Abstract
Composting has been recognised an alternative method to tannery sludge recycling and afterwards to be used in agriculture. As the tannery sludge contains salts and chromium, the application of composted tannery sludge (CTS) should be performed carefully to minimise negative effects on soil microbial properties. Therefore, this study evaluated the effects of 5-year repeated CTS amendment on soil microbial biomass (SMB) and enzyme activities in a tropical soil. CTS was applied during 5 years at 0, 2.5, 5, 10 and 20 Mg ha−1, and at the fifth year, the microbial biomass C (MBC) and N (MBN), basal and substrate-induced respiration (SIR), metabolic quotient (qCO2) and dehydrogenase (DHA) and fluorescein diacetate (FDA) hydrolysis were determined in the soil samples. Soil MBC and MBN showed the highest values with the amendment of 5 Mg ha−1 CTS. Soil respiration increased with the increase in CTS rates, while SIR showed the highest values with the amendment of 0, 2.5 and 5 Mg ha−1 CTS. DHA activity showed the highest values with the amendment up to 2.5 Mg ha−1, while FDA hydrolysis increased up to the rate of 5 Mg ha−1 CTS. The results show that after 5 years of permanent amendment of CTS, soils amended with 2.5 Mg ha−1 have SMB and enzymatic activities similar to those in unamended soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The tannery industry is important to the Brazilian economy, with annual assets of $2.5 billion (CICB 2013). However, it releases one million tons of tannery sludge annually, 3 % of which is solid waste (Santos et al. 2011). In particular, the tannery industry is a large contributor of chromium (Cr), and the accumulation of Cr in soil can cause long-term toxic effects on soil ecosystems (Patel and Patra 2014). In addition, tannery sludge contains salts, which, when associated with Cr, can affect soil biochemical processes.
Recently, composting has been used to facilitate tannery sludge recycling (Santos et al. 2011; Gonçalves et al. 2014) and may be an alternative method for recycling organic wastes such as tannery and textile sludge (Santos et al. 2011; Araújo et al. 2007). However, the application of composted wastes to agricultural soils should be performed carefully to minimise negative effects on soil microbial properties (Singh and Agrawal 2007). Soil microorganisms represent a large fraction of global terrestrial biodiversity and mediate a range of processes important for soil quality in both natural ecosystems and agricultural systems (Lavelle and Spain 2001). However, these microorganisms respond quickly to changes in soil management (Lopes et al. 2011), forest devastation (Zimmermann and Frey 2002), land degradation (Araujo et al. 2013a) and the application of wastes (Silva et al. 2014). Specifically, soil microbial biomass (SMB), the living component of the soil organic matter, is considered an early and sensitive indicator of soil stress caused by wastes (Santos et al. 2011). Moreover, SMB produces enzymes that play essential roles in various ecosystem processes (van der Heijden et al. 2008). Soil enzyme assays have been considered one of the most convenient techniques used as sensors for measuring the degree of soil pollution (Patel and Patra 2014), and their activities may be inhibited according to the nature and concentration of heavy metals. Thus, soil enzymes have long been considered an indicator of soil stress after waste management (Olcay et al. 2013).
Previous short-term studies were completed under controlled laboratory conditions and evaluated the effects of different composted industrial wastes on soil microbial properties (Araújo and Monteiro 2006; Araújo et al. 2007; Santos et al. 2011; Giacometti et al. 2012). Usually, the results showed positive effects of composted waste on soil microbial properties, suggesting no toxic or detrimental effects of the waste application on soil. However, the effects of repeated applications of composted wastes, mainly under field conditions, should be evaluated to define the long-term effects of the waste on soil microbial properties (Giacometti et al. 2012; Silva et al. 2014). Additionally, studies employing the repeated application of composted wastes such as composted tannery sludge (CTS) under field conditions are scarce, and the limited information from earlier studies does not provide a clear picture.
