Abstract
Dissipation of chlorpyriphos and cypermethrin in chilli was studied following three applications of a combination formulation of Nurelle-D 505 (chlorpyriphos 50 % + cypermethrin 5 %) at 1 and 2 L ha−1 at an interval of 15 days. Residues of chlorpyriphos and cypermethrin in chilli were estimated by gas–liquid chromatography and confirmed by gas chromatography–mass spectrometry. Half-life periods for chlorpyriphos were found to be 4.43 and 2.01 days, whereas for cypermethrin these values were observed to be 2.51 and 2.64 days at single and double the application rates, respectively. Residues of chlorpyriphos dissipated to more than 80 % after 10 days at both the dosages. However, residues of cypermethrin dissipated to the extent of more than 70 % in 7 days. Soil samples collected after 15 days of the last application did not show the presence of chlorpyriphos and cypermethrin at their respective determination limit of 0.01 mg kg−1. The use of chlorpyriphos and cypermethrin mixture at the recommended dosage does not seem to pose any hazards to the consumers, and a waiting period of 1 day is suggested to reduce the risk before consumption of green chilli.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chilli (Capsicum annuum L.), also called “red pepper,” is an important cash crop in India and is grown for its pungent fruits, which are used both green and ripe to impart pungency to the food. Different varieties are grown for vegetables spices, condiment, sauces, and pickles (Chaudhary 2000). Chilli is one of the most important condiments having immense commercial and therapeutic value (Reddy et al. 2007a). It is an important ingredient in day-to-day curries, pickles, and chutnies and is a rich source of vitamins A, C, and E (Kumar et al. 2000). As a condiment, it has become indispensable in every Indian home. The pungency is due to the active principle “capsicin” contained in the skin and the septa of the fruit. The world’s consumption of chillies is going up due to the increasing popularity of ethnic foods. The increased availability of oleoresins and spice oils of chilli has also enhanced its consumption in various food preparations. India is the largest producer of chillies in the world, but its production pattern is highly erratic.
The low production of chilli in the country could be attributed to several factors, one of the most important being the damage caused by various insect pests. The major pests attacking chilli crops are sucking pests and pod borers. The crop is ravaged by a wide array of insect pests, including 51 species of insects and two species of mites which belong to 27 families under nine orders in both the nursery as well as the main field (Reddy and Puttaswamy 1983, 1984), resulting in yield losses. Among these pests, thrips and mites are serious (Puttarudraih 1959). The indiscriminate and repeated use of conventional insecticides accumulates the toxic pesticide residue on an agriculture produce and poses serious threat to the health of the consumers. India is dominating the world market in chilli as a spice product. The trend in the export of chilli is very encouraging, but on other hand, the exports are rejected due to pesticide residues (Reddy et al. 2007b). In 2001, Germany and Greece rejected red chilli and chilli powder due to pesticide residues (Rao et al. 2005). Many farmgate chilli samples showed the presence of insecticide residues (Chahal et al. 1997, 1999; Singh et al. 1999; Shah et al. 2000).
Chlorpyriphos [O,O,-diethyl-O-(3,5,6-trichloro-2-pyridyl)-phosphorothioate] is an organophosphorus broad-spectrum insecticide registered for application to more than 40 different food crops (Fig. 1a). It is a stable compound in neutral and acidic conditions. It kills insects by direct contact or ingestion and by disrupting the normal functioning of the nervous system. It is affective against both sucking and chewing insects and has been widely used to control pests of various vegetables. It is non-systemic and fairly persistent (Anonymous 2000), but almost insoluble in water (2 mg L−1). Cholinesterase inhibition is the mode of action of chlorpyriphos and is the cause of potential toxicity in humans (Oliver et al. 2000). Chlorpyriphos acts by inactivating acetylcholinesterase to paralyze the synaptic response with chronic symptoms including nausea, headaches, vomiting, diarrhea, and general weakness (Cochran et al. 1995). Cypermethrin [(RS)-α-cyano-3-phenoxybenzyl (1RS,3RS; 1RS,3SR)-3-(2,2-dichloro-vinyl)-2,2-dimethyl-cyclopropane carboxylate] is a digestive and contact insecticide effective against a wide range of insect pests, particularly leaf- and fruit-eating Lepidoptera and Coleoptera in cotton, fruit, vegetables, vines, tobacco, and other crops (Fig. 1b; Worthing 1987). Cypermethrin is a pyrethroid classified as a class II, moderately hazardous pesticide according to the World Health Organization. It has widespread application in virtually all sectors of insect control in animals, in agriculture, in the home, and in the garden as a possible alternative of some organophosphate, carbamate, or organochlorine insecticides (Jee et al. 2005). Cypermethrin is widely used by farmers to control insect pests of vegetables. In India, a number of ready pre-mix formulations containing a mixture of an organophosphate and a synthetic pyrethroid are registered for use on various crops (Regupathy et al. 2004). One such ready pre-mix formulation is Nurelle-D 505 (chlorpyriphos 50 % + cypermethrin 5 %).
