Abstract
In regions with high livestock densities, the usage of antibiotics and metals for veterinary purposes or as growth promoters poses a risk in manured soils. We investigated to which degree the concentrations and depth distributions of Cu, Zn, Cr and As could be used as a tracer to discover contaminations with sulfonamides, tetracyclines and fluoroquinolones. Besides, we estimated the potential vertical translocation of antibiotics and compared the results to measured data. In the peri-urban region of Beijing, China, soil was sampled from agricultural fields and a dry riverbed contaminated by organic waste disposal. The antibiotic concentrations reached 110 μg kg−1 sulfamethazine, 111 μg kg−1 chlortetracycline and 62 μg kg−1 enrofloxacin in the topsoil of agricultural fields. Intriguingly, total concentrations of Cu, Zn, Cr and As were smaller than 65, 130, 36 and 10 mg kg−1 in surface soil, respectively, therewith fulfilling Chinese quality standards. Correlations between sulfamethazine concentrations and Cu or Zn suggest that in regions with high manure applications, one might use the frequently existing monitoring data for metals to identify potential pollution hotspots for antibiotics in topsoils. In the subsoils, we found sulfamethazine down to ≥2 m depth on agricultural sites and down to ≥4 m depth in the riverbed. As no translocation of metals was observed, subsoil antibiotic contamination could not be predicted from metal data. Nevertheless, sulfonamide stocks in the subsoil could be estimated with an accuracy of 35–200 % from fertilisation data and potential leaching rates. While this may not be sufficient for precise prediction of antibiotic exposure, it may very well be useful for the pre-identification of risk hotspots for subsequent in-depth assessment studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Veterinary antibiotics are administered for medical purposes, but in some countries (e.g. USA, Brazil, India) they are also used as growth promoters in animal husbandry (WHO 2001). Despite a ban on the use of veterinary antibiotics as feed additives in China, some of these substances are applied on a large scale in subtherapeutic doses (Zhu et al. 2013). Due to their pharmacokinetic properties, antibiotics are excreted to a major percentage and accumulate in animal waste, as was frequently documented by analysis of manure samples (e.g. Hu et al. 2010; Chen et al. 2012). Heavy metals, especially Cu and Zn, also exhibit growth-promoting properties and are therefore often added to animal fodder (Nicholson et al. 1999; Luo et al. 2009), and thus accumulated in manure (Cang et al. 2004; Xiong et al. 2010). Besides, there also is evidence for the usage of Cr and As as feed additives in China (Zhang et al. 2005). Hence, regions with intensive livestock farming and excessive disposal of animal wastes on arable land face the risk of soil contamination with veterinary antibiotics and several metals. The concomitant application of antibiotics and heavy metals suggests that more readily available data sets of heavy metal concentrations in soils with organic fertilisation background could possibly be used to identify potential hotspots of antibiotic contamination. If true, this would reduce the cost and time-intensive antibiotic analyses, thereby greatly accelerating environmental risk assessment studies.
In the peri-urban districts of the Beijing Municipality, P.R. China, intensive animal farming results in high animal densities in relation to the area of agricultural land (Heimann 2013; Roelcke et al. 2013). Thus, the available arable land receives a severe surplus of nutrients from organic fertilisers and surface water bodies are contaminated by the disposal of excessive amounts of organic residues (Kamphuis et al. 2004). Considering that leaching experiments have documented the mobility of various classes of antibiotics in soil (Boxall et al. 2002; Golet et al. 2003; Kay et al. 2004, 2005; Ostermann et al. 2013), a major threat in areas influenced by intensive animal husbandry is the contamination of ground- and drinking water with antibiotic substances (Hu et al. 2010; Hirsch et al. 1999; Tong et al. 2009). Similar risks arise from Cu and Zn, which have been detected in drainage water in column experiments (Kaschl et al. 2002; Zhao et al. 2009) and under field conditions (McBride et al. 1999; Aldrich et al. 2002). Despite potential leaching losses, strong sorption of pharmaceuticals and metals (Higgins 1984; Welp and Brümmer 1999) can lead to an accumulation in agricultural soils (Gupta and Charles 1999; Dalkmann et al. 2012). Yet, combined investigation of leaching and accumulation of antibiotics in agricultural soils is still scarce (Aust et al. 2008; Tai et al. 2010, 2011; Tamtam et al. 2011; Huang et al. 2013; Wu et al. 2013) and seldom includes the class of sulfonamides or the simultaneous assessment of heavy metals (Aust et al. 2008; Tamtam et al. 2011).
The objective of our study was to quantify concentrations of antibiotics, heavy metals and As in top- and subsoils of different agricultural management systems in a Chinese peri-urban region characterised by high livestock densities. In total, 12 important veterinary antibiotics of the classes sulfonamides, tetracyclines and fluoroquinolones, known to exhibit different sorption strength, as well as the growth-promoting metals Cu, Zn and Cr, and the metalloid As were investigated. We hypothesised (i) that the combined usage of organic and inorganic growth promoters enables us to use elevated metal and metalloid concentrations as proxy for a potential contamination with antibiotics, (ii) that after excessive manure applications even strongly sorbing substances are leached, and (iii) that subsoil stocks of antibiotics can be deduced from fertilisation data and average leaching rates.
