Abstract
Persistence and risk assessment of spirotetramat and imidacloprid in chilli fruits were studied following three applications of a mixture formulation of spirotetramat (12 %) and imidacloprid (12 %) at 1000 and 2000 mL ha−1. Residues of spirotetramat and imidacloprid in chilli were estimated by high-performance liquid chromatograph (HPLC). Residues of spirotetramat and imidacloprid dissipated to more than 65 % after 3 days at both the dosages. Residues of spirotetramat on chilli fruits were found to be below its limit of quantification (LOQ) of 0.03 mg kg−1 after 5 and 7 days for recommended and double the recommended dosages, respectively. Similarly, imidacloprid residues were found to be below its LOQ of 0.01 mg kg−1 at 7 and 10 days, respectively. Half-life periods for spirotetramat were found to be 1.91 and 1.30 days, whereas, for imidacloprid, these values were observed to be 1.41 and 1.65 days at recommended and double the recommended dosages, respectively. Red chilli samples collected after 20 days of the last application did not show the presence of spirotetramat and imidacloprid at their respective determination limit. As the theoretical maximum residue contributions on chilli fruits are found to be less than the maximum permissible intake values on initial deposits, a waiting period of 1 day may follow to reduce risk before consumption at the recommended dose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chilli (Capsicum annuum L), also called “red pepper,” is an important cash crop in India and used both green and ripe to impart pungency to the food. Different varieties are grown for vegetables, spices, condiment, sauces, and pickles (Chaudhary 2000). Chillies have high nutritive value, are rich in vitamins like vitamin A and C (Raju and Luckose 1991), and yield about 160 calories of energy from every 100 g of dried pods (Narayanan et al. 1999). India has immense potential to grow and export different types of chillies required to various markets around the world. Pests are a major restraint to chilli production, and crop is attacked by more than 21 insects and non-insect pests major being sucking pests (Dey et al. 2001).
The combination formulation of spirotetramat and imidacloprid, Movento Plus® (spirotetramat 12 % + imidacloprid 36 %, 480 soluble concentrate (SC)) has increased efficacy against different insect development phases with outstanding results against sucking pests (Lozano et al. 2008). As reported by Salles (2002), a combination of both active ingredients resulted in an increased efficacy in different phases of insect development. Besides, it gave a good control of plant viruses, transported by sucking insects besides the direct damage. The combination formulation is also a suitable tool for resistance management and has shown outstanding performance against sucking pests (Mohapatra et al. 2012).
Spirotetramat, cis-4-(ethoxycarbonyloxy)-8-methoxy-3-(2,5-xylyl)-1-azaspiro[4.5] dec-3-en-2-one, is a novel tetramic acid insecticide that inhibits lipid biosynthesis and affects juvenile stages and adult fecundity of a broad spectrum of sucking insects such as aphids, whiteflies, scales, mealy bugs, etc. (Fig. 1a; Brück et al. 2009; Ramanaidu and Cutler 2012). It is systemic in action, xylem and phloem mobile, allowing acropetal and basipetal translocation in the plant. After foliar application, spirotetramat penetrates through the leaf cuticle and is hydrolyzed to spirotetramat-enol, the form in which the compound enters the xylem and phloem and is translocated throughout the plant (Fig. 1b; Smiley et al. 2011). This full ambimobility or two-way systemicity (phloem and xylem transport) ensures the control of hidden and soil-living sucking pests after foliar application and protects new shoots (Brück et al. 2009). No cross resistance has been reported for spirotetramat to any other commercially available insecticide (van Waetermeulen et al. 2007). Moreover, only low adverse effects have been found on beneficial arthropods, which make the product suitable for modern IPM systems (Brück et al. 2009).
Imidacloprid [1-(6-chloro-3-pyridylmethyl)-N-nitroimidazolidin-2-ylideneamine], is the most widely used neonicotinoid insecticide, globally. It is used as seed treatment, soil drench, foliar spray, and tree injection in more than 120 countries for pest management on over 140 crops (Fig. 1c; Brittain and Potts 2011). In insects, postsynaptic binding to the nicotinic acetylcholine receptors (nAChRs) causes paralysis and ultimately death due to overstimulation of the central nervous system (Tomizawa et al. 1999). Imidacloprid is a systemic pesticide with physical or chemical properties that allow residues to move into treated plants and then throughout the plant via xylem transport and translaminar (between leaf surfaces) movement (Buchholz and Nauen 2002). Seed and foliar application is quite effective against insect herbivores and also helps in controlling pathogen carried by these insects (Girolami et al. 2009; Robson et al. 2007).