Accordingly, we began a long-term study of CTS application to evaluate its effects on soil microbial properties after consecutive CTS amendment. In particular, we hypothesised that soil microbial properties are affected by the consecutive and cumulative addition of CTS with respect to increased soil salinity and Cr content (Araujo et al. 2013b). In this study, we evaluated the effects of 5-year repeated CTS amendment on SMB and enzyme activities in a tropical soil.
Materials and methods
The experimental site is located at the Long-Term Experimental Field of the Agricultural Science Centre, Teresina, Piauí (05° 05 S; 42° 48′ W, 75 m). The regional climate is dry tropical (Köppen) and is characterised by two distinct seasons: rainy summer and dry winter, with annual average temperatures of 30 °C and rainfall of 1200 mm. The rainy season extends from January to April when 90 % of the total annual rainfall occurs. The soil is classified as a Fluvisol with the following composition at 0–20-cm depth: 10 % clay, 28 % silt and 62 % sand.
CTS was produced annually by mixing tannery sludge with sugarcane straw and cattle manure (ratio 1:3:1; v/v/v). The composting process was performed using the aerated-pile method for 85 days. The pile was 2 m long, 1 m wide and 1.5 m high. The pile (60 % humidity and C/N ratio 30:1) was turned twice a week during the first 30 days (where the temperature increased to 65 °C) and twice a month during the remaining 55 days (compost maturity). At the end of the composting process, 20 subsamples were randomly collected from the CTS to produce a composite sample. The water content was determined after oven-drying the samples at 105 °C for 24 h, the pH and electrical conductivity (EC) were directly read and total solids were measured by drying the samples at 65 °C (APHA 2005). The total organic C content was evaluated by dichromate oxidation of the samples under external heating (Nelson and Sommers 1996). The total N content was determined through the method of sulphuric acid digestion (Bremner 1996). The total Ca, Mg, K, P, S, Na, Zn, Cu, Cd, Pb, Ni and Cr concentrations were determined by atomic absorption spectrophotometry after nitric acid digestion of the samples in a microwave oven (USEPA 1996).
CTS was annually applied from 2009 using five rates: 0 (without CTS application), 2.5, 5, 10 and 20 Mg ha−1 of CTS (dry basis). The experimental site was arranged in a completely randomised design with four replicates. Plots were 10 m2 each, with 6 m2 of usable area for soil and plant sampling, and rows were spaced 1.0 m apart. In the fifth year (2013), CTS was applied 10 days before cowpea (Vigna unguiculata L.) sowing. The CTS was spread on the soil surface and incorporated into the 20-cm layer with a harrow. Cowpea was grown at a density of 5 plants m−1 (approximately 62,000 plants ha−1).
During the fifth year, soil samples were collected from each plot 60 days after CTS amendment. In each plot, four samples were collected (0–20 cm), sieved and stored at 4 °C prior to analysis. Before the analysis, the soil was pre-incubated (with a 100-mL jar of soda lime and bottles containing deionised water) in sealed plastic containers for 7 days with a moisture content of 60–70 % of the water-holding capacity to equilibrate the physiological activity of SMB (Forster 1995).
The levels of soil microbial biomass C (MBC) and N (MBN) were determined according to the methods developed by Vance et al. (1987) and Brookes et al. (1985), respectively, with 0.5 M K2SO4 extraction of the organic C and total N contents from fumigated and unfumigated soils. The coefficients of extraction (0.38 and 0.45) were used to convert the differences in C and N contents between the fumigated and unfumigated soils to microbial C and N, respectively. Substrate-induced respiration (SIR) was evaluated according to the procedure developed by Alef and Nannipieri (1995) by incubating the soil at 25 °C for 2 h in a glucose solution (0.1 mL g−1). The soil respiration was monitored through daily measurement of CO2 evolution under aerobic incubation at 25 °C for 7 days (Alef and Nannipieri 1995). The metabolic quotient (qCO2) was calculated as the ratio of the basal respiration to the MBC and is expressed as milligrams CO2 per kilogram biomass C per day. Fluorescein diacetate (FDA) hydrolysis was measured by spectrophotometry at 490 nm after incubation for 20 min at 30 °C, according to the method described by Schnurer and Rosswall (1982). Dehydrogenase (DHA) activity was determined by employing the method described by Casida et al. (1965), which is based on the spectrophotometric analysis of triphenyl tetrazolium formazan released by 5 g of soil after 24 h of incubation at 35 °C.