Materials and methods
Study region, soil sampling, basic soil characterisation
The Shunyi District is situated in the peri-urban area of the Beijing Municipality, P.R China, and is a major food production base for the Beijing market especially regarding the supply with pigs and ducks (Kamphuis et al. 2004). The agricultural land area of approximately 40,000 ha is mainly cultivated with wheat, maize, open field vegetables and fruit trees. The intensive production of animal products in the district results in a mean livestock density of 10.6 livestock units ha−1 (Hou et al. 2012). The mean annual precipitation is about 625 mm, 75 % of which about occurs in summer (June–August); the mean annual temperature is 11.5 °C (Kamphuis et al. 2004). In March 2009, 24 agricultural field sites including different crop rotations and fertilisation treatments were sampled in this area and a neighbouring district (approx. 39°56 N, 118° 20 E, Table 1); two of these sites were poplar plantations that had not been fertilised for at least 9 years and were sampled as control plots (Table 1). Soil samples were taken in depth intervals of 0–20, 20–60, 60–90, 90–120, 120–160 and 160–200 cm. Four to six subsamples were taken on a subsection of each field with horizontal distances between corings ranging from 5 to 20 m (depending on field size) and pooled to a composite sample. The dominating soil types in the experimental region were Eutric Cambisols (Heimann 2013) with slightly calcaric properties in the topsoil and often calcic properties in the subsoil. The pH values in the topsoil ranged from 5.9 to 7.5 (0.01 M CaCl2). The texture in the topsoil (typically 20 cm in this area) ranged from sandy loam to loam, with silt loam being the dominating texture. In the subsoil, loam and silt textures also dominated; however, here occasionally also pure sand was found (Heimann 2013). Detailed soil properties are listed in Online Resource 1. In addition to the agricultural fields, a site adjacent to a small stream in a dry riverbed was sampled as potential hotspot of contamination, as large volumes of effluent from a pig farm and its biogas plant had been discarded into this river over the past 20 years. Here, a single coring was carried out and sampled to a maximum depth of 4 m in 11 intervals (0–20, 20–50, 50–90, 90–130, 130–170, 170–210, 210–250, 250–290, 290–340, 340–370, 370–400 cm). To avoid cross-contamination between sampling depths, the auger was cleaned thoroughly between different depth intervals and only the inner part of the drilled soil core was used for analyses. Samples were transported in cooled boxes on ice immediately after their collection. Aliquots for the extraction of antibiotics were stored at −20 °C and lyophilised before extraction. Aliquots for the basic characterisation and extraction of metals and As were air-dried and sieved to a particle size of less than 2 mm.
A basic soil characterisation was carried out using aliquots of air-dried samples (≤2 mm), including soil texture (ISO 11277 2002), pH value (0.01 M CaCl2, glass electrode WTW 330i, SEN Tix 21, WTW Werkstätten GmbH, Weilheim, Germany) and carbonate content (ISO 10693 1994).
Extraction and analyses of metals and As
Copper, Zn, Cr and As concentrations extractable with both EDTA (Quevauviller et al. 1996) and aqua regia (ISO 11466 1995) were determined for all topsoil samples, and additionally down to the maximum sampling depth for those five agricultural sites with the highest topsoil concentrations (2 m), as well as for the contaminated riverbed (4 m). Quantification of these elements was done with ICP-OES (Horiba Jobin Yvon Model 75 Plus, Unterhaching, Germany).
Extraction of antibiotics
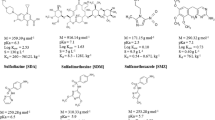
Standards of the investigated substances chlortetracycline, oxytetracycline, tetracycline, difloxacin (all in form of hydrochloride), enrofloxacin, sulfathiazole, sulfamethoxypyridazine, sulfadimethoxine, sulfamethoxazole, sulfadiazine and sulfamethazine were purchased from Sigma-Aldrich (Schnelldorf, Germany) while 4-epi-oxytetracycline, 4-epi-tetracycline, 4-epi-chlortetracycline and doxycycline (the latter three as hydrochloride) were obtained from Fisher Scientific (Schwerte, Germany). 13C6-Sulfadiazine and 13C/15N-difloxacinhydrochloride (Institute of Environmental Biology and Chemodynamics of RWTH Aachen University), 13C6-sulfamethazine, 13C6-sulfamethoxazole (Cambridge Isotope Laboratories, Saarbrücken, Germany) and D5-enrofloxacin (Sigma-Aldrich, Schnelldorf, Germany) served as internal standards. All substances had a declared purity of >95 %. Internal standard solution was prepared in acetonitrile at a concentration of 1 ng ml−1 and stored in the dark at −20 °C. During extraction, epimerisation of tetracyclines was likely; thus, when tetracycline, chlortetracycline and oxytetracycline concentrations are mentioned in the following, these concentrations refer to the sum of the original substance and the associated epimers. The 100 mg l−1 stock solution of the tetracyclines and their epimers in methanol with 0.5 % formic acid was stored at −20 °C.
The extraction of sulfonamides was carried out according to Förster et al. (2008) with 25 ml acetonitrile/Millipore water 1:4 (v/v) and 5 g of soil in a microwave oven at 150 °C (500 W) for 15 min. For topsoil samples, an aliquot of the supernatant was centrifuged (15,000×g, 20 min) to sediment particles before analysis. The limit of quantification (LOQ) for topsoil was 5 μg kg−1 dry soil. On 11 field sites with high topsoil concentrations, subsoil samples were analysed additionally. A cleanup and pre-concentration of extracts by solid phase extraction was added as extra step for the subsoil samples due to expected smaller concentrations compared to the topsoil. This improved the quantification limit to 0.4 μg kg−1. Prior to solid phase extraction, the microwave extract was centrifuged at room temperature (2,500×g, 20 min) to prevent the clogging of cartridges. Following a dilution of the extract to 500 ml with Millipore water to reduce the acetonitrile content to <1 %, a pH value of 4 was adjusted with formic acid and sodium hydroxide. For solid phase extraction, a Chromabond SB cartridge (1000 mg, Macherey & Nagel, Düren, Germany) was used for removal of interfering matrix components, followed in tandem by an Oasis hydrophilic-lipophilic-balanced reversed-phase (HLB) cartridge (200 mg, Waters, Eschborn, Germany). Cartridges were pre-conditioned with 6 ml methanol (pH 2.5) followed by 6 ml Millipore water (pH 2.5), before addition of the diluted and acidified extracts. Cartridges were air-dried under negative pressure for 30 min before discarding the Chromabond anion exchange cartridge in order to assure the complete amount of extract being loaded onto the SB cartridge. Elution of the HLB cartridges was carried out with 6 ml methanol. The volume of the eluate was reduced to 0.5 ml by rotary evaporation at 40 °C and the weight was determined after dilution to approximately 1 ml with acetonitrile/Millipore 1:4 (v/v). Finally, 50 μl of internal standard solution was added and the sample stored at −20 °C until analysis.