The indiscriminate and repeated use of insecticides accumulates the toxic pesticide residue on an agriculture produce and poses serious threat to health of the consumers. India is dominating the world market in chilli as spices product. The trend in export of chilli is very encouraging, but on other hand, the exports are rejected due to pesticide residues (Reddy et al. 2007a). Considerable concern is being expressed over the magnitude of pest control chemicals left in foodstuffs following their use on crops. It is important to ensure that the levels of harvest time residues of pesticides on foodstuffs do not pose any hazard to consumers and are admissible in domestic as well as international trade. Therefore, the present studies were undertaken to know the persistence and risk of spirotetramat and imidacloprid on chilli fruits under subtropical conditions of Punjab, India.
Materials and methods
Chemicals and reagents
Solvents like dichloromethane and acetonitrile (HPLC grade), sodium chloride (AR grade ≥99.9 %) (NaCl), and analytical grade activated magnesium sulfate (MgSO4) were obtained from E. Merck (India) Limited, Mumbai. Sodium sulfate anhydrous and charcoal decolorizing powders activated were purchased from S. D. Fine Chemicals, Mumbai. Primary secondary amine (PSA) sorbent and activated graphitic carbon black (GCB, 400 mesh) were obtained from Agilent Technologies Pvt. Ltd., Bangalore, India. All common solvents were redistilled in all-glass apparatus before use. The suitability of the solvents and other chemicals was ensured by running reagent blanks before actual analysis.
The analytical standards of spirotetramat (purity 99.2 %), its metabolite spirotetramat cis enol (purity 99.1 %) and imidacloprid (purity 98.6 %) along with the formulation mixture of spirotetramat 12 % + imidacloprid 12 % (240 SC) were supplied by M/s Bayer CropScience India Ltd., Mumbai, India. Analysis of acetonitrile extract of the formulation showed only spirotetramat and imidacloprid and none of its metabolic product and was found to be accurate with respect to its active ingredient.
Instrumentation
High-performance liquid chromatography (HPLC) was used for the estimation of spirotetramat, spirotetramat cis enol, and imidacloprid residues. The high-performance liquid chromatograph (Model DGU-2045) equipped with reverse phase (RP) C18 column and photo diode array (PDA) detector, dual pump was supplied by M/s Shimadzu Corporation, Kyoto, Japan. The HPLC column, a Luna 5 μm C18 column (250 × 4.6 mm size, 5.20 ± 0.30 μm particle size, 2.20 ± 0.30 (90/10 %) particle distribution, 95 ± 15 A° pore diameter, 430 ± 40 m2 g−1 surface area, <55.0 ppm metal content, 19.00 ± 0.70 % total carbon, and 3.25 ± 0.50 μmoles m−2 surface coverage), was obtained from M/S Spincotech Pvt. Ltd. Chennai, India. The sample injector was equipped with a 20-μL loop. For instrument control, data acquisition, and processing, LC Solution software was used.
Field experiment
Chilli (variety CH-1) was raised at Entomological Research Farm, Punjab Agricultural University, Ludhiana, following recommended agronomic practices. There were three replications for each treatment arranged in a randomized block design, and the size of each plot was 100 m2.
The first application of mixture formulation of spirotetramat (12 %) + imidacloprid (12 %) at 1000 and 2000 mL ha−1 was made at fruit initiation stage followed by another two applications at 10-day intervals. Pesticide was sprayed as foliar application with the help of a Knapsack sprayer fitted with hollow cone nozzle. In control plots, only water was sprayed. The range of maximum temperature was 27.0–43.5 °C, minimum temperature was 19.8–32.0 °C, maximum relative humidity was 48–100 %, minimum relative humidity was 19–95 %, and total rainfall was 276.9 mm.