The average values of the soil chemical properties (0–20-cm depth) after 5 years of CTS amendment are shown in Table 2. Soil pH was estimated in water (1:2.5 v/v) and measured using a pH meter (Tedesco et al. 1995). Soil EC was evaluated in water (1:2 v/v) according to the method described by Richards (1954). Soil organic C was determined by wet combustion using a mixture of 5 mL of 0.167 mol L−1 potassium dichromate and 7.5 mL of concentrated sulphuric acid under heating (170 °C for 30 min) (Yeomans and Bremner 1988). The total Cr concentration was analysed according to USEPA (1996) after the digestion of soil with HNO3, HCl and H2O2. The concentration of Cr from soil samples was analysed using atomic absorption spectrophotometry.
The data were statistically analysed using analysis of variance (ANOVA), and the means were compared using least significant difference values calculated at the 5 % level. The data from the variables were analysed using multivariate ordination non-metric multidimensional scaling (NMS) with Sorensen distances. Ordination was performed using the PC-ORD v. 6.0 program.
Results
The chemical characteristics of CTS are shown in Table 1. CTS exhibited alkalinity and high organic C, Ca, Na and Cr content. Therefore, soil chemical properties, such as soil pH, EC, soil organic C (SOC) and Cr content, increased after 5 years of CTS amendment (Table 2). Considering the highest CTS rate, after 5 years of CTS amendment, soil pH increased 1.3 units, while the soil EC increased threefold compared with unamended soil. Similarly, compared with the unamended soil, SOC and Cr content increased four and eightfold, respectively, after amendment with 20 Mg ha−1 CTS.
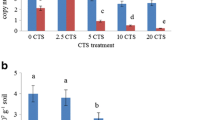
Soil microbial properties showed different responses to consecutive CTS amendment over 5 years (Table 3). Soil MBC and MBN showed the highest values with the amendment of 5 Mg ha−1 CTS. At the highest CTS rates, SMB decreased significantly. Soil respiration and SIR exhibited different responses to CTS amendment. Soil respiration increased with the amendment of 20 Mg ha−1 CTS, while SIR showed the highest values with the amendment of 0, 2.5 and 5 Mg ha−1 CTS. The increase in soil respiration influenced the metabolic quotient, which increased with the amendment of 10 and 20 Mg ha−1 CTS. Soil enzymes showed different responses to CTS amendment, while DHA activity showed the highest values with the amendment up to 5 Mg ha−1. FDA hydrolysis increased up to the rate of 10 Mg ha−1 CTS. Afterwards, both soil enzymes decreased with the increase in CTS rates.
NMS analysis explained approximately 97 % of the variation and defined four distinct groups according to the biological and chemical properties (Fig. 1). The first group consisted of 0 and 2.5 Mg ha−1 CTS and was linked with soil enzymes and SIR. The second group consisted of 5 Mg ha−1 CTS and grouped with SMB. The third and fourth groups consisted of 10 and 20 Mg ha−1 CTS, respectively, and grouped with soil respiration, metabolic quotient, soil pH, EC, SOC and Cr content.