There is currently an increasing concern and awareness on the hazards of pesticides to consumers. Even with the adoption of integrated pest management, farmers still believe in the control of pests using pesticide because of its quick effect. The application of pesticides pre- or post-harvest could, however, leave residues on food products, which pose a potential risk to the health of consumers (Lindsay 1997). The presence of pesticidal residues in food commodities is of concern to human health due to the toxic nature of pesticides. Hence, it is imperative to study the persistence of pesticides on edible crops to ensure human safety. It is important to ensure that the levels of harvest time residues of pesticides on foodstuffs do not pose any hazard to consumers and are admissible in domestic as well as international trade. In this context, the present study was carried out to investigate the residual behavior and risk assessment of chlorpyriphos and cypermethrin on green chilli fruits at different time intervals.
Materials and methods
Chemicals and reagents
The certified reference standards of chlorpyriphos (purity, 99.4 %) and cypermethrin (purity, 93.6 %) were supplied by M/S De-Nocil Crop Protection Limited (Mumbai, India). Formulation Nurelle-D 505 (chlorpyriphos 50 % and cypermethrin 5 %) was also obtained from M/S De-Nocil Crop Protection Limited. The analysis of the formulation in acetone extract with respect to its active ingredient of chlorpyriphos and cypermethrin was estimated using gas–liquid chromatography (GLC). The results showed that the concentrations of chlorpyriphos and cypermethrin in the formulation were correct as claimed by the manufacturer.
Solvents and reagents like acetone, dichloromethane, hexane, methanol, sodium chloride (ASC reagent grade, ≥99.9 %), and sodium sulfate anhydrous (AR grade) were all procured from Merck (Darmstadt, Germany). Activated charcoal decolorizing powder was obtained from Qualigens Fine Chemicals (Mumbai). All common solvents were redistilled in an all-glass apparatus before use. The suitability of the solvents and other chemicals was ensured by running reagent blanks before actual analysis.
Preparation of standard solution
A standard stock solution of chlorpyriphos and cypermethrin having a concentration of 1 mg mL−1 was prepared in acetone. The standard solutions required for constructing a calibration curve (2.00, 1.50, 1.00, 0.50, 0.25, and 0.10 μg mL−1) were prepared from stock solution by serial dilution using acetone and were stored at 4 °C.
Field trials
Chilli (var. CH-1) was raised at Entomological Research Farm, Punjab Agricultural University, Ludhiana, following recommended agronomic practices (Anonymous 2010). There were three replications for each treatment (i.e., control, recommended, and double the recommended dosages) arranged in a randomized block design; the size of the each plot was 100 m2. The soil under crop was of light texture with low content of organic matter. Other relevant properties of the soil were organic carbon = 0.30 %, pH 8.0, sand = 78.0 %, silt = 10.2, clay = 11.8 %, and EC = 0.30 dS m−1.
The first application of Nurelle-D 505 (chlorpyriphos 50 % + cypermethrin 5 %) at 1 and 2 L ha−1 was made at fruit formation stage followed by another two applications at an interval of 15 days. In control plots, only water was sprayed. Pesticide was sprayed as foliar application in three replications with the help of a knapsack sprayer fitted with a hollow cone nozzle.
Sampling procedure
Green and red chilli
About 1 kg of green chilli was collected randomly separated from the control and treated plots of each treatment at 0 (2 h), 1, 3, 5, 7, and 10 days after the third application of the insecticide. Red chilli samples were also collected at the time of harvest (15 days). The samples from each treatment plot were collected from each plot separately, packed in polyethylene bags, and brought to the laboratory for processing. Samples were extracted and cleaned up immediately after sampling.