Sampling procedure
About 500 g of green chillies were collected randomly from different plots separately at 0 (2 h), 1, 3, 5, 7, and 10 days after the third application of the insecticide. Red chilli samples were also collected at the time of harvest (20 days). Samples were extracted and cleaned up immediately after sampling. A representative 50 and 15 g samples of chilli fruits were processed for spirotetramat and imidacloprid, respectively.
Extraction and cleanup for spirotetramat residues
The samples were processed and analyzed at Pesticide Residue Analysis Laboratory, Department of Entomology, Punjab Agricultural University, Ludhiana. The extraction and cleanup of chilli fruit samples for residues of spirotetramat and spirotetramat cis enol were carried out as per methodology described by Singh et al. (2013). A representative 50-g sample of chopped and macerated chilli fruits was extracted with acetonitrile followed by liquid-liquid partitioning with dichloromethane. The dichloromethane extract was treated with activated charcoal to remove colored impurities and filtered through Whatman filter paper No. 1 along with rinsings of acetonitrile. The eluate was concentrated to near dryness and then reconstituted to 5 mL of acetonitrile for estimation on HPLC.
Extraction and cleanup for imidacloprid residues
The extraction and cleanup of chilli fruit samples were prepared following the QuEChERS (quick, easy, cheap, effective, rugged, and safe) method for the determination of imidacloprid residues. A subsample of 15 g of chilli fruit was weighed into a 50-mL centrifuge tube, and then, 30 mL acetonitrile was dispensed into it. The sample was homogenized using high-speed homogenizer (Heidolph Silent Crusher-M®) for 2–3 min at 14,000–15,000 rpm. Sodium chloride (NaCl) 10 ± 0.1 g was added for phase separation. The contents were centrifuged at 2500–3000 rpm for 3 min. An aliquot of 15 mL acetonitrile layer was transferred over 10 ± 0.1 g anhydrous sodium sulfate (Na2SO4) in a test tube. The acetonitrile extract subjected to cleanup by dispersive solid-phase extraction (DSPE). An aliquot of 6 mL acetonitrile was taken in a test tube containing 0.15 ± 0.01 g PSA sorbent, 0.90 ± 0.01 g anhydrous MgSO4, and 0.05 ± 0.01 g graphitic carbon black, and the contents were thoroughly vortexed on a vortex shaker. Again, it was centrifuged at 2500–3000 rpm for 1 min. Four-milliliter aliquot of this acetonitrile extract was evaporated to dryness using low-volume evaporator at 40 °C. Volume was made up to 2 mL with acetonitrile.
Estimation of spirotetramat and imidacloprid residues
The HPLC analysis was carried out at column temperature 25 °C under condition acetonitrile with pump flow at 0.50 mL min−1. The instrument was set at wavelength 210 and 272 nm for spirotetramat and imidacloprid, respectively. An injection volume of 20 μL was used in all experiments. Residues of these insecticides were quantified by comparison of peak height/peak area of standards with that of unknown or spiked samples run under identical conditions.
Results and discussion
Efficiency of the method
In the present investigations, recovery experiments were carried out at different levels to establish the reliability and validity of analytical method and to know the efficiency of extraction and cleanup procedures. Chilli fruit samples were spiked with spirotetramat, spirotetramat cis enol, and imidacloprid at different levels and analyzed separately as per the methodology described above. The control samples from untreated plots and reagent blanks were also processed in the same way so as to find out the interferences, if any, due to the substrate and reagents, respectively. The mean percent recoveries of these insecticides from chilli fruit samples at the fortification level of 0.01 to 0.30 mg kg−1 ranged from 84.63 to 91.67. The average recovery values were found to be more than 85 %; thus, the results have been presented as such without applying any correction factor. The precision of the method was determined by repeatability studies of the method and expressed by relative standard deviation (RSD) values. The RSD for repeatability ranged from 1.67 to 5.54 % for these insecticides for different spiking levels as shown in Table 1. The limit of quantification (LOQ) of both spirotetramat and spirotetramat cis enol in chilli fruits was 0.03 mg kg−1, whereas the limit of detection (LOD) of both spirotetramat and spirotetramat cis enol was 0.01 mg kg−1. Similarly, LOQ of imidacloprid was found to be 0.01 mg kg−1 and LOD being 0.003 mg kg−1. The cleaned-up procedure for this methodology was found to be efficient and as such no significant matrix effect was observed.