Tetracyclines were extracted by pressurised liquid extraction (ASE 350 system, Dionex, Idstein, Germany) using the method of Jacobsen et al. (2004) with slight modifications. Stainless steel extraction cells (34 ml) were filled from bottom to top with (i) methanol-rinsed cellulose filters (Dionex, Idstein, Germany), (ii) glass wool (Labomedic, Bonn, Germany) and (iii) 5 g of lyophilised soil mixed with 5 g of acid-washed sea sand (VWR, Langenfeld, Germany). A 1:1 (v/v) mixture of methanol and 0.2 M citric acid with a pH of 4.7 adjusted with sodium hydroxide was used for extraction in two cycles (10 and 3 min static extraction) at 10,340 kPa and room temperature. Due to a lack of appropriate internal tetracycline standards, the method of standard addition (see Eq. 1) was applied for quantification of tetracyclines to overcome observed matrix effects. To this end, 25 μl of standard solution (1 ng μl−1) was added to one of two aliquots, which were then analysed on the same day without further treatment.
Fluoroquinolones were also extracted by pressurised liquid extraction following the method of Golet et al. (2002) with slight modifications. Extraction cells (34 ml) were filled with methanol-rinsed cellulose filters and 5 g of lyophilised soil mixed with 5 g of acid-washed sea sand. After five extraction cycles (10 min static extraction time and 180 s nitrogen purge), 50 ng of internal standard solution was added to 950 μl aliquot of the extractant. Samples were stored frozen and received no further treatment until measurement. The LOQ for tetracyclines and fluoroquinolones was 15 μg kg−1.
The recoveries of all soil extraction methods were determined by thoroughly mixing air-dried soil samples with defined antibiotic amounts and extracting the antibiotics with the respective methods 24 h after application. For sulfonamides, recoveries of 69 % (sulfathiazole), 73 % (sulfadiazine), 76 % (sulfamethazine), 80 % (sulfamethoxazole), 84 % (sulfamethoxypyridazine) and 86 % (sulfadimethoxine) were achieved. The recoveries for tetracyclines were 25 % for oxytetracycline, 33 % for chlortetracycline, 37 % for doxycycline and 47 % for tetracycline, while for fluoroquinolones the recovery ranged from 41 % (enrofloxacin) to 73 % (difloxacin).
Analyses and quantification of antibiotics
For separation and quantification of the antibiotic concentrations, a HPLC (Surveyor autosampler plus and Surveyor MS pump plus, Thermo Scientific, Dreieich, Germany) coupled with a tandem mass spectrometer (TSQ Quantum Ultra, Thermo Scientific, Dreieich, Germany) was used. Tetracyclines were ionised by electro spray ionisation (ESI), sulfonamides by an atmospheric pressure chemical ionisation source (APCI) and fluoroquinolones by heated electro spray ionisation (HESI), all run in the positive ion mode and with an injection volume of 10 μl. Sulfonamides were separated using a Nucleodur Sphinx RP column (3 μm, Macherey & Nagel, Düren, Germany) at a temperature of 15 °C, with mobile phases chosen according to Jacobsen et al. (2004). The mobile phases A (Millipore water/methanol—1:20 (v/v) with 0.5 % formic acid), B (methanol/Millipore water—1:20 (v/v) with 0.5 % formic acid) and C (acetonitrile with 0.1 % formic acid) were run with a flow of 300 μl min−1 from 100 % B to 95 % A and 5 % C during the first 22 min, then back to 100 % B within 4 min, which was kept constant for 4 min. Column conditions and mobile phases were the same for tetracyclines, but the gradient was run from 100 % B to 95 % A and 5 % C within 16 min and then back to 100 % B in 10 min, which stayed constant for 4 min. Fluoroquinolones were separated with an XBridge C18 column (3.5 μm, Waters, Eschborn, Germany). Methanol and Millipore water, each with 0.1 % formic acid, served as mobile phases D and E. The gradient was run from 5 % D and 95 % E to 60 % D within 5 min, increasing to 80 % D in a further 10 min and then reaching 95 % within one more minute. This composition stayed constant for 50 s and re-changed to initial percentages within 10 s, which was then run for the last 8 min.
For the analysis of sulfonamides, the APCI and MS system was set to a spray voltage of 4,000 V and sheath and auxiliary gas pressures of 25 and 20 arbitrary units, respectively. Optimum temperatures for vaporiser and capillary were 355 and 200 °C, respectively. Argon served as collision gas with a pressure of 0.25 Pa. Tetracyclines were ionised under a spray of 3,500 V, vaporiser and capillary temperatures both reached 300 °C, and a sheath gas of 30 arbitrary units was applied. The collision pressure was set to 0.21 Pa.
Sulfonamide and fluoroquinolone concentrations were quantified by the response ratio of the study compound and the internal standards. 13C6-Sulfadiazine served as internal standard for sulfadiazine and sulfathiazole, 13C6-sulfamethazine as standard for sulfamethazine, 13C6-sulfamethoxazole as standard for sulfamethoxazole, sulfadimethoxine and sulfamethoxipyridazine, while 13C/15N-difloxacinhydrochloride and D5-enrofloxacin served as internal standards for quantification of difloxacin and enrofloxacin, respectively. An external standard sequence with concentrations of 1–75 ng ml−1 for sulfonamides and 1–100 ng ml−1 for fluoroquinolones was used. As pre-tests showed that the standard sequence for tetracyclines was linear in the relevant concentration range, their concentrations were quantified by the method of single point standard addition according to the following Eq. (1):
where y 0 is the unknown concentration of the sample (ng vial−1), X is the amount of standard added (25 ng vial−1), PA(sample) is the peak area of the sample and PA(standard) is the peak area of the sample with standard addition.
The following procedure for data analysis for tables and figures was applied: arithmetic means of antibiotic concentrations were calculated in case that all analytical replicates were higher than the limit of quantification. If all replicates were smaller than the limit of quantification, this was depicted as <LOQ (e.g. <5 μg kg−1, for sulfonamides) in tables. If one or two of the analytical replicates were smaller than the limit of quantification, this was presented as <maximum value measured of the analytical replicates. For the calculation of mean values in figures, only those analytical replicates that exceeded the LOQ were considered.