Soil
Soil samples (1 kg) were collected 15 days after application of the insecticide. Soil samples were collected separately from 10 to 15 sites of each treated plot with the help of a tube auger at a depth of about 0–15 cm; the soil from the 10–15 sites were pooled and sieved and extraneous matter, including stones/pebbles, were removed. After thorough mixing, a subsample of about 1 kg was taken from each pooled sample from each treatment plot and transported to the laboratory. One part of the field sample was subjected to extraction while another part was analyzed to determine the moisture content. In the end, the results of chlorpyriphos and cypermethrin residues in soil were expressed on a dry weight basis.
Extraction and cleanup
Green and red chilli
The samples were processed and analyzed at the Pesticide Residue Analysis Laboratory, Department of Entomology, Punjab Agricultural University, Ludhiana. A representative 20-g sample of chopped and macerated green or red chilli was dipped separately overnight into 100 mL acetone in an Erlenmeyer flask and kept for 24 h. The extract was filtered into a 1-L separatory funnel along with rinsing of acetone. The filtrate in the separatory funnel was diluted with 600 mL brine solution (almost saturated sodium chloride solution) and the contents partitioned two times into dichloromethane using 100 and 50 mL. The combined organic layers were passed through anhydrous sodium sulfate and collected in a 500-mL beaker. Again, the aqueous layer was partitioned twice with hexane using 100 and 50 mL each time. The upper layer of hexane was drained into a 500-mL beaker through one and half-inch layer of anhydrous sodium sulfate supported on a pre-washed glass wool in a funnel. Sodium sulfate was washed with an additional 25 mL of hexane. The extract was treated with 300 mg of activated charcoal powder for about 1 h. When the solution became clear, it was filtered through Whatman filter paper no. 1. The clear extract so obtained was concentrated using a rotary vacuum evaporator at <30 °C. The final volume was reconstituted to about 5 mL using hexane.
Soil
Soil samples (20 g) were dipped in a 100-mL mixture of methanol and water (2:1, v/v) overnight. The contents were filtered into a 1-L separatory funnel, diluted with 600 mL of 5 % sodium chloride solution, and partitioned into dichloromethane and hexane using 100 mL each time. The organic layers were combined and the residue was dissolved in hexane and estimated on a gas chromatograph using a flame thermionic detector (FTD) and an electron capture detector (ECD) for chlorpyriphos and cypermethrin, respectively.
Estimation by GLC
Analyses of chlorpyriphos and cypermethrin were carried out on a GLC (Shimadzu model GC-2010) equipped with FTD and ECD 63Ni, respectively, supplied by M/S Shimadzu (Japan).
Flame thermionic detector
Chromatographic separation in a capillary column provides good results. A capillary column, Altech EC-5 (30-m × 0.25-mm i.d. × 0.25-μm film thickness of 5 % phenyl and 95 % methyl polysiloxane, non-polar phase), was used. The flow rates of nitrogen, air, and hydrogen were 40, 100, and 60 mL min−1, respectively. The temperatures for the injector, column, and detector were maintained at 280, 220 and 300 °C, respectively.
Electron capture detector
A capillary column, Rtx-5 (30-m × 0.53-mm i.d. × 0.25-μm film thickness of 5 % phenyl and 95 % methyl polysiloxane), with a split ratio of 1:10 was used for the estimation of cypermethrin. The working conditions of GC were: injector temperature, 290 °C; column temperature, 250 °C; and detector temperature, 300 °C. Carrier gas (N2) flow was maintained at 30 mL min−1 with a split ratio of 1:10. Before use, the column was primed with several injections of a standard solution of chlorpyriphos until a consistent response was obtained. Suitable aliquots of the cleaned up samples were then injected into the ECD mode of the detector. The compound in the sample was identified and quantified by comparison of the retention time and peak heights of the sample chromatograms with those of standard runs under identical operating conditions.