Persistence studies of spirotetramat residues on green chilli fruits
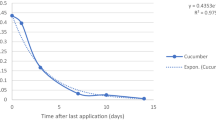
The overall results of the analysis of green chilli fruits following the third application spirotetramat 12 % + imidacloprid 12 % at 1000 and 2000 mL ha−1 are presented in Table 2. The mean initial deposits of spirotetramat were 0.55 and 1.22 mg kg−1 on the green chilli fruits following the third application with respect to spirotetramat at 120 and 240 g a.i. ha−1. These deposits dissipated to 0.25 and 0.58 mg kg−1 after 1 day at recommended and double the recommended dosages, respectively, thereby showing a loss of more than 50 %. The residues of spirotetramat reached below its LOQ of 0.03 mg kg−1 in 5 and 7 days at recommended and double the recommended dosages, respectively (Table 2). Metabolite of spirotetramat, spirotetramat cis enol, was not detected in any of the sample.
These results are not in agreement with those of Mohapatra et al. (2012) who studied the persistence of spirotetramat in mango fruits following application of spirotetramat 12 % + imidacloprid 12 % (240 SC) at 90 and 180 g a.i. ha−1 and reported that the residues were found to be below determination limit of 0.05 mg kg−1 at 10 days for both the dosages. This may be due to variation in substrates in which pesticide applied and varied weather conditions. Similarly, the major metabolite, spirotetramat cis enol, was not detected in mango. Pandiselvi et al. (2010) studied the residues of spirotetramat in cotton plant, seed, lint, and oil following application of spirotetramat 12 % + imidacloprid 36 % (480 SC) at 750 and 1500 mL ha−1 (90 and 180 g a.i. ha−1). The initial residues of spirotetramat on cotton plant were found to be 0.04 and 0.08 mg kg−1 at recommended and double the recommended dosages, respectively. These residues were found to be below determination limit of 0.03 mg kg−1 at 3 and 5 days, respectively. Cotton seed, lint, and oil samples collected at the time of harvest showed no detectable residues of spirotetramat.
Persistence studies of imidacloprid residues on green chilli fruits
The average initial deposits of imidacloprid on green chilli fruits were found to be 0.73 and 1.49 mg kg−1, respectively, following three applications of a combination mixture of spirotetramat 12 % + imidacloprid 12 % at 120 and 240 g a.i. ha−1 with respect to imidacloprid at 10-day intervals. These imidacloprid residues were dissipated to 0.43 and 1.00 mg kg−1 at recommended and double the recommended dosages, respectively, after 1 day of application. More than 65 % of these residues got dissipated in the third days at both these dosages. Residues of imidacloprid dissipated below LOQ of 0.01 mg kg−1 after 7 and 10 days at recommended and double the recommended dosages, respectively (Table 2).
Persistence of imidacloprid in chickpea pods and leaves was studied following three applications at 7-day interval of Solomon 300 OD (β-cyfluthrin 9 % + imidacloprid 21 %) at 200 and 400 mL ha−1. The average initial deposits of imidacloprid on chickpea pods were found to be 0.29 and 0.49 mg kg−1 at recommended and double the recommended dosages, respectively. More than 85 % of these residues got dissipated in 5 days at both these dosages. Residues of imidacloprid dissipated below LOQ of 0.01 mg kg−1 after 10 and 15 days at recommended and double the recommended dosages, respectively. Pesticides were not detected at harvest time. Imidacloprid residues were not detected at the pre-harvest interval (PHI) of 20 days in both the dosages (Chahil et al. 2014).
Dissipation of imidacloprid on brinjal was studied following three applications of a combination formulation of Solomon 300 OD (β-cyfluthrin 9 % + imidacloprid 21 %) at 42 and 84 g a.i. ha−1. Initial deposits were 0.24 and 0.37 mg kg−1 at single and double dose, respectively. Limit of quantification (LOQ) of imidacloprid was 0.01 mg kg−1. Imidacloprid residues took 10 days to reach LOQ at both the dosages (Mandal et al. 2010). Sahoo et al. (2012) studied the imidacloprid residues on okra fruits following application of Solomon 300 OD at 200 and 400 mL ha−1 which dissipated to below detectable level of 0.01 mg kg−1 after 5 and 7 days at single and double the dosages.
