Abstract
Repeated application of composted tannery sludge (CTS) changes the soil chemical properties and, consequently, can affect the soil microbial properties. The aim of this study was to evaluate the responses of soil microbial biomass and ammonia-oxidizing organisms to repeated application of CTS. CTS was applied repeatedly during 6 years, and, at the sixth year, the soil microbial biomass, enzymes activity, and ammonia-oxidizing organisms were determined in the soil. The treatments consisted of 0 (without CTS application), 2.5, 5, 10, and 20 t ha−1 of CTS (dry basis). Soil pH, EC, SOC, total N, and Cr concentration increased with the increase in CTS rate. Soil microbial biomass did not change significantly with the amendment of 2.5 Mg ha−1, while it decreased at the higher rates. Total and specific enzymes activity responded differently after CTS application. The abundance of bacteria did not change with the 2.5-Mg ha−1 CTS treatment and decreased after this rate, while the abundance of archaea increased significantly with the 2.5-Mg ha−1 CTS treatment. Repeated application of different CTS rates for 6 years had different effects on the soil microbial biomass and ammonia-oxidizing organisms as a response to changes in soil chemical properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The tannery industry represents an important part of Brazilian economy involving approximately 3.2 billion dollars in 2014. However, it releases about 50,000 t of dry tannery sludge (TS) annually (Santos et al. 2011). The most common method for TS disposal is landfilling, although soil application has been suggested as an alternative method (Singh and Agrawal 2008). The main concern with applying TS to soil is its high content of chromium (Cr), salts, and carbonates, which can accumulate in soil and cause long-term toxic effects on soil ecosystems (Patel and Patra 2014).
In the last 10 years, composting has been used as a suitable alternative to TS processing before soil application (Santos et al. 2011; Silva et al. 2014). However, the composting process does not fully eliminate TS toxicity, and some previous studies have shown an increase in Cr accumulation, soil pH, and salinity after the application of composted tannery sludge (CTS) (Araujo et al. 2013). Therefore, the application of CTS should be performed carefully to minimize the effects on plant and soil microorganisms.
Microorganisms represent a large fraction of soil biodiversity and play important processes for soil sustainability in the ecosystem (Lavelle and Spain, 2001). Also, soil microorganisms respond quickly to environmental impact by waste application (Kelly et al. 2011; Santos et al. 2011). Moreover, the activity of soil enzymes indicates that biochemical functions and some important enzymes, such as dehydrogenases or enzymes involved in the hydrolysis of fluorescein diacetate (FDA), are associated with biogeochemical cycles and consequently may reflect changes in the soil metabolic processes (Karaca et al. 2011). Also, these enzymes occur in all intact and viable microbial cells and may be correlated with oxidative potential of soil microorganisms (Gianfreda et al. 2005). Thus, they may be sensitive indicators of the effect of waste application on soil microbial activity. Previous studies have shown that soil microbial biomass and enzymatic activity may respond positively (García-Gil et al., 2000) or negatively (Tejada and Gonzalez 2007) to waste application in soil. The negative effect of wastes on soil microbial biomass and enzymes has often been related by the direct effects of metals, such as Cr, which accumulates in soil (Shi et al. 2002).
Recently, ammonia-oxidizing bacteria (AOB) and ammonia-oxidizing archaea (AOA) have been studied as part of an integrated method to evaluate soil health in a wide variety of soil conditions, including application of wastes (Tourna et al. 2008). Ecologically, AOA and AOB mediate the oxidation of ammonia to nitrite, the rate-limiting step of nitrification (Carney et al. 2004) and, therefore, transforming nitrogen nutrients in agricultural soils (Tago et al. 2015). Also, these organisms have been reported to be more sensitive to different pollutants than other microbial parameters (van Beelen and Doelman 1997) and, therefore, can be used as indicators of environmental impact of pollutants on soil (Liu et al. 2014). Kelly et al. (2011) assessed the effect of sewage sludge on AOB and AOA, after 3 years of repeated application, and found that the copy numbers of AOA were higher than AOB in response to application of sewage sludge. However, few studies on the response of AOB and AOA to the application of industrial organic wastes in soil have been published and, specifically, it is unclear what are the effects of repeated application of composted tannery sludge on AOB and AOA.