Confirmation by GC-MS
Confirmation of chlorpyriphos and cypermethrin was carried out on a GC (Shimadzu 2010) coupled with a mass detector (Fisons MD-800, quadrupole mass detector) equipped with a capillary column (GC-MS-QP 2010 plus, Shimadzu, Rtx-5 Sil MS). A capillary column (30-m × 0.25-mm i.d. × 0.25-μm film thickness) was used for the confirmation of these insecticides. The confirmation of chlorpyriphos and cypermethrin was done using gas chromatography–mass spectrometry (GC-MS) in a single-ion monitoring mode. The system software used was GCMS solution, version 2.5. Helium was used as a carrier gas with a flow rate of 0.94 mL min−1. The GC-MS operating conditions for chlorpyriphos were: the injector temperature was maintained at 285 °C and the oven was in temperature programming from 80 °C (3-min hold) to 180 °C at a rate 20 °C min−1 (2-min hold) and 180 to 200 °C at a rate 5 °C min−1 (10-min hold). The GC-MS operating conditions for cypermethrin were: oven (program) initial temperature was 80 °C and held for 3 min, ramped 20 °C min−1 to 180 °C and held for 2 min, then ramped 2 °C min−1 to 190 °C, held for 2 min, then again ramped 5 °C min−1 to 280 °C, held for 10 min; injector temperature was 285 °C. Helium was used as a carrier gas with a flow rate of 0.7 mL min−1. Injection volume was 1 μL in splitless mode. Detector voltage was maintained at 0.9 kV in selective ion monitoring (SIM) mode. The samples were injected and ionized using 70 eV. The compounds were identified based on a m/z ratio of total ion chromatograph and fragmentations of SIM compared with fragmentations of different mass numbers obtained with different reference standard runs under identical conditions. The mass spectra of standard chlorpyriphos and cypermethrin showed the most abundant ions as the base peak. The molecular mass of chlorpyriphos is 350.6, which was confirmed by its ion dissociate into (m/z) 314, 286, and 197. Similarly, cypermethrin has a molecular mass of 416.0 confirmed by m/z 181 and 163. These ion values were compared with the samples spiked with chlorpyriphos and cypermethrin and the samples collected from the treated plots for the confirmation of chlorpyriphos and cypermethrin residues.
Statistical analysis
The degradation kinetics of chlorpyriphos and cypermethrin in chilli were determined by plotting residue concentrations against time, and the maximum squares of correlation coefficients found were used to determine the equations of best-fit curves. For all the samples studied, exponential relations were found to apply, corresponding to a first-order rate equation. Confirmation of the first-order kinetics was further made graphically from the linearity of the plots of C against time. The persistence of these insecticides is generally expressed in terms of half-life (t 1/2) or DT50, i.e., time for the disappearance of pesticide to 50 % of its initial concentration. The rate equation was calculated from the first-order rate equation: C t = C 0 e −kt, where C t represents the concentration of the pesticide residue (in milligrams per kilogram) at time t (in days), C 0 represents the initial concentration (in milligrams per kilogram), and k is the first-order rate constant (per day) independent of C t and C 0. The half-life (t 1/2) was determined from the k value for each experiment, being t 1/2 = ln2/k.
Results and discussion
Quality control and quality assurance of the analytical method
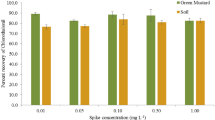
Green chilli, red chilli, and soil samples were spiked with chlorpyriphos and cypermethrin at three concentration levels (0.01, 0.05, and 0.10 mg kg−1) and analyzed as per the methodology described above to estimate the trueness of the method. Percent recoveries of chlorpyriphos and cypermethrin in green chilli, red chilli, and soil were found to be consistent and more than 85 %. Quantification was accomplished using a standard curve, prepared by diluting the stock solution. Good linearity was achieved with a correlation coefficient of 0.999 for chlorpyriphos and 0.995 for cypermethrin (Fig. 2). The precision of the method was determined by repeatability studies of the method and expressed as relative standard deviation (RSD) values. The RSD for repeatability ranged from 2.12 to 4.51 % for chlorpyriphos and fro 3.07 to 4.37 % for cypermethrin for the different spiking levels, as shown in Table 1. The percent recoveries of chlorpyriphos and cypermethrin in green chilli, red chilli, and soil were found to be consistent and more than 85 %. Therefore, the results have been presented as such without applying any correction factor.
Limit of detection and quantification
In general, the residues of chlorpyriphos and cypermethrin were determined by a comparison of the peak areas of the reference standards with those of the unknown or spiked samples run under identical working conditions of the instruments employed. For chlorpyriphos and cypermethrin, half-scale deflection was obtained for 0.5 ng, which could be easily identified from the baseline as 0.1 ng of the compound produced 10 % deflection, which can be measured. When 20 g of chilli fruit or soil was extracted, cleaned up, and the final volume made to 4 mL, 2 μL (10 mg) of the sample when injected did not produce any background interference. Thus, the limit of quantification was found to be 0.01 mg kg−1 and the limit of detection found to be 0.003 mg kg−1.