Prediction of sulfamethazine subsoil stocks, statistics
To estimate sulfamethazine stocks (SMZ(subsoil stock)) in microgram per square metre surface area and 20–200 cm depth from organic fertiliser application and potential leaching rates, we applied the following equation:
where SMZ(manure concentration) and the leaching rate are results from a previous field lysimeter study carried out in the same region (Ostermann et al. 2013): The average sulfamethazine concentration in fresh matter pig manure was found to be 3,800 μg kg−1 and of the applied sulfamethazine an average rate of 1.3 % was leached below 20 cm depth. The information about the annually applied manure amount (fertiliser(amount)) and the number of years with this fertilisation practice (fertilisation(duration)) were derived from a farmers’ survey carried out for the sampled sites. Degradation rates were not included in the equation as these are usually unknown under field conditions due to unknown formation of non-extractable and bound residues. Instead, we considered the dissipation rate, which is integral part of the leaching rate. Since the leaching rate was determined under field conditions, it effectively accounted for the dissipation of antibiotics during the soil passage after manure application.
Due to non-linearity of the data, a Spearman rank correlation test was carried out with the Statistica 8.0 software package (Statsoft, Hamburg, Germany) to elucidate correlations between the metal contents and the antibiotic concentrations in the topsoil.
Results and discussion
Topsoil concentrations of antibiotics in different agricultural management systems
All three investigated classes of antibiotics were found on the field sites. From the class of sulfonamides, sulfamethazine and sulfadiazine were detected, from the class of tetracyclines, chlortetracyline and oxytetracycline, and the fluoroquinolone, enrofloxacin were also frequently detected (Table 1). On 58 % of the field sites, at least one antibiotic substance was found, with sulfonamides being detected most frequently (on 46 % of all fields), typically in the lower microgram per kilogram range (Table 1). Other sites in China investigated by Li et al. (2011) exhibited distinctly higher contaminations, with at least one tetracycline, two sulfonamides and three quinolones present in the topsoil of all of the fields, most of which receiving manure as fertiliser. Chen et al. (2012) equally detected several antibiotic substances in all of their investigated fields. In contrast to other antibiotics screening studies, one third of our sampled field sites only received mineral fertilisers or industrially produced organic fertilisers, thus explaining why we found antibiotics less frequently.
The type of fertilisation had a strong influence on the occurrence and concentrations of antibiotics in the topsoil (Fig. 1). All fields receiving pig manure, chicken manure or both invariably contained at least one antibiotic substance in contrast to fields receiving other fertiliser types (Table 1). This was in accordance with the prevalence of antibiotics in animal wastes. Wei et al. (2011) detected antibiotics in wastewater from pig husbandry facilities with a higher frequency than in wastewater from cattle or chicken facilities, while Zhao et al. (2010) observed higher sulfonamide concentrations in chicken manure than in cattle or pig manure. Our study did not only reveal a widespread soil contamination as a consequence of fertilisation with pig and chicken manure but also the highest concentrations were detected in these fields (110 μg kg−1 sulfamethazine, 111 μg kg−1 chlortetracycline and 62 μg kg−1 enrofloxacin) (Table 1). The high concentration levels can be traced back to the high manure application rates typical for this region, especially in vegetable cultivation (Hou et al. 2012; Heimann 2013) (Table 1).
Distribution of sulfamethazine (SMZ), sulfadiazine (SDZ), chlortetracycline (CTC), oxytetracycline (OTC) and enrofloxacin (ENRO) concentrations in the topsoil of 24 agricultural fields receiving pig and/or chicken manure (top; n = 10) or other fertilisers (cattle or sheep manure, mineral fertiliser or no fertilisation) (bottom; n = 14). The boxes represent 25th and 75th percentile with a line showing the median when possible and whiskers representing the 10th and 90th percentile. For calculation of mean values, only data that exceeded the limit of quantification were considered here
In one case, the sulfamethazine concentration, and, in two cases, the chlortetracycline concentrations in topsoils exceeded the trigger value of 100 μg kg−1 for a detailed environmental risk assessment during the registration process of veterinary medicals (EMEA 2008). Li et al. (2011) found similar maximum concentrations for sulfamethazine and chlortetracycline in Chinese vegetable fields, whereas their maximum enrofloxacin concentrations were much higher than ours and reached 1,348 μg kg−1. In contrast, Hu et al. (2010) found distinctly higher tetracycline concentrations (1,079 μg kg−1), much lower sulfonamide concentrations and fluoroquinolone concentrations in the same range as our findings for sites in northern China. These differences probably resulted from regional variations concerning types and intensities of antibiotic applications, as becomes clear from manure analyses in different regions of China (Tong et al. 2009; Zhao et al. 2010; Wei et al. 2011). Altogether, these studies substantiate the risk of high antibiotic concentrations on agricultural fields, but they also corroborate that there is quite a pronounced spatial heterogeneity among plots, depending on fertilisation history.
Enrofloxacin was also detected on two fields that had exclusively received mineral fertiliser for at least five years (Plot 4 and 7); sulfamethazine was detected on one site that had not been fertilised during the last nine years (Plot 2) (Table 1). These findings point to the potential persistence of these compound classes in the soil environment (e.g., Förster et al. 2009; Rosendahl et al. 2012) and imply a long-term impact of applications of manure contaminated with antibiotics.
To scan for maximum pollution loads, soil of a dry riverbed that had received a constant influx of pig farm and biogas plant effluent for the past 20 years was sampled as potential local contamination hotspot. This site exhibited high sulfamethazine (844 μg kg−1) and chlortetracycline (2,375 μg kg−1) concentrations in 0–20 cm. The chlortetracycline concentrations exceeded the critical trigger value for detailed environmental risk assessment by a factor greater than 20. Sulfadiazine, oxytetracycline, enrofloxacin, sulfamethoxypyridazine, tetracycline and difloxacin were also found, but their concentrations did not reach the critical trigger value. The finding of the latter three substances indicates that the upstream river had probably received manifold farm wastewaters in addition to the biogas plant effluent of the experimental pig farm, as these substances did not occur in any other soil sampled in the investigated region.