Discussion
CTS showed high pH, Ca and Na content due to the use of hydroxides, carbonates and salts during the tanning process (Santos et al. 2011). Additionally, the residue had high Cr content because this metal is used during the tanning process. The Cr content in CTS was twice the upper limit for Cr prescribed by Brazilian regulations (CONAMA 2009) and is characteristic of raw tannery sludge, which is not pre-treated by the industry. However, as shown in Table 2, soil pH increased to above 7.0 as CTS rates increased, and when soil pH was alkaline, Cr remained inert in soil in forms with low mobility (Gonçalves et al. 2014).
The repeated use of CTS over 5 years elevated the soil pH. However, soil pH after CTS amendment was lower than that reported by Martines et al. (2010) using untreated tannery sludge with high pH and Ca content. Most likely, the composting process influenced the quality of waste, as composting stabilises the organic waste. CTS increased soil salinity as a consequence of the presence of salts such as sodium chloride (Gonçalves et al. 2014). Although the use of CTS over 5 years had increased soil salinity, the EC values detected in the present study are below the limit of 2.0 dS m−1 proposed by Richards (1954) to indicate saline soils. Thus, CTS amendment over 5 years did not salinise soil. The increase in SOC after 5 years of CTS amendment may be attributed to the direct effect of organic C in the CTS composition.
SMB responds rapidly and sensitively to organic residue inputs (Ros et al. 2006). Additionally, SIR may indicate active microbial biomass (Svensson and Friberg 2007) and both microbial biomass and toxicity in soils (Martí et al. 2007). The increases in SMB and SIR with the addition of 5 Mg ha−1 CTS after 5 years most likely occurred due to the permanent addition of C sources that favoured the soil microorganisms, as reported previously (Ros et al. 2006; Santos et al. 2011; Giacometti et al. 2012). In addition, our results show that soil microorganisms remained active after CTS amendment. However, the decreases in microbial biomass and SIR with the highest CTS rates (10 and 20 Mg ha−1 CTS) may suggest that high Cr content and soil salinity influenced the SMB. According to García-Gil et al. (2000), the accumulation of heavy metals after long-term waste amendment decreases SMB. Scherer et al. (2011) studied the long-term effects of sewage sludge and compost amendment on SMB. They detected a reduction in microbial biomass and assumed that it occurred due to increased Zn, Cr and Cd content in the soil after repeated sewage sludge amendment.
As SMB alone may be insufficient to assess the status of microbial activity, soil respiration and the metabolic quotient can provide more information. Our data showed the highest soil respiration with the amendment of 20 Mg ha−1 and may suggest negligible influences of Cr content and soil salinity. However, we detected an increase in metabolic quotient after the amendment of 10 and 20 Mg ha−1, which could be an indicator of soil stress from CTS on SMB (Santos et al. 2011). Based on these results, permanent CTS amendment at 10 and 20 Mg ha−1 may stress soil microorganisms. According to Ros et al. (2006), who studied the effect of sewage sludge on SMB and reported an increase in metabolic quotient, metals might be toxic to soil microorganisms, and at high rates, CTS might promote Cr toxicity. Similar results were obtained by Fernandes et al. (2005), Araújo and Monteiro (2006) and Santos et al. (2011), who found higher metabolic quotients for soils that received the higher sewage, textile and tannery sludge rates, respectively.