Therefore, we began a long-term study of CTS application to evaluate its effects on soil microorganisms after repeated amendment. A previous study showed that repeated applications of CTS have increased the soil pH, salinity, and Cr accumulation in soil (Araujo et al. 2013). Therefore, the main question of this study is how soil microbial biomass and ammonia-oxidizing organisms respond to changes in soil chemical properties, after 6 years of repeated application of different CTS rates.
Materials and methods
Experimental site
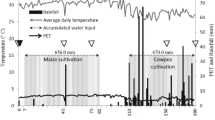
The experimental site is located at the Long-Term Experimental Field of the Agricultural Science Center, Teresina, Piauí (05° 05′ S; 42° 48′ W, 75 m). The regional climate is dry tropical (Köppen) and is characterized by two distinct seasons: rainy summer and dry winter, with annual average temperatures of 30 °C and rainfall of 1200 mm. The rainy season extends from January to April, when 90 % of the total annual rainfall occurs. The soil is classified as a Fluvisol with the following composition at 0–20-cm depth: 10 % clay, 28 % silt, and 62 % sand.
Composted tannery sludge
CTS was produced annually by mixing tannery sludge with sugarcane straw and cattle manure (ratio 1:3:1 v/v/v). The composting process was performed using the aerated pile method for 85 days. The pile was 2 m long, 1 m wide, and 1.5 m high. The pile was turned twice a week during the first 30 days and twice a month during the remaining 55 days. At the end of the composting process, 20 subsamples were randomly collected from the CTS to produce a composite sample. The water content was determined after oven drying the samples at 105 °C for 24 h; the pH and electrical conductivity (EC) were directly read; and total solids were measured by drying the samples at 65 °C (APHA 2005). The total organic C content was evaluated by dichromate oxidation of the samples under external heating (Yeomans and Bremner 1988). The total N content was determined using the Kjeldahl method after sulfuric acid digestion of the samples. The total Ca, Mg, K, P, S, Na, Zn, Cu, Cd, Pb, Ni, and Cr concentrations were determined by atomic absorption spectrophotometry after nitric acid digestion of the samples in a microwave oven (EPA, 1996). The chemical properties of CTS are shown in Table 1.
Land application
CTS was applied annually from 2009 to 2014 using five rates: 0 (without CTS application), 2.5 (2.5CTS), 5 (5CTS), 10 (10CTS), and 20 (20CTS) t ha−1 of CTS (dry basis). The experimental site was arranged in a completely randomized design with four replicates. Plots were 20 m2 each, with 12 m2 of usable area for soil and plant sampling, and rows were spaced 1.0 m apart. In the sixth year (2014), CTS was applied 10 days before cowpea (Vigna unguiculata) sowing. The CTS was spread on the soil surface and incorporated into the 20-cm layer with a harrow. Cowpea was planted at a density of 5 plants m−1 (approximately 62,000 plants ha−1).
Soil sampling
At the sixth year, CTS was amended in January 2014, and the soil samples were collected from each plot 60 days after CTS amendment. In each plot, four samples were collected (0–20 cm), sieved (2 mm), and stored at 4 °C prior to analysis. A portion of the soil samples was stored in polythene bags and kept at −20 °C for real-time PCR analysis. Before analysis, the soil was pre-incubated (with a 100-mL jar of soda lime and bottles containing deionized water) in sealed plastic containers for 7 days with a moisture content of 60–70 % of the water-holding capacity to equilibrate the physiological activity of soil microbial biomass.