Heavy metals as proxies for antibiotic loads in the surface soils
The sites containing antibiotics also displayed significant amounts of heavy metals. Total Cu concentrations in topsoils ranging from 12 to 65 mg kg−1 (mean = 22.9 mg kg−1) and Zn concentrations ranging from 56 to 130 mg kg−1 (mean = 74.7 mg kg−1) (Online Resource 2) were well in line with other findings on agricultural sites in China (Huang and Jin 2008; Teng et al. 2010; Fang et al. 2011). EDTA-extractable concentrations of Cu ranged from 3.2 to 45.6 mg kg−1 and of Zn from 1.4 to 74.5 mg kg−1. The higher total concentrations of Cu and Zn were found on the open-field vegetable sites receiving high amounts of manure (Table 1), which was in agreement with findings by Cang et al. (2004) and Luo et al. (2009), who documented the usage of these feed additives in Chinese pig and chicken husbandry. The concentration ranges observed for Cr and As were less wide with 22–36 mg kg−1 soil (Cr) and 4–10 mg kg−1 (As); the observed As concentrations were distinctly lower than on other agricultural sites where regular manure application resulted in a significant increase (Gupta and Charles 1999).
The Chinese environmental quality standard for soils (GB15618-1995, described by Huang and Jin 2008) was consulted to evaluate the environmental risk associated with the observed metal and As concentrations. This quality standard defines total concentrations of 35 mg kg−1 Cu, 100 mg kg−1 Zn, 90 mg kg−1 Cr and 15 mg kg−1 As also extracted with aqua regia as background values for natural soils. The concentration of total Cu in the agricultural topsoils exceeded the “natural background concentration” at 4 of the 24 investigated sites (17 %), for the concentration of Zn the background value was exceeded at three sites (13 %) (Online Resource 2). Neither for Cr nor for As did the concentrations observed in the fields reach or exceed the respective background values (Online Resource 2), suggesting that the application of Cr and As as growth promoter with the animal feed was not common in the investigated region. The second criterion of this Chinese quality standard, which ensures the safety for agricultural production (total concentrations of Cu: 50 mg kg−1 at pH <6.5 and 100 mg kg−1 at pH >6.5; Zn: 200 mg kg−1 at pH <6.5, 250 mg kg−1 at pH 6.5–7.5, and 300 mg kg−1 at pH >7.5), was not reached (Huang and Jin 2008). For EDTA-extractable fractions, no standardised threshold values exist, which limits the evaluation of these results. Total concentrations indicate that environmental risks associated with the use of heavy metals were obviously lower than those associated with antibiotics on the investigated sites. The dry riverbed again formed an exception. Here, also the topsoil concentration of Cu (287 mg kg−1) exceeded the second quality criterion and that of Zn (820 mg kg−1 Zn) even the third criterion (500 mg kg−1) by far. Equally, EDTA-extractable concentrations were much larger than for the other sites reflecting the massive anthropogenic input of organic wastes (Fig. 2). Elevated contents of Cr and As were again not observed. We therefore focused on correlating antibiotic concentrations with those of Cu and Zn for testing the suitability of elevated heavy metal concentrations as proxy to detect antibiotic contaminations.
Relationship between sulfamethazine and total and EDTA-extractable Cu or Zn concentrations in the topsoil of 24 agricultural field sites in peri-urban Beijing, China. Vertical lines represent the background value for total concentration in uncontaminated, natural soils according to the Chinese environmental quality standard GB15618, 1995; described by Huang and Jin (2008) (35 mg kg−1 for Cu and 100 mg kg−1 for Zn). For calculation of mean values, only data exceeding the limit of quantification were considered here
The high concentrations of antibiotics on field sites that had been fertilised with pig or chicken manure coincided with elevated Cu and Zn concentrations. This supports the assumption that these manure types were a common source for these contaminants. Indeed, we found significant correlations between sulfamethazine and total Cu concentrations (Spearman rank correlation r 2 = 0.72, p < 0.001) as well as between sulfamethazine and total Zn concentrations (r 2 = 0.76, p < 0.001) for topsoils (Fig. 2). Similar relationships were found with the occurrence of EDTA-extractable heavy metals (Spearman rank correlation for Cu and sulfamethazine: r 2 = 0.74, p < 0.001; and for Zn and sulfamethazine: r 2 = 0.77, p < 0.001). Thus, contrary to our expectation, using EDTA-extractable concentrations instead of total concentrations did not improve the correlation between metals and antibiotics. A correlation with concentrations of other antibiotic substances could not be tested due to the only sporadic detection of the latter (Table 1).
The quantification of heavy metals is easier and less cost intensive than the quantification of antibiotics. Thus, the observed correlations give support to the idea that it might be possible to identify regional hotspots of contaminations with veterinary antibiotics. Despite the large number of factors influencing concentrations of antibiotics and heavy metals in manure and soils (e.g. husbandry conditions, degradation, sorption, variable background values of metals), the suggested method seems suitable to pre-select sampling sites on the basis of available data on Cu and Zn concentrations.
The use of total heavy metal concentrations can be advantageous compared with the use of EDTA-extractable amounts because (i) the majority of existing soil heavy metal data sets comprises total metal concentrations instead of EDTA or salt-extractable fractions, and (ii) in those cases where data on EDTA or salt-extractable fractions are available, a wide range of methods was usually applied (e.g. EDTA, DTPA, CaCl2, NaNO3, NH4NO3) and results obtained from these methods differ significantly (Menzies et al. 2007).
When applying backwards the proposed localisation of sites with high antibiotic contaminations from total Cu and Zn data to our fields, we would have been able to exclude all those sites from a risk assessment that lacked the detections of any antibiotics (Fig. 2). Noteworthy, however, we would have also pre-excluded sites with concentrations of sulfamethazine reaching 80 μg kg−1 soil.
Depth distributions of antibiotics, Cu and Zn
As lysimeter experiments in the same study area suggested that antibiotics may also leach into the subsoil (Ostermann et al. 2013), we extended our screening below the topsoils. Indeed, sulfamethazine was quantifiable below the plough horizon (i.e. >20 cm depth) on 46 % of all the investigated agricultural field sites (n = 24, Table 1) and reached the maximum sampling depth interval of 160–200 cm on six (25 %) of the field sites (Table 1). Despite lower topsoil concentrations, sulfadiazine was also detected twice in 160–200 cm soil depth. To our knowledge, the occurrence of sulfonamides down to such a depth has not been reported so far, although their mobility in soil has well been documented in leaching experiments (Boxall et al. 2002; Kay et al. 2004, 2005). The maximum detection depth for sulfonamides so far was 120 cm; at this depth of a Luvisol, Tamtam et al. (2011) found sulfamethoxazole with a concentration of less than 2 μg kg−1 after long-term irrigation with wastewater.