Soil enzymes behaved differently to CTS amendment, which may be associated with the distinctive natures of DHA and FDA. Dehydrogenase is an intracellular enzyme involved in microbial oxidoreductase metabolism, and its activity depends on the metabolic state of the soil biota (García-Gil et al. 2000). Therefore, the metabolic state of SMB might have been affected with the increase in CTS amendment, which is likely due to the accumulation of Cr (Santos et al. 2011) and salts in soil (Tripathi et al. 2007). There is evidence that dehydrogenase activity is inhibited by the toxic effects of heavy metals added by organic residues (Marzadori et al. 1996). FDA hydrolysis reflects the activity of several enzymes, including lipases, esterases and proteases, and its activity increases with SMB (Schnurer and Rosswall 1982). The results showed that microbial metabolism was negatively affected by Cr and salts, which is supported by reductions in FDA and DHA activities. Some studies have demonstrated detrimental effects of Cr on DHA and FDA activities (Kizilkaya et al. 2004; Santos et al. 2011). Stępniewska and Wolińska (2005) investigated the effect of Cr on Eutric Cambisol, and DHA demonstrated a tendency to decrease with increase of Cr concentration. Inhibition of DHA by Cr was also reported by Wyszkowska et al. (2001), who noted that decrease of enzymatic activity in soil should be considered as very unfavourable in terms of soil fertility because soils with high organic matter content show high enzymatic activity. In addition, soil salinity may negatively influence the enzymatic activity, especially DHA activity. The reduction in the enzyme activity as a result of salinity could be due to the osmotic dehydration of the microbial cells, causing the release of intracellular enzymes and, consequently, decreased enzyme activity (Frankenberger and Bingham 1982).
The NMS analysis showed different responses for the effect of CTS amendment over 5 years, suggesting that at low rates (2.5 and 5 Mg ha−1), the CTS was beneficial to SMB and enzymes. Previous studies with industrial wastes found a strong correlation between microbial biomass and soil enzymes (Ros et al. 2006; Scherer et al. 2011; Patel and Patra 2014). Additionally, we identified a grouping between the 0 and 2.5 Mg ha−1 CTS with soil enzymes and substrate-induced respiration. Similarly, when evaluating tannery sludge, Patel and Patra (2014) found a significant correlation between dehydrogenase and SIR. However, CTS amendment at high rates (10 and 20 Mg ha−1) grouped with chemical properties and metabolic quotient and indicated that consecutive CTS amendment increases soil organic matter, Cr accumulation in soil and soil salinity and alkalinity. Consequently, the metabolic quotient increased and could indicate stress on soil microorganisms.
Conclusion
The results show that after 5 years of permanent amendment of CTS, soils amended with 2.5 Mg ha−1 have SMB and enzymatic activities similar to those in unamended soil, suggesting that low quantities of CTS amendment did not produce detrimental effects on soil microbial properties. However, these microbial properties decreased after long-term amendment with 10 and 20 Mg ha−1 of CTS. This decrease may have occurred because of the increases in Cr concentration and EC in the soil.
References
Alef, K., & Nannipieri, P. (1995). Methods in soil microbiology and biochemistry. New York: Academic.
APHA. (2005). Standard methods for the examination for water and wastewater. Washington: American Public Health Association. 1600 pp.
Araújo, A. S. F., & Monteiro, R. T. R. (2006). Microbial biomass and activity in a Brazilian soil plus untreated and composted textile sludge. Chemosphere, 64, 1043–1046.
Araújo, A. S. F., Monteiro, R. T. R., & Carvalho, E. M. S. (2007). Effect of textile sludge composted on growth, nodulation and nitrogen fixation of soybean and cowpea. Bioresource Technology, 98, 1028–1032.
Araujo, A. S. F., Cesarz, S., Leite, L. F. C., Borges, C. D., Tsai, S. M., & Eisenhauer, N. (2013a). Soil microbial properties and temporal stability in degraded and restored lands of Northeast Brazil. Soil Biology & Biochemistry, 66, 175–181.
Araujo, A. S. F., Silva, M. D. M., Leite, L. F. C., Araujo, F. F., & Dias, N. S. (2013b). Soil pH, electric conductivity and organic matter after three years of consecutive applications of composted tannery sludge. African Journal of Agricultural Research, 8, 1204–1208.
Bremner, J. M. (1996). Nitrogen-total. In J. M. Bigham (Ed.), Methods of soil analysis, part 3 (pp. 1085–1121). Madison: Soil Science Society of America, American Society of Agronomy.
Brookes, P. C., Landman, A., Pruden, G., & Jenkinson, D. S. (1985). Chloroform fumigation and the release of soil nitrogen, a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biology & Biochemistry, 17, 837–842.