Chlorpyriphos residues on green chilli samples
The overall results of the analysis of green chilli fruits following the third application of Nurelle-D 505 (chlorpyriphos 50 % + cypermethrin 5 %) at 1 and 2 L ha−1 are presented in Table 2. The mean initial deposits of chlorpyriphos were observed to be 0.59 and 2.02 mg kg−1 on green chilli fruits following the third application with respect to chlorpyriphos at 500 and 1,000 g a.i. ha−1. These deposits dissipated to 0.27 and 0.55 mg kg−1 after 5 days at single and double dosages, respectively, thereby showing losses of about 54.24 and 72.61 %. More than 75 % of these residues got dissipated in 1 week at both these dosages. These initial residues were dissipated to 84.75 and 88.61 % at the recommended and double the recommended dosages, respectively, after 10 days of the last application of the pesticide (Table 2). The results are in agreement with those of Raina and Raina (2008) who reported the dissipation of chlorpyriphos following its application at 500 and 1,000 g a.i. ha−1 for two consecutive years, 2004 and 2005. The average initial deposits varied from 0.56 to 0.86 and from 1.29 to 1.43 mg kg−1, respectively. No residues were detected 15 days after the last application of the pesticide at both dosages. Samriti et al. (2011) reported that the average initial deposits of chlorpyriphos on okra were 0.067 and 0.129 mg kg−1 following the application of chlorpyriphos at 200 and 400 g a.i. ha−1. These residues declined below the determination limit of 0.01 mg kg−1 after the 10th and 15th day of application in single and double doses, respectively. Following spray application of ready mix formulation Action-505EC (chlorpyriphos 50 % + cypermethrin 5 %) at 400 and 800 g a.i. ha−1, chlorpyriphos on tomato showed average initial deposits of 0.117 and 0.253 mg kg−1. These residues reduced to 0.010 and 0.038 mg kg−1 on the seventh day, accounting for the loss of more than 82–91 %. The residues persisted beyond 10 and 15 days at the single and double doses, respectively (Gupta et al. 2011).
Cypermethrin residues on green chilli samples
The average initial deposits of cypermethrin on green chilli were found to be 0.32 and 0.44 mg kg−1, respectively, following three applications at an interval of 15 days of Nurelle-D 505 (a combination mixture of chlorpyriphos 50 % + cypermethrin 5 %) at 50 and 100 g a.i. ha−1 with respect to cypermethrin. The initial deposits were dissipated to 0.14 and 0.20 mg kg−1 after 3 days at the single and double dosages, respectively, thereby showing a loss of about 56.25 and 54.55 %. About 70 % of the residues of cypermethrin dissipated in 1 week after the last spray at both the dosages. Residues of cypermethrin dissipated below the determination limit of 0.01 mg kg−1 after 10 days at the recommended dose, but a 0.10 mg kg−1 residue was present after 10 days on the sample with double the recommended dose (Table 3). These results are in agreement with those of Singh et al. (1990) who studied the residues of cypermethrin on cauliflower. Following the application of cypermethrin on cauliflower crop at a rate of 50 g a.i. ha−1, the maximum initial deposits of these insecticides on the heads and leaves were 1.10 and 0.75 mg kg−1, respectively.
The initial deposit of 0.42 mg kg−1 from cypermethrin treatment at 60 g a.i. ha−1 on chickpea declined to 0.34 mg kg−1 after 2 days of its application and further dissipated to 0.11 mg kg−1 by 15 days, thereby representing losses of 19.05 and 73.81 %, respectively (Kumar et al. 1998). Khan et al (1999) reported that the initial deposit of cypermethrin on okra was 1.31 mg kg−1 following its application at 0.012 %. These residues dissipated to 0.78, 0.50, 0.17, and 0.05 mg kg−1 at 1, 3, 7, and 10 days, respectively. The average initial deposits of cypermethrin on tomato were observed to be 0.081 and 0.098 mg kg−1 following the application of Roket 44 EC (profenofos 40 % + cypermethrin 5 %) at 40 and 80 g a.i. ha−1 cypermethrin. After 15 days, the residues were detected in the case of the single-dose application sample; however, at the double dose, the residues persisted beyond 15 days. Similarly, following the application of Action-505 EC (chlorpyriphos 50 % + cypermethrin 5 %) at 40 and 80 g a.i. ha−1, cypermethrin on tomato showed average initial deposits of 0.060 and 0.086 mg kg−1. More than 80 % dissipation was recorded on the seventh day, although the residues persisted beyond 15 days at the high dose (Gupta et al. 2011).