In contrast to the sulfonamides, tetracyclines and fluoroquinolones were not detected below the topsoil on the agricultural fields, which is in accordance with their reported strong sorption to soil constituents (Thiele-Bruhn 2003). However, Tamtam et al. (2011) reported the detection of a quinolone antibiotic down to 1 m depth on a long-term wastewater irrigation site, and Tai et al. (2010 and 2011) and Huang et al. (2013) also found tetracyclines and fluoroquinolones down to 80 cm depth (which was the maximum sampling depth). Our exclusive detection of tetracyclines and fluoroquinolones in topsoils can be explained by the promoting effect of bivalent cations on the sorption of these agents. All investigated soils contained carbonates, and the sorption of tetracyclines, which are zwitterionic or negatively charged at the given soil pH (Jia et al. 2006; Zhang et al. 2011), is usually enhanced by metal bridging with these cations. The same applies to fluoroquinolones (Nowara et al. 1997; Vasudevan et al. 2009; Guaita et al. 2011).
In the dry riverbed soil, sulfamethazine was detected even down to the maximum sampled depth interval of 370–400 cm (Table 2). The observed mobility of sulfonamide antibiotics in soils poses the threat that these pollutants might enter groundwater bodies and thus potentially influence drinking water quality.
Screening the subsoils for heavy metal loads revealed that neither in any of the agricultural fields nor even in the riverbed the concentrations of Cu and Zn in subsoils exceeded the proposed Chinese background value for natural soils. Hence, using Cu and Zn concentrations for localizing hotspots of antibiotic loads would be restricted to the surface soil and may not be extended to subsoil depths. In part, this may be due to a peculiarity of the investigated soil in the region, as most of the sites exhibited pH values in the surface soil of >7, thus promoting the precipitation and retardation of Cu and Zn in the slightly calcareous topsoils. Nevertheless, the occurrence of lime is also abundant in many other top- and subsoils worldwide, i.e. we generally think that it would be risky to also predict antibiotic leaching from heavy metal screening data.
Prediction of subsoil contamination with sulfonamides using input and leaching data
Results from our previous field lysimeter study carried out in the same experimental region showed that 1.3 % of manure-derived sulfamethazine was translocated below 20 cm depth after 600 mm precipitation (Ostermann et al. 2013). Hence, we tested a simple approach to predict the vertical translocation of sulfamethazine by comparing calculated and measured subsoil stocks as outlined in Eq. 2. Measured stocks were deduced from subsoil sulfamethazine concentrations assuming a soil bulk density of 1.4 g cm−3 (Fig. 3). By combining the average sulfamethazine concentration of regionally sampled pig manure (3.8 mg kg−1 fresh matter; Ostermann et al. 2013) with questionnaire information from the sampled field sites (annual amount of pig manure application and number of years with this unchanged manure fertilisation, Table 1), we calculated the theoretical subsoil sulfamethazine stock for those sites fertilised with pig manure. Despite this very simple approach, the measured subsoil stocks were in most cases well reproduced by the calculated stocks (Fig. 3); the largest error in these calculations was an underestimation by the factor of 2.8 (plot 23) and an overestimation by the factor of 2.1 (plot 22). Hence, using the potential translocation rate of antibiotics that was determined on a single field site (Ostermann et al. 2013) also helped to assess sulfamethazine loads for the subsoils of other fields in the same region with an accuracy of 35 to 200 % of the measured concentration. Such an accuracy is not adequate for in-depth process studies on the combined fate and effects of these antibiotics. However, after validating the approach with a larger set of data points, it may be sufficient for providing a promising qualitative tool for a future pre-assessment of hotspots of antibiotic translocation into the subsoil and potentially into the groundwater in regions with similar climatic conditions and soil texture.
Over the last decade, numerous data sets on topsoil antibiotic concentrations have been collected; however, these studies seldom include information on subsoil concentrations. Our predictions indicate that for those studies that also surveyed basic management data (applied manure amount, duration of fertilisation history and antibiotic concentration in the manure), subsoil antibiotic concentrations can be estimated with a sufficient accuracy. This approach offers a promising outlook to predict sulfamethazine contamination in the subsoil on a large scale based on existing data sets. As antibiotic pools in the subsoil are less affected by microbial metabolism or degradation compared to the topsoil pool (Kasteel et al. 2010), it is essential to include these potentially persistent pools in advanced risk assessment studies.
Conclusions
Soils in the peri-urban region of Beijing with intensive animal husbandry exhibited frequent occurrence of antibiotics as well as elevated levels of Cu and Zn that slightly exceeded the Chinese natural background levels. Concentrations of these heavy metals, which are applied for growth-promoting purposes in animal husbandry, could be used as promising tool for identification of potential antibiotics pollution hotspots. Notably, the occurrence of sulfonamides was not restricted to the surface soil; we failed, however, to detect a significant leaching of the investigated heavy metals in these calcareous soils, i.e. Cu and Zn have been proxies only for a contamination with antibiotics in surface soils but not in subsurface ones. Yet, the measured stocks of sulfamethazine in subsoils corresponded to stocks deduced from fertilisation data, average concentrations in manure and leaching rates assessed in a former lysimeter study. Altogether, it is thus also possible to identify sites with distinct translocation of sulfonamides from available farm and literature data. In contrast, tetracyclines and fluoroquinolones were not translocated to greater depths despite high topsoil concentrations.
References
Aldrich, A. P., Kistler, D., & Sigg, L. (2002). Speciation of Cu and Zn in drainage water from agricultural soils. Environmental Science and Technology, 36, 4824–4830.
Aust, M. O., Godlinski, F., Travis, G. R., Hao, X., McAllister, T. A., Leinweber, P., & Thiele-Bruhn, S. (2008). Distribution of sulfamethazine, chlortetracycline and tylosin in manure and soil of Canadian feedlots after subtherapeutic use in cattle. Environmental Pollution, 156, 1243–1251.
Boxall, A. B. A., Blackwell, P. A., Cavallo Kay, P., & Tolls, J. (2002). The sorption and transport of a sulphonamide antibiotic in soil systems. Toxicology Letters, 131, 19–28.
Cang, L., Wang, Y., Zhou, D., & Dong, Y. (2004). Heavy metals pollution in poultry and livestock feeds and manures under intensive farming in Jiangsu Province, China. Journal of Environmental Sciences, 16, 371–374.