Soil microbial biomass and enzymes
The levels of soil microbial biomass C (MBC) and microbial biomass N (MBN) were determined according to the methods developed by Vance et al. (1987) and Brookes et al. (1985), respectively, with 0.5-M K2SO4 extraction of the organic C and total N contents from fumigated and unfumigated soils. The coefficients of extraction (0.38 and 0.45) were used to convert the differences in C and N contents between the fumigated and unfumigated soils to microbial C and N, respectively. The soil respiration was monitored through daily measurement of CO2 evolution under aerobic incubation at 25 °C for 7 days (Alef and Nannipieri 1995). The respiratory quotient (qCO2) was calculated as the ratio of the basal respiration (BR) to the MBC and is expressed as mg CO2 kg−1 biomass C d−1. Fluorescein diacetate (FDA) hydrolysis was measured by spectrophotometry at 490 nm after incubation for 20 min at 30 °C, according to the method described by Schnurer and Rosswall (1982). Dehydrogenase (DHA) activity was determined by employing the method described by Casida et al. (1964), which is based on the spectrophotometric analysis of triphenyl tetrazolium formazan released by 5 g of soil after 24 h of incubation at 35 °C. Phosphatase (PHO) activity was estimated by quantifying the ρ-nitrophenol resulting from the enzymatic hydrolysis of ρ-nitrophenyl phosphate (Tabatabai and Bremner 1969) assessed after incubating the soil for 1 h at 37 °C. The specific enzymatic activity was estimated by dividing the total enzyme activities over the microbial biomass C (Katsalirou et al. 2010).
Soil chemical analyses
A portion of the soil samples was air-dried, sieved through a 2-mm screen, and homogenized for chemical analyses. The average values of the soil chemical properties (0–20-cm depth) after 6 years of CTS amendment are shown in Table 2. Soil pH was estimated in water (1:2.5 v/v) and measured using a pH meter. Soil EC was evaluated in water (1:2 v/v) according to the method described by Richards (1954). Soil organic C and total N were determined by wet combustion using a mixture of 5 mL of 0.167 M potassium dichromate and 7.5 mL of concentrated sulfuric acid under heating (170 °C for 30 min) (Yeomans and Bremner 1988; Bremner 1996). The total Cr concentration was analyzed according to EPA (1996) after the digestion of soil with HNO3, HCl, and H2O2. The concentration of Cr from soil samples was analyzed using atomic absorption spectrophotometry.
Real-time PCR
Soil DNA was extracted from 0.5 g (total humid weight) of soil using the Power Soil DNA Isolation Kit (MoBIO Laboratories, Carlsbad, CA, USA), according to the manufacturer’s instructions. The DNA extraction was performed in triplicate for each soil sample. The quality and relative quantity of the extracted DNA were determined using a Thermo Scientific NanoDrop 2000.
The qPCR was performed on an Applied Biosystems (Applied Biosystems, NJ, USA) ABI 7300 sequence detection system using SYBR Green detection. The qPCR was performed in 20-μL reaction mixtures containing the following components: 10 μL of SYBR GreenER™ qPCR SuperMix (Invitrogen, NJ, USA) and 0.5 μM of each primer. Primer set 341F/534R was used for bacterial 16S ribosomal RNA (rRNA) gene amplification. The qPCR assay to estimate archaeal 16S rRNA gene content used the primer set Arch771F/957R. The archaeal amoA gene amplifications were carried out using primers Arch-amoAF/AR, while the primers A189 and amoA-2R′ were used for the bacterial amoA gene quantification. The cycling parameters used were 50 °C for 2 min, 95 °C for 10 min, followed by 50 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 60 s, with a detection step at the end of each cycle.
A melting curve analysis was performed after each assay to ensure that only the products of the desired melting temperature were generated from the SYBR Green qPCR. The R 2 values for the standard curves were 0.99 or better for all runs. All reactions were run in triplicate with a standard curve spanning 101–106 copy numbers for bacterial and archaeal 16S rRNA genes or 100–105 copy numbers for bacterial and archaeal amoA genes. The standard curves for quantifying gene copy numbers were determined by cloning the PCR products in a plasmid using the procedures reported by Okano et al. (2004). The population sizes of total bacteria, total archaea, AOA, and AOB were estimated as the normalized copies per gram of dry soil.