Chlorpyriphos and cypermethrin residues on red chilli samples
After 15 days, red chilli samples were found to contain residues of chlorpyriphos at 0.08 and 0.11 mg kg−1, respectively, at the recommended and double the recommended doses (Table 2). The residues of cypermethrin in red chilli collected after 15 days of the last spray were found to be below its determination limit of 0.01 mg kg−1 at both the dosages (Table 3). The residues of fipronil and profenofos in dried red chillies (collected at harvest) were below the detectable limit (Reddy et al 2007a). Similarly, the residues of endosulfan, dicofol, dimethoate, and λ-cyhalothrin in harvested red chillies were below the detectable levels in all the treatments (Reddy et al. 2007b).
Chlorpyriphos and cypermethrin residues in soil samples
Since Nurelle-D 505 (chlorpyriphos 50 % + cypermethrin 5 %) was not directly applied to the soil, its residues were estimated only after 15 days of treatment of the crop. Residues of chlorpyriphos and cypermethrin were found to be <0.01 mg kg−1 for both these insecticides at the single and double dosages collected 15 days after the last spray (Tables 2 and 3).
Following the application of Action 505 EC (chlorpyriphos 50 % + cypermethrin 5 %) on tomato crop at a single dose, no chlorpyriphos residue was detected on soil at 0 day. At the double dose, 0.012 mg kg−1 residues were detected in soil on 0 day, which dissipated with time, and no residue was detected in the samples collected after the seventh day. Similarly, following the application of Roket 44EC (profenofos 40 % + cypermethrin 5 %) and Action-505EC on tomato crop at the recommended dose, no residue of cypermethrin was detected in soil even on 0 day. At the double dose, no residue was detected in soil when Roket 44EC was used; however, when Action-505EC was used, 0.032 mg kg−1 residues were detected in soil on 0 day, which dissipated to a non-detectable level on the seventh day (Gupta et al. 2011).
Half-life values of chlorpyriphos and cypermethrin on green chilli
The dissipation of the residues of chlorpyriphos and cypermethrin from chilli fruits did not follow first-order kinetics. The correlation coefficient varied from 0.889 to 0.973 (Figs. 3 and 4). The half-life value (t 1 /2) is usually defined as the time required for half the given quantity of material to dissipate (Gunther and Blinn 1955). The t 1/2 values of chlorpyriphos and cypermethrin were calculated using Hoskins’ (1961) formula. The half-lives (t 1/2) of chlorpyriphos on chilli were observed to be 4.43 and 2.01 days, respectively, when applied at 500 and 1,000 g a.i. ha−1 (Table 2 and Fig. 3). The half-lives (t 1/2) of cypermethrin on chilli were observed to be 2.51 and 2.64 days, respectively, following the application of cypermethrin at 50 and 100 g a.i. ha−1 (Table 3 and Fig. 4). The half-life values of chlorpyriphos on cauliflower varied from 1.4 to 1.5 days and from 1.5 to 1.6 days for the single (500 g a.i. ha−1) and double doses (1,000 g a.i. ha−1), respectively (Raina and Raina 2008). The half-life values of chlorpyriphos on okra fruits were 3.25 and 3.46 days following application at 200 and 400 g a.i. ha−1 (Samriti et al. 2011). The half-life value of cypermethrin on okra fruits was 2.25 days following the application of cypermethrin at 0.012 % at 10-day interval initiating from 30 days after showing (Samriti et al. 2011), and the half-life values of cypermethrin on chickpea green pods were 8.36 and 9.40 days following the application of cypermethrin at 60 and 90 g a.i. ha−1, respectively (Kumar et al. 1998).