Chen, Y. S., Zhang, H. B., Luo, Y. M., & Song, J. (2012). Occurrence and assessment of veterinary antibiotics in swine manures: a case study in East China. Chinese Science Bulletin, 57, 606–614.
Dalkmann, P., Broszat, M., Siebe, C., Willasheck, E., Sakinc, T., Huebner, J., Amelung, W., Grohmann, E., & Siemens, J. (2012). Accumulation of pharmaceuticals, enterococcus, and resistance genes in soils irrigated with wastewater for zero to 100 years in Central Mexico. PLoS ONE, 7, doi:10.1371/journal.pone.0045397
EMEA (European Medicines Agency). (2008). Revised guideline on the environmental impact assessment for veterinary medicinal products, available at: http://www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500004386.pdf. Accessed 19 Oct 2012.
Fang, S. B., Hu, H., Sun, W. C., & Pan, J. J. (2011). Spatial variations of heavy metals in the soils of vegetable-growing land along urban–rural gradient of Nanjing, China. International Journal of Environmental Research and Public Health, 2011(8), 1805–1816.
Förster, M., Laabs, V., Lamshöft, M., Pütz, T., & Amelung, W. (2008). Analysis of aged sulfadiazine residues in soils using microwave extraction and liquid chromatography tandem mass spectrometry. Analytical and Bioanalytical Chemistry, 391, 1029–1038.
Förster, M., Laabs, V., Lambshöft, M., Groeneweg, J., Zuehlke, S., Spiteller, M., Krauss, M., Kaupenjohann, M., & Amelung, W. (2009). Sequestration of manure-applied sulfadiazine residues in soils. Environmental Science and Technology, 43, 1824–1830.
Golet, E. M., Strehler, A., Alder, A. C., & Giger, W. (2002). Determination of fluoroquinolone antibacterial agents in sewage sludge and sludge-treated soil using accelerated solvent extraction followed by soild-phase extraction. Analytical Chemistry, 74, 5455–5462.
Golet, E. M., Xifra, I., Siegrist, H., Alder, A. C., & Giger, W. (2003). Environmental exposure assessment of fluoroquinolone antibacterial agents from sewage to soil. Environmental Science and Technology, 37, 3243–3249.
Guaita, D. P., Sayen, S., Boudesocque, S., & Guillon, E. (2011). Copper(II) influence on flumequine retention in soils: macroscopic and molecular investigations. Journal of Colloid and Interface Science, 357, 453–459.
Gupta, G., & Charles, S. (1999). Trace elements in soils fertilized with poultry litter. Poultry Science, 1999(78), 1695–1698.
Heimann, L. (2013). Optimization of nutrient and carbon management of agricultural land in the peri-urban region of Beijing with intensive animal husbandry. Ph.D. dissertation. Technische Universität Braunschweig, Germany. Agraria - Studien zur Agrarökologie Vol. 34, Dr. Kovac (publisher), Hamburg, 198 p.
Higgins, A. J. (1984). Land application of sewage sludge with regard to cropping systems and pollution potential. Journal of Environmental Quality, 1984(13), 441–448.
Hirsch, R., Ternes, T., Haberer, K., & Kratz, K. L. (1999). Occurrence of antibiotics in the aquatic environment. The Science of the Total Environment, 225, 109–118.
Hou, Y., Gao, Z., Heimann, L., Roelcke, M., Ma, W., & Nieder, R. (2012). Nitrogen balances of smallholder farms in major cropping systems in a peri-urban area of Beijing, China. Nutrient Cycling in Agroecosystems, 2012(92), 347–361.
Hu, X., Zhou, Q., & Luo, Y. (2010). Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environmental Pollution, 158, 2992–2998.
Huang, S. W., & Jin, J. Y. (2008). Status of heavy metals in agricultural soils as affected by different patterns of land use. Environmental Monitoring and Assessment, 139, 317–327.
Huang, X., Liu, C., Li, K., Liu, F., Liao, D., Liu, L., Zhu, G., & Liao, J. (2013). Occurrence and distribution of veterinary antibiotics and tetracycline resistance genes in farmland soils around swine feedlots in Fujian Province, China. Environmental Science and Pollution Research International, 20, 9066–9074.
ISO 10693. (1994). Soil quality—determination of carbonate content—volumetric method. Berlin: Beuth.
ISO 11277. (2002). Soil quality—determination of soil particle size distribution in mineral soil material—method by sieving and sedimentation. Berlin: Beuth.
ISO 11466. (1995). Soil quality—extraction of trace elements soluble in aqua regia. Berlin: Beuth.
Jacobsen, A. M., Halling-Sørensen, B., Ingerslev, F., & Hansen, S. H. (2004). Simultaneous extraction of tetracycline, macrolide and sulfonamide antibiotics from agricultural soils using pressurised liquid extraction, followed by solid-phase extraction and liquid chromatography–tandem mass spectrometry. Journal of Chromatography A, 1038, 157–170.
Jia, D. A., Zhou, D. M., Wang, Y. J., Zhu, H. W., & Chen, J. L. (2006). Adsorption and cosorption of Cu(II) and tetracycline on two soils with different characteristics. Geoderma, 146, 224–230.
Kamphuis, B., Jongbloed, A., & van Keulen, H. (2004). Agriculture and water in Shunyi District, Beijing, available at: http://edepot.wur.nl/40559. Accessed 1 Feb 2012.
Kaschl, A., Römheld, V., & Chen, Y. (2002). The influence of soluble organic matter from municipal solid waste compost on trace metal leaching in calcareous soils. The Science of the Total Environment, 291, 45–57.
Kasteel, R., Mboh, C. M., Unold, M., Groeneweg, J., Vanderborght, J., & Vereecken, H. (2010). Transformation and sorption of the veterinary antibiotic sulfadiazine in two soils: a short-term batch study. Environmental Science and Technology, 44, 4651–4657.
Kay, P., Blackwell, P. A., & Boxall, A. B. A. (2004). Fate of veterinary antibiotics in a macroporous tile drained clay soil. Environmental Toxicology and Chemistry, 23, 1136–1144.