Spirotetramat and imidacloprid residues on red chilli samples
After 20 days, spirotetramat and imidacloprid residues on red chilli samples were found to be below their determination limit of 0.03 and 0.01 mg kg−1, respectively (Table 2). Similarly, the residues of fipronil and profenofos in dried red chillies (collected at harvest) were below detectable limit (Reddy et al. 2007b). The residues of endosulfan, dicofol, dimethoate, and λ-cyhalothrin in harvested red chillies were also below detectable levels in all the treatments (Reddy et al. 2007a). Jyot et al. (2013) studied the residues of chlorpyriphos and cypermethrin in red chilli following application of Nurelle-D 505 (chlorpyriphos 50 % + cypermethrin 5 %) at 1 and 2 L ha−1. Residues of cypermethrin in red chilli collected after 15 days of the last spray were found to be below its determination limit of 0.01 mg kg−1 at both the dosages. But red chilli samples were found to contain residues of chlorpyriphos at 0.08 and 0.11 mg kg−1 at recommended and double the recommended dosages, respectively, at 15 days (Jyot et al. 2013).
Half-life values of spirotetramat and imidacloprid
Statistical data on regression analysis and half-life for the dissipation of spirotetramat and imidacloprid on chilli fruits were given in Table 3. The persistence of spirotetramat and imidacloprid are generally expressed in terms of half-life (T 1/2) or DT50, i.e., time for disappearance of pesticide to 50 % of its initial concentration. The T 1/2 of spirotetramat and imidacloprid was calculated using Hoskins formula (1966) for each treatment separately. The half-life of spirotetramat on chilli fruits was observed to be 1.91 and 1.30 when applied at 120 and 240 g a.i. ha−1 (Table 3). Pandiselvi et al. (2010) reported the half-life values of 2.6 and 3.0 days on cotton plant following application of spirotetramat at 90 and 180 g a.i. ha−1, respectively. The half-life values of spirotetramat in mango were 3.3 and 5.2 days following application of mixture formulation (spirotetramat 12 % + imidacloprid 12 %, 240 SC) at 90 and 180 g a.i. ha−1, respectively (Mohapatra et al. 2012). Half-life of imidacloprid on chilli fruits was observed to be 1.41 and 1.65 when applied at 120 and 240 g a.i. ha−1 (Table 3). Half-life of imidacloprid on chickpea pods was observed to be 2.07 and 2.31 and leaves were 1.75 and 1.72 days, respectively, when applied at 42 and 84 g a.i. ha−1 (Chahil et al. 2014).
Risk assessment of spirotetramat and imidacloprid
The use of pesticides on food crops leads to unwanted residues, which may constitute barriers to exporters and domestic consumptions when they exceed maximum residue limits (MRLs). However, MRLs for spirotetramat and imidacloprid on chilli fruits are not available; hence, the risk assessment was calculated. Theoretical maximum residues contribution (TMRC) were calculated and compared with maximum permissible intake (MPI) to evaluate the risk to the consumer for the spirotetramat and imidacloprid on chilli fruits. However, acceptable daily intake (ADI) for spirotetramat has been observed to be 0.05 mg kg−1 body weight day−1 (European Food Safety Authority 2013). Maximum permissible intake (MPI) was obtained by multiplying the ADI with the weight of an average Indian person (55 kg) (Mukherjee and Gopal 2000). MPI was calculated to be 2750 μg person−1 day−1 without any appreciable risk of life. An average Indian consumes 2.5 g of green chilli for an Indian balanced diet (Anonymous 2002). The TMRC values on 0 day are found to be 1.375 and 3.050 μg person−1 day−1, in case of recommended dose (120 g a.i. ha−1) and double the recommended dose (240 g a.i. ha−1), respectively (Table 4). Both the values are low as compared to MPI; hence, the insecticide is well below the acceptable risk level at the time of consumption of such chilli fruits.