Risk assessment of chlorpyriphos and cypermethrin on green chilli
Human health risk situations are a function of hazard and exposure to that hazard. Theoretical maximum residues contribution (TMRC) values were calculated and compared with the maximum permissible intake (MPI) to evaluate the risk to the consumer of chlorpyriphos and cypermethrin on chilli. The prescribed acceptable daily intake (ADI) values of chlorpyriphos and cypermethrin are 0.01 and 0.05 mg/kg body weight per day, respectively (Sharma 2007). MPI was obtained by multiplying the ADI with the weight of an average Indian person (55 kg; Mukherjee and Gopal 2000). MPI was calculated to be 0.55 and 2.75 mg per person per day. Taking 2.5 g as the chilli consumption for an Indian balanced diet (Anonymous 2002) and maximum residues of chlorpyriphos on chilli, the TMRC values on 0 day are found to be 0.0015 and 0.0056 mg per person per day in the case of the recommended dose (500 g a.i. ha−1) and the double the recommended dose (1,000 g a.i. ha−1), respectively. The TMRC values of cypermethrin on chilli on 0 day are found to be 0.00085 and 0.0013 mg per person per day in the case of the single dose (50 g a.i. ha−1) and the double dose (100 g a.i. ha−1), respectively. These values are significantly quite low as compared to the MPI; hence, the insecticide will not cause adverse effect after consumption of such green chilli fruits. The TMRC values were also found to be well below the MPI, even if double doses were considered. As the theoretical maximum residue contributions on chilli fruits are found to be less than the toxicological estimated MPI values of 0.55 and 2.75 mg per person per day, respectively, consumer health risks are minimal at the recommended dose on chilli. These studies, therefore, suggest that the use of chlorpyriphos and cypermethrin at the recommended dosages does not seem to pose any hazards to the consumers; a waiting period of 1 day is suggested to reduce the risk before consumption of green chilli. These results are in agreement with those of Gupta et al. (2011) who suggested a 1-day waiting period for the safe consumption of tomato fruits following the application of Roket 44 EC (profenofos 40 % + cypermethrin 5 %) and Action-505EC (chlorpyriphos 50 % + cypermethrin 5 %). These findings are in close conformity with the findings of Singh et al. who reported that the maximum initial deposits of cypermethrin on cauliflower leaves were less than their respective MRL value of 1.0 mg kg−1 prescribed for Brassica leafy vegetables; it took 1 day for their residues on cauliflower heads to decline below this level.
Conclusions
The half-life values for chlorpyriphos following three applications at the recommended and double the recommended dosages on chilli fruits were observed to be 4.43 and 2.01 days, respectively, whereas in the case of cypermethrin, these values were 2.51 and 2.64 days. The TMRC values were quite low as compared to the MPI; hence, the insecticide will not cause adverse effects after consumption of such green chilli fruits. A waiting period of 1 day is suggested to reduce the risk before consumption of green chilli fruits. Therefore, application of the chlorpyriphos and cypermethrin mixture at the recommended dose on chilli is quite safe from crop protection and environmental contamination points of view and consumption by the consumer.
References
Anonymous. (2000). Chlorpyriphos. Office of Prevention, Pesticide and Toxic Substance, US Environmental Protection Agency Washington, DC, June 20.
Anonymous. (2002). Opinion of the Scientific Committee on Food on capsaicin. European Commission Health & Consumer Protection Directorate-General. http://ec.europa.eu/food/fs/sc/scf/out120_en.pdf. Accessed 15 May 2012.
Anonymous. (2010) Package of practices for cultivation of vegetables. Punjab Agricultural University, Ludhiana, India, pp. 41–43.
Chahal, K. K., Singh, B., Kang, B. K., Battu, R. S., & Joia, B. S. (1997). Insecticide residues in farm gate vegetable samples in Punjab. Pesticide Research Journal, 9, 256–260.
Chahal, K. K., Singh, B., Battu, R. S., & Kang, B. K. (1999). Monitoring of farmgate vegetables for insecticide residues in Punjab. Indian Journal of Ecology, 26, 50–55.
Chaudhary, B. (2000). Solanaceous fruits and cole crops vegetables (pp. 63–84). India: National Book Trust.
Cochran, R. C., Kishiyama, J., Aldous, C., Carr, W. C., & Pfeifer, K. F. (1995). Chlorpyriphos: hazard assessment based on a review of the effects of short-term and long-term exposure in animals and humans. Food and Chemical Toxicology, 33, 165–172.
Gunther, F. A., & Blinn, R. C. (1955). Analysis of insecticides and acaricides. New York: Interscience Publishers. 696 pp.
Gupta, S., Gajbhiye, V. T., Sharma, R. K., & Gupta, R. K. (2011). Dissipation of cypermethrin, chlorpyriphos, and profenofos in tomato fruits and soil following application of pre-mix formulations. Environmental Monitoring and Assessment, 174, 337–345.
Hoskins, W. M. (1961). Mathematical treatments of loss of pesticide residues. FAO Plant Protection Bulletin, 9, 163–168.
Jee, J. H., Masroor, F., & Kang, J. C. (2005). Responses of cypermethrin-induced stress in haematological parameters of Korean rockfish, Sebastes schlegeli (Hilgendorf). Aquatic Research, 36, 898–905.