Statistical analyses
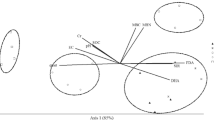
The data were statistically analyzed using analysis of variance (ANOVA), and the means were compared using least significant difference values calculated at the 5 % level. In addition to analyzing each variable separately, the data from the variables were analyzed using the non-metric multidimensional scaling (NMS) technique of multivariate ordination, employing Sørensen distance. Before the analysis, data were standardized by total within each variable, in order to cancel the differences in the variable units. The standardized data matrix was composed of the biological and chemical variables. Ordination NMS was performed in the autopilot mode, with option “slow and thorough” in the PC-ORD v. 6.0 program. The dimensionality is determined by comparing the final stress values among the best solution for each axis. The final ordination was rotated to maximize variables represented and separation of treatments on axes 1 and 2. Relationships between biological and chemical variables were explored with the joint plot feature. In joint plot, the length and direction of each vector are related to correlations of variables with the ordination axis (McCune and Grace 2002).
Results
Soil pH, EC, SOC, total N, and Cr concentration increased with the increase in CTS rate (Table 2). CTS application increased soil pH from 6.5 to 8.3 and EC from 0.63 to 1.85 dS m−1. Also, SOC content increased from 9.5 to 35.5 g kg−1, whereas total N ranged from 1.3 to 5.7 g kg−1 and Cr accumulated from 6.5 to 57.1 mg kg−1. Compared with unamended soil (control), soil pH, EC, SOC, total N, and Cr increased 0.3, 2.9, 3.7, 4.3, and 8.7 times, respectively, with the application of 20 Mg ha−1 CTS.
The results of soil microbial biomass and activity are shown in Table 3. MBC and MBN did not change significantly with the amendment of 2.5-Mg ha−1 CTS. At higher CTS application rates, MBC decreased 30–36 % and MBN decreased 48–74 % as compared with unamended soil. Soil basal respiration increased 82 % with the increase in CTS rates, and it influenced the respiratory quotient, which also increased 80 % across the CTS treatment. Total and specific enzyme activity responded differently after CTS application (Table 4). Total DHA decreased, while the specific DHA did not vary after CTS application. However, total and specific FDA and phosphatase increased significantly after CTS application.
The abundance of bacteria and archaea varied significantly and showed different patterns according to different CTS rates (Fig. 1). The abundance of bacteria did not change with the 2.5-Mg ha−1 CTS treatment and decreased after this rate. The abundance of archaea increased significantly with the 2.5-Mg ha−1 CTS treatment and decreased after this rate. AOB gene copy numbers did not vary significantly with CTS amendment, while AOA gene copy number was similar between 0 CTS and 2.5-Mg ha−1 CTS treatments and decreased at higher CTS application rates.
NMS analysis explained 75 % of the total variation, where the axis 1 explained almost the total of variation (67 %) and clustered basically two distinct groups according to the biological and chemical properties (Fig. 2; Table 5). The first group consisted of 0 and 2.5-Mg ha−1 CTS and was linked with the soil microbial biomass and abundance of bacteria, archaea, and AOA. The second group consisted of 5-, 10-, and 20-Mg ha−1 CTS and grouped with SOC, TN, pH, Cr, PHO, and qCO2.
NMS based on biological and chemical properties of soil. The two-dimensional solution had a final stress of 2.95. SOC soil organic C, TN total N, P phosphorus, Cr chromium, pH soil pH, MBC microbial biomass C, MBN microbial biomass N, BR basal respiration, qCO 2 respiratory quotient, qmic microbial quotient, PHO phosphatase activity, PHO_S specific phosphatase activity, %Arch percentage of archaea from the total prokaryote, AOA ammonia-oxidizing archaea, AOB ammonia-oxidizing bacteria
Discussion
The chemical properties of CTS show high pH, sodium, organic matter, and Cr content (Table 1) due to different compounds used during the tanning process, such as hydroxides, carbonates, salts, and Cr. Thus, repeated application of CTS increased soil pH, EC, SOC, total N, and total Cr accumulation. The increase in SOC and total N showed a positive effect of organic matter amendment from compost after 6 years of application. Similar results were obtained by Bouzaiane et al. (2007), who reported an increase in the soil organic C and total N after 3 years of municipal solid waste compost application. Cr accumulated in the soil and its content is considered high; however, this metal may remain inert, with low mobility since soil pH increased, and it may become the metal insoluble and with reduced toxicity (Hayes and Traina 1998). In addition, the values of Cr found in soil are below the limit proposed by EPA (2002), which is 230 mg kg−1. Soil salinity also increased after CTS application, but the values remained below 2.0 dS m−1, which is the limit to indicate saline soils (Richards 1954).
