. doi:10.1111/j.1399-3054.2008.01183.×.
Rossielo, R. O. P., Araujo, A. P., Manzatto, C. V., & Fernandes, M. S. (1995). Comparação dos métodos fotoelétricos e da inserção na determinação da área, comprimento e raio médio radicular. Pesquisa Agropecuária Brasileira, 30, 633–638.
Sharma, P., & Dubey, R. S. (2007). Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of Al. Plant Cell Reports, 26, 2027–2038. doi:10.1007/s00299-007-0416-6.
Silva, S., Pinto, G., Correia, B., Pinto-Carnides, O., & Santos, C. (2013). Rye oxidative stress under long term Al exposure. Journal of Plant Physiology, 170, 879–889. doi:10.1016/j.jplph.2013.01.015.
Simonovicova, M., Huttova, J., Mistrik, I., Siroka, B., & Tamas, L. (2004). Root growth inhibited by aluminium is probably caused by cell death due to peroxidase-mediated hydrogen peroxide production. Protoplasma, 224, 91–98. doi:10.1007/s00709-004-0054-6.
Sivaguru, M., Liu, J., & Kochian, L. V. (2013). Targeted expression of SbMATE in the root distal transition zone is responsible for sorghum aluminum resistance. The Plant Journal, 76, 297–307. doi:10.1111/tpj.12290.
Tabaldi, L. A., Cargnelutti, D., Gonçalves, J. F., Pereira, L. B., Castro, G., Maldaner, J., et al. (2009). Oxidative stress is an early symptom triggered by aluminum in Al-sensitive potato plantlets. Chemosphere, 76, 1402–1409. doi:10.1016/j.chemosphere.2009.06.011.
Tamás, L., Simonovicová, J., & Huttová, I. M. (2003). Changes in the composition of cell wall proteins in barley roots during germination and growth in aluminum presence. Plant, Soil & Environment, 49, 327–331.
Tamás, L., Simonovicová, M., Huttová, J., & Mistrik, I. (2004). Aluminum stimulated hydrogen peroxide production of germinating barley seeds. Environmental and Experimental Botany, 51, 281–288. doi:10.1016/j.envexpbot.2003.11.007.
Tamás, L., Huttová, J., Mistrik, I., Simonovicová, M., & Siroká, B. (2006). Aluminum induced drought and oxidative stress in barley roots. Journal of Plant Physiology, 163, 781–784. doi:10.1016/j.jplph.2005.08.012.
Vitorello, V. A., Capaldi, F. R., & Stefanuto, V. A. (2005). Recent advances in aluminum toxicity and tolerance in higher plants. Brazilian Journal of Plant Physiology, 17, 129–143. doi:10.1590/S1677-04202005000100011.
Wang, J. W., & Kao, C. H. (2007). Protective effect of ascorbic acid and glutathione on AlCl3-inhibited growth of rice roots. Biologia Plantarum, 51, 493–500. doi:10.1007/s10535-007-0104-y.
Yamamoto, Y., Kobayashi, Y., & Matsumoto, H. (2001). Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiology, 125, 199–208. doi:10.1104/pp. 125.1.199.
Yamamoto, Y., Kobayashi, Y., Devi, S. R., Rikiishi, S., & Matsumoto, H. (2002). Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiology, 128, 63–72. doi:10.1104/pp. 010417.
Yin, Y. G., Kobayashi, Y., Sanuki, A., Kondo, S., Fukuda, N., Ezura, H., et al. (2010). Salinity induces carbohydrate accumulation and sugar-regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv. ‘Micro-Tom’) fruits in an ABA- and osmotic stress-independent manner. Journal of Experimental Botany, 61, 563–574. doi:10.1093/jxb/erp333.
Zhang, B., Wang, X., Li, X., Ni, Y., & Li, H. (2009). Aluminum uptake and disease resistance in Nicotiana rustica leaves. Ecotoxicology and Environmental Safety, 73, 655–663. doi:10.1016/j.ecoenv.2009.12.028.
Acknowledgments
This work was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant number 2009/54676-0). We thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (R.A.A, F.A.M, and L.B) and FAPESP (R.C.N) for the fellowship and scholarships granted.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nogueirol, R.C., Monteiro, F.A., Gratão, P.L. et al. Tropical soils with high aluminum concentrations cause oxidative stress in two tomato genotypes. Environ Monit Assess 187, 73 (2015). https://doi.org/10.1007/s10661-015-4282-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4282-3