Khan, M. A. M., Reddy, D. J., & Rao, S. V. (1999). Dissipation of cypermethrin residues in okra fruits. Pesticide Research Journal, 11, 84–85.
Kumar, P., Singh, S. P., & Tanwar, R. S. (1998). Dissipation of cypermethrin residue on chickpea. Pesticide Research Journal, 10, 242–245.
Kumar, K. P., Reddy, D. J., Reddy, K. N., Babu, T. R., & Narendranath, V. V. (2000). Dissipation and decontamination of triazophos and acephate residues in chilli (Capsicum annum Linn). Pesticide Research Journal, 12, 26–29.
Lindsay, D. G. (1997). Pesticide residue in food: the need for fairer cost–benefit analysis. Pesticide Outlook, 8, 6–10.
Mukherjee, I., & Gopal, M. (2000). Environmental behaviour and translocation of imidacloprid in eggplant, cabbage and mustard. Pest Management Science, 56, 932–936.
Oliver, G. R., Bolles, H. G., & Shurdut, B. A. (2000). Chlorpyriphos: probabilistic assessment of exposure and risk. Neurotoxic, 21, 203–208.
Puttarudraih, M. (1959). Short review on the chilli leaf curl complex and spray programme for its control. Mysore Journal of Agricultural Science, 34, 93–95.
Raina, A. K., & Raina, M. (2008). Dissipation of chlorpyriphos on cauliflower (Brassica oleracea L. var. botrytis). Pesticide Research Journal, 20, 263–265.
Rao, C. S., Bour, T. B., & Reddy, K. N. (2005). Pesticide residues: impact on Indian Agriculture exports in WTO era. Proceedings of the National Symposium on Pesticide Residues and Their Risk Assessment, January 20–21, 2005, pp. 54–57.
Reddy, D. N. R., & Puttaswamy, M. (1983). Pests infesting chilli in the transplanted crop. Mysore Journal of Agricultural Science, 17, 246–251.
Reddy, D. N. R., & Puttaswamy, M. (1984). Pests infesting chilli (Capsicum annum L.) in nursery. Mysore Journal of Agricultural Science, 18, 122–125.
Reddy, K. D., Reddy, K. N., & Mahalingappa, P. B. (2007a). Dissipation of fipronil and profenofos residues in chillies (Capsicum annum L.). Pesticide Research Journal, 19, 106–107.
Reddy, K. N., Satyanarayana, S., & Reddy, K. D. (2007b). Persistence of some insecticides in chillies. Pesticide Research Journal, 19, 234–236.
Regupathy, A., Ramasubramanian, T., & Ayyasamy, R. (2004). Rationale behind the use of insecticide mixtures for the management of insecticide resistance in India. International Journal of Food Agriculture Environment, 2, 278–284.
Samriti, Chauhan, R., & Kumari, B. (2011). Persistence and effect of processing on reduction of chlorpyriphos residues in okra fruits. Bulletin of Environmental Contamination and Toxicology, 87, 198–201.
Shah, P. G., Raj, M. F., Patel, B. A., Patel, B. K., Diwan, K. D., Patel, J. A., & Talati, J. G. (2000). Pesticidal contamination status in farm gate vegetables in Gujarat. Pesticide Research Journal, 12, 95–99.
Sharma, K. K. (2007). Pesticide residue analysis manual. Directorate of Information and Publications of Agriculture, Indian Council of Agricultural Research, New Delhi, 294 pp.
Singh, P. P., Singh, B., & Battu, R. S. (1990). Residues of cypermethrin, fenvalerate and deltamethrin on cauliflower. Phytoparasitica, 18, 153–158.
Singh, B., Gupta, A., Bhatnagar, A., & Parihar, N. S. (1999). Monitoring of pesticide residues in farm gate samples of chillies. Pesticide Research Journal, 11, 207–209.
Worthing, C. R. (1987). Pesticide manual. A world compendium. UK: British Crop Protection Council. 1081 pp.
Acknowledgments
The authors are thankful to the Professor and Head of the Department of Entomology, PAU, Ludhiana, for providing the necessary research facilities. Financial assistance provided by the Indian Council of Agricultural Research (ICAR), New Delhi. India, is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jyot, G., Mandal, K., Battu, R.S. et al. Estimation of chlorpyriphos and cypermethrin residues in chilli (Capsicum annuum L.) by gas–liquid chromatography. Environ Monit Assess 185, 5703–5714 (2013). https://doi.org/10.1007/s10661-012-2977-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2977-2