Soil microbial biomass and activity were significantly influenced by repeated application of CTS and showed distinct responses according to CTS rates. Microbial biomass C and N were not affected with the application of the lowest rate of CTS. This may mean that CTS added C and N sources for the maintenance of soil microorganisms (Giacometti et al. 2012), while the slight increase in soil pH, salinity, and Cr accumulation, at the lowest CTS rate, did not harm the soil microbial biomass. However, soil microbial biomass decreased in 5CTS, 10CTS, and 20CTS treatments, and it may be related to the increase in soil pH (Pankhurst et al. 2001) and Cr accumulation (Scherer et al. 2011). Scherer et al. (2011) evaluated the effect of repeated application of untreated and composted sewage sludge on soil microbial biomass and found a decrease in microbial biomass due to presence of high concentrations of Zn, Cr, and Cd in the soil. However, the decrease in soil microbial biomass cannot be attributed only to elevated accumulation of Cr with the highest CTS rate, but also probably to the humification and stability of organic matter in the stable compost which was resistant to microbial utilization attack as reported by Bouzaiane et al. (2007), evaluating municipal solid waste compost and farmyard manure on soil microbial biomass and activity.
Soil basal respiration was stimulated after CTS application, and it may suggest high biological activity due to C and nutrient inputs from CTS. However, high soil respiration may have occurred as an ecological disorder due to repeated CTS application, and it was measured through qCO2. Increased qCO2 has been suggested to correlate with low efficiency of substrate utilization by soil microbial biomass under environmental stress (Islam and Weil, 2000). Thus, the increase in qCO2 in soils under highest CTS rates may suggest a negative effect of composted tannery sludge, probably due to the Cr toxicity.
Soil enzyme activity showed different patterns according to CTS application and may be related to the characteristics of each enzyme. As an intracellular enzyme involved in the metabolisms of microorganisms, the dehydrogenase activity is related to the soil microbial biomass (García-Gil et al., 2000). Total DHA activity decreased with CTS application, probably due to Cr accumulation. Some studies have previously demonstrated detrimental effects of Cr on DHA (Stępniewska and Wolińska, 2005; Santos et al. 2011). Stępniewska and Wolińska (2005) evaluated the influence of Cr on Cambisol and found a trend in decrease in DHA activity with increase in Cr concentration.
Interestingly, the specific DHA activity did not vary across the treatments and suggests that, although SMB decreased, the amount of enzyme per unit of microbial biomass was not affected by CTS application. The specific enzymatic activity per microbial biomass C is a measurement which permits comparison of the metabolic status of microorganisms (Raiesi and Beheshti 2014), indicating the physiological capacity of enzymes related to the current or most recent viable microbial biomass (Waldrop et al. 2000). Therefore, our results indicate that repeated CTS application affected total soil microbe numbers but did not affect the microbes’ ability to produce dehydrogenase.
On the other hand, our results showed that despite the decrease in soil microbial biomass after CTS application, the activity of FDA hydrolysis and phosphatase was not affected. As extracellular enzymes, FDA, which is hydrolyzed by lipases, esterases, and proteases (Schnurer and Rosswall, 1982), and phosphatase, which catalyze the hydrolysis of both esters and anhydrides of phosphoric acid (Schmidt and Laskowski 1961), are not subjected to factors controlling microbial biomass (Nannipieri et al. 2011), such as CTS application. In addition, the turnovers of extracellular enzymes are lower than that of soil microbial biomass, and, therefore, these enzymes can persist in the soil after the death of soil microbial biomass (Bonmati et al. 1991).
The abundance of bacteria and archaea showed that CTS application in the lowest rates did not affect these groups of microorganisms and followed the same trend found for soil microbial biomass. It confirms the relationship between the abundance of bacteria and archaea and the soil microbial biomass (Rousk et al. 2010). Thus, the application of CTS changed soil chemical properties, and it contributes to the decrease in the abundance of bacteria and archaea in soil.