ADI for imidacloprid has been observed to be 0.06 mg kg−1 body weight day−1 (Sharma 2013). Likewise, an adult of 55 kg can tolerate an intake of 3300 μg day−1 without any appreciable risk to life. The TMRC values on 0 day are found to be 1.825 and 3.725 μg person−1 day−1 in case of imidacloprid at 120 and 240 g a.i. ha−1, respectively (Table 4). Both these values are low as compared to MPI. As the theoretical maximum residue contributions on chilli fruits are found to be less than the toxicologically estimated MPI value of 3300 μg person−1 day−1, the consumer health risks are minimal at both the dosages on chilli.
These studies, therefore, suggest that the use of spirotetramat and imidacloprid mixture formulation at recommended and double the recommended dosages does not seem to pose any hazards to the consumers and a waiting period of 1 day is suggested to reduce the risk before consumption of chilli fruits.
Conclusions
Half-life values for spirotetramat and imidacloprid following two applications at recommended dosages on chilli fruits were observed to be 1.91 and 1.41 days, respectively. Residue data showed that the initial deposits of spirotetramat and imidacloprid were below the theoretical maximum residues contribution at both recommended and double the recommended dosages. A waiting period of 1 day is suggested to reduce the risk before consumption of chilli fruits. Therefore, application of spirotetramat and imidacloprid mixture formulation at the recommended dose on chilli is quite safe from crop protection and environmental contamination point of view.
References
Anonymous. (2002). Opinion of the scientific committee on food on capsaicin. European Commission Health & Consumer Protection Directorate-General, (http://ec.europa.eu/food/fs/sc/scf/out120_en.pdf)
Brittain, C., & Potts, S. G. (2011). The potential impacts of insecticides on the life-history traits of bees and the consequences for pollination. Basic and Applied Ecology, 12, 321–331.
Brück, E., Elbert, A., Fischer, R., Krueger, S., Kühnhold, J., Klueken, A. M., Nauen, R., Niebes, J. F., Reckmann, U., Schnorbach, H. J., Steffens, R., & Waetermeulen, X. (2009). Movento®, an innovative ambimobile insecticide for sucking insect pest control in agriculture: biological profile and field performance. Crop Protection, 28, 838–844.
Buchholz, A., & Nauen, R. (2002). Translocation and translaminar bioavailability of two neonicotinoid insecticides after foliar application to cabbage and cotton. Pest Management Science, 58, 10–16.
Chahil, G. S., Mandal, K., Sahoo, S. K., Battu, R. S., & Singh, B. (2014). Risk assessment of β-cyfluthrin and imidacloprid in chickpea pods and leaves. Ecotoxicology and Environmental Safety, 101, 177–183.
Chaudhary, B. (2000). Solanaceous fruits and cole crops vegetables (pp. 63–84). India: National Book Trust.
Dey, P. K., Sarkar, P. K., & Somchoudhury, A. K. (2001). Efficacy of different treatment schedules of profenofos against major pests of chilli. Pestology, 25, 26–29.
European Food Safety Authority. (2013). Reasoned opinion on the modification of the existing MRLs for spirotetramat in strawberries, bananas, table olives, pineapples and shallots. EFSA Journal, 11, 3361–3398.
Girolami, V., Greatti, M., Bernardo, A. D., Tapparo, A., & Giorio, C. (2009). Translocation of neonicotinoid insecticides from coated seeds to seedling guttation drops: a novel way of intoxication for bees. Journal of Economic Entomology, 102, 1808–1815.
Hoskins, W. M. (1966). Mathematical treatment of the rate of loss of pesticide residues. FAO Plant Protection Bulletin, 9, 163–168.
Jyot, G., Mandal, K., Battu, R. S., & Singh, B. (2013). Estimation of chlorpyriphos and cypermethrin residues in chilli (Capsicum annuum L.) by gas-liquid chromatography. Environmental Monitoring and Assessment, 185, 5703–5714.
Lozano, F., Kemper, K., & Tundisi, H. (2008). Field development of Movento Plus for sucking pest insect control in Brazil. Bayer Crop Science Journal, 61, 329–341.
Mandal, K., Chahil, G. S., Sahoo, S. K., Battu, R. S., & Singh, B. (2010). Dissipation kinetics of spirotetramat and imidacloprid in brinjal and soil under subtropical conditions of Punjab, India. Bulletin of Environmental Contamination and Toxicology, 84, 225–229.