Casida, L. E., Klein, D. A., & Santoro, T. (1965). Soil dehydrogenase activity. Soil Science, 98, 371–376.
CICB (2013). Informações Econômicas. Centro das Indústrias de Curtume do Brasil. http://www.cicb.org.br/?p=10817. Accessed 20 October 2014.
CONAMA (2009). Define critérios e procedimentos para o uso de lodos de esgoto gerados em estações de tratamento de esgoto sanitário e seus produtos derivados. Resolução N° 375 Diário Oficial da União, DF, N° 167. p. 141–146. Conselho Nacional do Meio Ambiente (Conama), Brasília, Brazil.
Fernandes, S. A. P., Bettiol, W., & Cerri, C. C. (2005). Effect of sewage sludge on microbial biomass, basal respiration, metabolic quotient and soil enzymatic activity. Applied Soil Ecology, 30, 65–77.
Forster, J. C. (1995). Soil sampling and storage. In K. Alef & P. Nannipieri (Eds.), Methods in applied soil microbiology and biochemistry (p. 49). London: Academic.
Frankenberger, W. T., & Bingham, F. T. (1982). Influence of salinity on soil enzyme activities. Soil Science Society of American Journal, 46, 1173–1177.
García-Gil, J. C., Plaza, C., Soler-Rovira, P., & Polo, A. (2000). Long-term effects of municipal solid waste compost application on soil enzyme activities and microbial biomass. Soil Biology & Biochemistry, 32, 1907–1913.
Giacometti, C., Cavani, L., Gioacchini, P., Ciavatta, C., & Marzadori, C. (2012). Soil application of tannery land plaster, effects on nitrogen mineralization and soil biochemical properties. Applied Environmental Soil Science, 1, 1–9.
Gonçalves, I. C. R., Araujo, A. S. F., Nunes, L. A. P. L., & Melo, W. J. (2014). Soil microbial biomass after two years of consecutive application of composted tannery sludge. Acta Scientiarum Agronomy, 36, 35–41.
Kizilkaya, R., Askin, T., Bayrakli, B., & Saglam, M. (2004). Microbiological characteristics of soils contaminated with heavy metals. European Journal of Soil Biology, 40, 95–102.
Lavelle, P., & Spain, A. (2001). Soil ecology. Dordrecht: The Netherlands, Kluwer Academic Publishers.
Lopes, E. L. N., Fernandes, A. R., Ruivo, M. L. P., Cattanio, J. H., & Souza, G. F. (2011). Microbial biomass and soil chemical properties under different land use systems in northeastern Pará. Revista Brasileira de Ciência do Solo, 35, 1127–1139.
Martí, E., Sánchez, M., Sierra, J., Cruanas, R., & Garau, M. A. (2007). Ecotoxicological tests assessment of soils polluted by chromium (VI) and pentachlorophenol. Science of Total Environment, 378, 53–57.
Martines, A. M., Nogueira, M. A., Santos, C. A., Nakatani, A. S., Andrade, C. A., Coscione, A. R., Cantarella, H., Sousa, J. P., & Cardoso, E. J. B. N. (2010). Ammonia volatilization in soil treated with tannery sludge. Bioresource Technology, 101, 4690–4696.
Marzadori, C., Ciavatta, C., Montecchio, D., & Gessa, C. (1996). Effects of lead pollution on different soil enzyme activities. Biology & Fertility of Soils, 22, 53–58.
Nelson, D. W., & Sommers, L. E. (1996). Total carbon, organic carbon, and organic matter. In A. L. Page (Ed.), Methods of soil analysis, part 2 (2nd ed.). Madison: American Society of Agronomy.
Olcay, F., Şagban, T., Dindar, E., & Başkaya, H. S. (2013). Biostimulating effects of wastewater sludges on stressed soils. Journal of Biology and Environmental Science, 7, 153–161.