CTS application promoted different responses of AOB and AOA gene copy numbers, suggesting that AOA are more susceptible to environmental changes through waste amendment, such as metal accumulation. Previous studies have been reported that AOA and AOB are affected by metal pollution, but AOA were more strongly reduced in Cu- (Van Beelen et al. 2004; Liu et al. 2014), and Zn- (Kelly et al. 2011) polluted soils.
The NMS clustered two different responses of soil microorganisms to repeated application of CTS and showed that under 0CTS and 2.5CTS, there were no detrimental effects of CTS on soil microbial biomass and ammonia-oxidizing organisms, except AOB. On the other hand, CTS applied in highest rates influenced some important soil chemical properties, i.e., increased pH and Cr, which were negatively correlated with the soil microbial biomass and ammonia-oxidizing organisms and AOA. This negative relationship between soil microorganisms and Cr suggests that in Cr-contaminated soil the conversion of C into microbial biomass is lower (Ipsilantis and Coyne 2007). Interestingly, our results found that AOA and AOB showed different patterns according to CTS rates. AOA were positively influenced in the lowest CTS rate but was limited by the highest CTS rate. On the contrary, AOB were not limited by CTS rates, showing higher tolerance to CTS application. Possible limiting factors for AOA in the highest CTS rates might have been pH and Cr accumulation. Thus, soil pH influenced AOA but not AOB. AOA are not usually correlated with soil pH since AOA are present over a wider pH range, while AOB increase from neutral to alkaline soils (Yao et al. 2011). On the other hand, contrary to others studies (He et al. 2007; O’Sullivan et al. 2013; Rousk et al. 2010), our results showed a negative correlation between pH and AOA abundance. Also, there was a negative correlation between AOA and Cr, similar to the results reported previously by Van Beelen et al. (2004), who showed that AOA were more affected by contamination from Cu and Cr in a sludge-amended soil, suggesting that AOB have a higher heavy metal tolerance than AOA (Mertens et al. 2009).
Conclusion
Repeated application of different CTS rates for 6 years had different effects on the soil microbial biomass and ammonia-oxidizing organisms as a response to changes in soil chemical properties, i.e., increased soil pH, SOC, TN, and Cr accumulation. Soil microbial biomass and ammonia-oxidizing bacteria and AOA were unaffected by the application of 2.5-Mg ha−1 CTS, but decreased at higher application rates. Ammonia-oxidizing archaea increased with the application of 2.5-Mg ha−1 CTS, while AOB remained unaffected. The results indicate that AOA and AOB likely occupy different niches and respond differently to repeated application of composted tannery sludge.
References
Alef K, Nannipieri P (1995) Methods in soil microbiology and biochemistry. Academic, New York
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Washington DC
Araujo ASF, Silva MDM, Leite LFC, Araujo FF, Dias NS (2013) Soil pH, electric conductivity and organic matter after three years of consecutive applications of composted tannery sludge. Afr J Agric Res 8:1204–1208
Bonmati M, Ceccanti B, Nannipieri P (1991) Spatial variability of phosphates, urease, organic carbon and total nitrogen in soil. Soil Biol Biochem 23:391–396
Bouzaiane O, Cherif H, Ayari F, Jedidi N, Hassen A (2007) Municipal solid waste compost dose effects on soil microbial biomass determined by chloroform fumigation-extraction and DNA methods. Ann Microbiol 57:681–686
Bremner JM (1996) Nitrogen-total. In: Bigham JM (ed) Methods of soil analysis, part 3. Soil Science Society of America. American Society of Agronomy, Madison, pp. 1085–1121
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen, a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Carney KC, Matson PA, Bohannan BJM (2004) Diversity and composition of tropical soil nitrifiers across a plant diversity gradient and among land-use types. Ecol Lett 7:684–694