Mohapatra, S., Deepa, M., Leka, S., Nethravathi, B., Radhika, B., & Gourishanker, S. (2012). Residue dynamics of spirotetramat and imidacloprid in/on mango and soil. Bulletin of Environmental Contamination and Toxicology, 89, 862–867.
Mukherjee, I., & Gopal, M. (2000). Environmental behavior and translocation of imidacloprid in eggplant, cabbage and mustard. Pest Management Science, 56, 932–936.
Narayanan, S. S., Hedge, S., Sadananda, A. R., & Chelliah, S. (1999). Commerce and utility considerations of chillies. Kisan World, 26, 73–75.
Pandiselvi, S., Sathyanarayanan, S., & Ramesh, A. (2010). Determination of spirotetramat and imidacloprid residues in cotton seed, lint, oil and soil by HPLC UV method and their dissipation in cotton plant. Pesticide Research Journal, 22, 168–173.
Raju, K. V., & Luckose, C. K. (1991). Trends in area, production and exports of chillies from India. Agricultural Situation in India, 45, 767–772.
Ramanaidu, K., & Cutler, G. C. (2012). Different toxic and hormetic responses of Bombus impatiens to Beauveria bassiana, Bacillus subtilis and Spirotetramat. Pest Management Science, 69, 949–954.
Reddy, K. N., Satyanarayana, S., & Reddy, K. D. (2007a). Persistence of some insecticides in chillies. Pesticide Research Journal, 19, 234–236.
Reddy, K. D., Reddy, K. N., & Mahalingappa, P. B. (2007b). Dissipation of fipronil and profenofos residues in chillies (Capsicum annum L.). Pesticide Research Journal, 19, 106–107.
Robson, J. D., Wright, M. G., & Almeida, R. P. P. (2007). Effect of imidacloprid foliar treatment and banana leaf age on Pentalonia nigronervosa (Hemiptera, Aphididae) survival. New Zealand Journal of Crop and Horticultural Science, 35, 415–422.
Sahoo, S. K., Chahil, G. S., Mandal, K., Battu, R. S., & Singh, B. (2012). Estimation of spirotetramat and imidacloprid in okra fruits and soil by chromatography technique. Journal of Environmental Science and Health. Part. B, 47, 42–50.
Salles, L. A. (2002). Os insetos como vetores de patógenos de plantas. Cultivar, 13, 3–6.
Sharma, K.K. (2013). Pesticide residue analysis manual. Directorate of Information and Publications of Agriculture, Indian Council of Agricultural Research, New Delhi, p. 251.
Singh, B., Mandal, K., Sahoo, S. K., Bhardwaj, U., & Battu, R. S. (2013). Development and validation of HPLC method for determination of spirotetramat and spirotetramat cis enol in various vegetables and soil. Journal of Association of Official Analytical Chemists - J. AOAC Int., 96, 670–675.
Smiley, R. W., Marshall, J. M., & Yan, G. P. (2011). Effect of foliarly applied spirotetramat on reproduction of Heterodera avenae on wheat roots. Plant Disease, 95, 983–989.
Tomizawa, M., Latli, B., & Casida, J. E. (1999). Structure and function of insect nicotinic acetylcholine receptors studied with nicotinic insecticide affinity probes. In I. Yamamoto & J. E. Casida (Eds.), Nicotinoid insecticides and the nicotinic acetylcholine receptor (pp. 271–292). Tokyo: Springer.
Van Waetermeulen, X., Bruck, E., Elbert, A., Fischer, R., Krueger, S., Kuhnhold, J., Nauen, R., Niebes, J. F., Reckmann, U., Schnorbach, H. J., & Steffens, R. (2007). Spirotetramat, an innovative fully systemic insecticide for sucking insect pest control in agriculture: biological profile and field performance. XVI International Plant Protection Congress Proceeding, 1, 60–67.
Acknowledgments
The authors are thankful to M/s Bayer CropScience, India, and the Indian Council of Agricultural Research, India, for sponsoring the project. The authors are also thankful to the Head of the Department of Entomology, PAU, Ludhiana, for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chahil, G.S., Mandal, K., Sahoo, S.K. et al. Risk assessment of mixture formulation of spirotetramat and imidacloprid in chilli fruits. Environ Monit Assess 187, 4105 (2015). https://doi.org/10.1007/s10661-014-4105-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-014-4105-y