Patel, A., & Patra, D. D. (2014). Influence of heavy metal rich tannery sludge on soil enzymes vis-à-vis growth of Tagetes minuta, an essential oil bearing crop. Chemosphere, 112, 323–332.
Richards, L. A. (1954). Diagnosis improvements of saline and alkaline soils. Washington: Department of Agriculture. 160p.
Ros, M., Pascual, J. A., Garcia, C., Hernandez, M. T., & Insam, H. (2006). Hydrolase activities, microbial biomass and bacterial community in a soil after long-term amendment with different composts. Soil Biology & Biochemistry, 38, 3443–3452.
Santos, J. A., Nunes, L. A. P. L., Melo, W. J., & Araujo, A. S. F. (2011). Tannery sludge compost amendment rates on soil microbial biomass of two different soils. European Journal of Soil Biology, 47, 146–151.
Scherer, H. W., Metker, D. J., & Welp, G. (2011). Effect of long-term organic amendments on chemical and microbial properties of a luvisol. Plant Soil Environment, 57, 513–518.
Schnurer, J., & Rosswall, T. (1982). Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Applied & Environmental Microbiology, 43, 1256–1261.
Silva, M. D. M., Barajas-Aceves, M., Araujo, A. S. F., Araujo, F. F., & Melo, W. J. (2014). Soil microbial biomass after three years of consecutive composted tannery sludge amendment. Pedosphere, 24, 469–475.
Singh, R. P., & Agrawal, M. (2007). Effects of sewage sludge amendment on heavy metal accumulation and consequent responses of Beta vulgaris plants. Chemosphere, 67, 2229–2240.
Stępniewska, Z., & Wolińska, A. (2005). Soil dehydrogenase activity in the presence of chromium (III) and (VI). International Agrophysics, 19, 79–83.
Svensson, K., & Friberg, H. (2007). Changes in active microbial biomass by earthworms and grass amendments in agricultural soil. Biology & Fertility of Soils, 44, 223–228.
Tedesco, M. J., Gianello, C., & Bissani, C. A. (1995). Analises de solos, plantas e outros materiais. Porto Alegre: UFRGS. 252p.
Tripathi, R. D., Srivastava, S., Mishra, S., Singh, N., Tuli, R., Gupta, D. K., & Maathuis, F. J. M. (2007). Arsenic hazards, strategies for tolerance and remediation by plants. Trends Biotechnology, 25, 158–165.
USEPA (1996). Acid digestion of sediments, sludge’s and soils. Method 3050b. Washington, EPA, 12p.
van der Heijden, M. G. A., Bardgett, R. D., & van Straalen, N. M. (2008). The unseen majority, soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology Letters, 11, 296–31.
Vance, E. D., Brookes, P. C., & Jenkinson, D. S. (1987). An extraction method for measuring soil microbial biomass C. Soil Biology & Biochemistry, 19, 703–707.
Wyszkowska, J., Kucharski, J., Jastrzębska, E., & Hlasko, A. (2001). The biological properties of soil as influenced by chromium contamination. Polish Journal of Environmental Studies, 10, 37–42.
Yeomans, J. C., & Bremner, J. M. (1988). A rapid and precise method for routine determination of organic carbon in soil. Communication in Soil Science and Plant Analysis, 19, 1467–1476.
Zimmermann, S., & Frey, B. (2002). Soil respiration and microbial properties in an acid forest soil, effects of wood ash. Soil Biology & Biochemistry, 34, 1727–1737.
Acknowledgments
This research was funded by “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq-Brazil) and “Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior” (CAPES). A.S.F Araújo and W.J. Melo are supported by a personal grant from CNPq-Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Araujo, A.S.F., Miranda, A.R.L., Oliveira, M.L.J. et al. Soil microbial properties after 5 years of consecutive amendment with composted tannery sludge. Environ Monit Assess 187, 4153 (2015). https://doi.org/10.1007/s10661-014-4153-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-014-4153-3