Casida LE, Klein DA, Santoro T (1964) Soil dehydrogenase activity. Soil Sci 98:371–376
CONAMA (2009) Define critérios e procedimentos para o uso de lodos de esgoto gerados em estações de tratamento de esgoto sanitário e seus produtos derivados. Resolução N° 375 Diário Oficial da União, DF, N° 167. Conselho Nacional do Meio Ambiente (Conama), Brasília, Conama pp. 141–146
García-Gil JC, Plaza C, Soler-Rovira P, Polo A (2000) Long-term effects of municipal solid waste compost application on soil enzyme activities and microbial biomass. Soil Biol Biochem 32:1907–1913
Giacometti C, Cavani L, Gioacchini P, Ciavatta C, Marzadori C (2012) Soil application of tannery land plaster, effects on nitrogen mineralization and soil biochemical properties. App Environ Soil Sci 1:1–9
Gianfreda L, Rao MA, Piotrowska A, Palumbo G, Colombo C (2005) Soil enzyme activities as affected by anthropogenic alterations: intensive agricultural practices and organic pollution. Sci Total Environ 341:265–279
Hayes KF, Traina SJ (1998) Metal speciation and its significance in ecosystem health. In: Huang PM (ed.) Soil chemistry and ecosystem health. Soil Sci Soc Am J 45–84
He JZ, Shen JP, Zhang LM, Zhu YG, Zheng YM, Xu MG (2007) Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ Microbiol 9:2364–2374
Ipsilantis I, Coyne MS (2007) Soil microbial community response to hexavalent chromium in planted and unplanted soil. J Environ Qual 36:638–645
Islam KR, Weil RR (2000) Land use effects on soil quality in a tropic forest ecosystem of Bangladesh. Agric Ecosyst Environ 79:9–16
Karaca A, Cema CC, Turgay OC, Kizilkaya R (2011) Soil enzymes as indicator of soil quality. In: Shukla GA (ed) Soil enzymology. Springer, Berlin, pp. 35–56
Katsalirou E, Deng S, Nofziger DL, Gerakis A (2010) Long-term management effects on organic C and N pools and activities of C-transforming enzymes in prairie soils. Eur J Soil Biol 46:335–341
Kelly JJ, Policht K, Grancharova T, Hundal LS (2011) Distinct responses in ammonia-oxidizing archaea and bacteria after addition of biosolids to an agricultural soil. Appl Environ Microbiol 77:6551–6558
Lavelle P, Spain A (2001) Soil ecology. Springer Nertheland: Dordrecht, The Netherlands
Liu Y, Liu Y, Ding Y, Zheng J, Zhou T, Pan G (2014) Abundance, composition and activity of ammonia oxidizer and denitrifier communities in metal polluted rice paddies from South China. PLoS One 9:e102000
McCune B, Grace JB (2002) Analysis of Ecological Communities. Gleneden Beach, OR: MjM Software Design
Mertens J, Broos K, Wakelin SA, Kowalchuk GA, Springael D, Smolders E (2009) Bacteria, not archaea, restore nitrification in a zinc-contaminated soil. ISME J 3:916–923
Nannipieri P, Giagnoni L, Landi L, Renella G (2011) Role of phosphatase enzymes in soil. In: Bunemann EK (ed) Phosphorus in action. Soil biology, vol 26. Springer, Berlin, pp. 215–243
Okano Y, Hristova KR, Leutenegger CM, Jackson LE, Denison RF, Gebreyesus B, Lebauer D, Scow KM (2004) Application of real-time PCR to study effects of ammonium on population size of ammoniaoxidizing bacteria in soil. Appl Environ Microbiol 70:1008–1016
O’Sullivan CA, Wakelin SA, Fillery RP, Roper MM (2013) Factors affecting ammonia-oxidizing microroganisms and potential nitrification rates in southern Australian agricultural soils. Soil Res 51: 240–252
Pankhurst CE, Yu S, Hawke BG (2001) Capacity of fatty acid profiles and substrate utilisation patterns to describe differences in soil microbial communities associated with increased salinity or alkalinity at three locations win South Australia. Biol Fertil Soils 33:204–217
Patel A, Patra DD (2014) Influence of heavy metal rich tannery sludge on soil enzymes vis-à-vis growth of Tagetes minuta, an essential oil bearing crop. Chemosphere 112:323–332
Raiesi F, Beheshti A (2014) Soil specific enzyme activity shows more clearly soil responses to paddy rice cultivation than absolute enzyme activity in primary forests of Northwest Iran. Appl Soil Ecol 75:63–70
Richards LA (1954) Diagnosis improvements of saline and alkaline soils. Department of Agriculture, Washington 160p
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–1351
Santos JA, Nunes LAPL, Melo WJ, Araujo ASF (2011) Tannery sludge compost amendment rates on soil microbial biomass of two different soils. Eur J Soil Biol 47:146–151
Scherer HW, Metker DJ, Welp G (2011) Effect of long-term organic amendments on chemical and microbial properties of a luvisol. Plant Soil Environ 57:513–518
Schmidt G, Laskowski M (1961) Phosphatase ester cleavage (survey). In: Boyer PD, Lardy H, Myrback K (eds) The enzymes, 2nd edn. Academic, New York, pp. 3–35
Schnurer J, Rosswall T (1982) Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol 43:1256–1261
Shi W, Bischoff M, Turco R, Konopka A (2002) Long-term effects of chromium and lead upon the activity of soil microbial communities. Appl Soil Ecol 21:169–177
Silva MDM, Barajas-Aceves M, Araujo ASF, Araujo FF, Melo WJ (2014) Soil microbial biomass after three years of consecutive composted tannery sludge amendment. Pedosphere 24:469–475
Singh RP, Agrawal M (2008) Potential benefits and risks of land application of sewage sludge. Waste Manag 28:347–358
Stępniewska Z, Wolińska A (2005) Soil dehydrogenase activity in the presence of chromium (III) and (VI). Int Agrophys 19:79–83
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Tago K, Okubo T, Shimomura Y, Kikuchi Y, Hori T, Nagayama A, Hayatsu M (2015) Environmental factors shaping the community structure of ammonia-oxidizing bacteria and archaea in sugarcane field soil. Microbes Environ 30:21–28
Tejada M, Gonzalez JL (2007) Application of different organic wastes on soil properties and wheat yield. Agron J 99:1597–1606
Tourna M, Freitag TE, Nicol GW, Prosser JI (2008) Growth, activity and temperature responses of ammonia oxidising archaea and bacteria in soil microcosms. Environ Microbiol 10:1357–1364
EPA (1996) Acid digestion of sediments, sludge’s and soils. Method 3050b. EPA, Washington 12p
EPA (2002) Supplemental guidance for developing soil screening levels for superfund sites. Office of Solid Waste and Emergency Response, Washington, D.C.
Van Beelen P, Doelman P (1997) Significance and application of microbial toxicity tests in assessing ecotoxicological risks of contaminants in soil and sediment. Chemosphere 34:455–499
Van Beelen P, Wouterse M, Posthuma L, Rutgers M (2004) Location-specific ecotoxicological risk assessment of metal-polluted soils. Environ Toxicol Chem 23:2769–2779
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Waldrop MP, Balser TC, Firestone MK (2000) Linking microbial community composition to function in a tropical soil. Soil Biol Biochem 32:1837–1846
Yao HY, Gao YM, Nicol GW, Campbell CD, Prosser JI, Zhang LM, Han WY, Singh BK (2011) Links between ammonia oxidizer community structure, abundance, and nitrification potential in acidic soils. Appl Environ Microbiol 77:4618–4625
Yeomans JC, Bremner JM (1988) A rapid and precise method for routine determination of organic carbon in soil. Commun Soil Sci Plant Anal 19:1467–1476
Acknowledgments
The authors thank “Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior—CAPES” (PNPD grant 23038.007660/2011-51) and “Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq” (grants Universal 471347/2013-2 and Postdoctoral Research Fellowship (grants PDE 200484/2014-1) for financial support to this project.
This research was funded by “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (Proc. CNPq 471447/2013-2) and “Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior” (Proc. CAPES 23038.007660/2011-51). A.S.F Araújo is supported by a personal grant from CNPq—Brazil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Araújo, A.S.F., Lima, L.M., Santos, V.M. et al. Repeated application of composted tannery sludge affects differently soil microbial biomass, enzymes activity, and ammonia-oxidizing organisms. Environ Sci Pollut Res 23, 19193–19200 (2016). https://doi.org/10.1007/s11356-016-7115-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7115-1