Kay, P., Blackwell, P. A., & Boxall, A. B. A. (2005). A lysimeter experiment to investigate the leaching of veterinary antibiotics through a clay soil and comparison with field data. Environmental Pollution, 134, 333–341.
Li, X., Wu, X., Mo, C., Tai, Y., Huang, X., & Xiang, L. (2011). Investigation of sulfonamide, tetracycline, and quinolone antibiotics in vegetable farmland soil in the Pearls River Delta area, southern China. Journal of Agricultural Food Chemistry, 59, 7268–7276.
Luo, L., Ma, Y., Zhang, S., Wei, D., & Zhu, Y. G. (2009). An inventory of trace element inputs to agricultural soils in China. Journal of Environmental Management, 90, 2524–2530.
McBride, M., Richards, B., Steenhuis, T., & Spiers, G. (1999). Long-term leaching of trace elements in a heavily sludge-amended silty clay loam soil. Soil Science and Plant Nutrition, 164, 613–623.
Menzies, N. W., Donn, M. J., & Kopittke, P. M. (2007). Evaluation of extractants for estimation of the phytoavailable trace metals in soils. Environmental Pollution, 145, 121–130.
Nicholson, F., Chambers, B., Williams, J., & Unwin, R. (1999). Heavy metal contents of livestock feeds and animal manures in England and Wales. Bioresource Technology, 70, 23–31.
Nowara, A., Burhenne, J., & Spiteller, M. (1997). Binding of fluoroquinolone carboxylic acid derivated to clay minerals. Journal of Agricultural and Food Chemistry, 45, 1459–1463.
Ostermann, A., Siemens, J., Welp, G., Xue, Q., Lin, X., Liu, X., & Amelung, W. (2013). Leaching of veterinary antibiotics in calcareous Chinese croplands. Chemosphere, 91, 928–934.
Quevauviller, P., Lachia, M., Barahona, E., Rauret, G., Ure, A., Gomez, A., & Muntau, H. (1996). Interlaboratory comparison of EDTA and DTPA procedures prior to certification of extractable trace elements in calcareous soil. The Science of the Total Environment, 178, 127–132.
Roelcke, M., Heimann, L., Ostermann, A., Hou, Y., Müller-Hansen, K., Lu, H.Y., Knaus, M., Clemens, J., Nieder, R., Goldbach, H., Ma, W.Q., Dong, R.J., Liu, X.J. & Zhang, F.S. (2013). Organic residue management options in an Chinese peri-urban region with high nutrient and pollutant loads, In: Dong, R.J. and Raninger, B. (eds.), Biogas engineering and application, Volume 3, p. 295–309. China Agricultural University Press, Beijing, ISBN: 978-7-5655-0751-9, 361 pp.
Rosendahl, I., Siemens, J., Kindler, R., Groeneweg, J., Zimmermann, J., Czerwinski, S., Lambshoeft, M., Laabs, V., Wilke, B.-M., Vereecken, H., & Amelung, W. (2012). Persistence of the fluoroquinolone antibiotic difloxacin in soil and lacking effects on nitrogen turnover. Journal of Environmental Quality, 41, 1275–1283.
Tai, Y., Mo, C., Li, Y., Wu, X., Zou, X., Gao, P., & Huang, X. (2010). Concentration and distribution of quinolone antibiotics in long-term manure-amended soil. China Environmental Science, 30, 816–821. in Chinese.
Tai, Y., Mo, C., Li, Y., Wu, X., Duan, X., Qu, X., & Huang, X. (2011). Concentrations and distribution of tetracycline antibiotics in vegetable field soil chronically fertilized with manures. Environmental Science, 2011(32), 1182–1187. in Chinese.
Tamtam, F., van Oort, F., Le Bot, B., Dinh, T., Mompelat, S., Chevreuil, M., Lamy, I., & Thiry, M. (2011). Assessing the fate of antibiotic contaminants in metal contaminated soils four years after cessation of long-term waste water irrigation. The Science of the Total Environment, 409, 540–547.
Teng, Y., Ni, S., Wang, J., Zuo, R., & Yang, J. (2010). A geochemical survey of trace elements in agricultural and non-agricultural topsoil in Dexing area, China. Journal of Geochemical Exploration, 104, 118–127.
Thiele-Bruhn, S. (2003). Pharmaceutical antibiotic compounds in soil—a review. Journal of Plant Nutrition and Soil Science, 166, 145–167.
Tong, L., Li, P., Wang, Y., & Zhu, K. (2009). Analysis of veterinary antibiotic residues in swine wastewater and environmental water samples using optimized SPE-LC/MS/MS. Chemosphere, 74, 1090–1097.
Vasudevan, D., Bruland, G. L., Torrance, B. S., Upchurch, V. G., & MacKay, A. A. (2009). PH-dependent ciprofloxacin sorption to soils. Geoderma, 2009(151), 68–76.
Wei, R., Ge, F., Huang, S., Chen, M., & Wang, R. (2011). Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China. Chemosphere, 2011(82), 1408–1414.
Welp, G., & Brümmer, G. (1999). Adsorption and solubility of ten metals in soil samples of different composition. Journal of Plant Nutrition and Soil Science, 162, 155–161.
WHO (World Health Organization), 2001, Monitoring antimicrobial usage in food animals for the protection of human health, available at: http://whqlibdoc.who.int/hq/2002/WHO_CDS_CSR_EPH_2002.11.pdf. Accessed 28 May 2012.
Wu, L., Pan, X., Chen, L., Huang, Y., Teng, Y., Luo, Y., & Christie, P. (2013). Occurrence and distribution of heavy metals and tetracyclines in agricultural soils after typical land use change in east China. Environmental Science and Pollution Research International, 20, 8342–8354.
Xiong, X., Li, Y., Li, W., Lin, C., Han, W., & Yang, M. (2010). Copper content in animal manures and potential risk of soil copper pollution with animal manure use in agriculture. Resources, Conservation and Recycling, 54, 985–990.
Zhang, S., Zhang, F., Liu, X., Wang, Y., Zou, S., & He, X. (2005). Determination and analysis on main harmful composition in excrement of scale livestock and poultry feedlots. Plant Nutrition and Fertilizing Science, 11, 822–829. in Chinese.
Zhang, Z., Sun, K., Gao, B., Zhang, G., Liu, X., & Zhao, Y. (2011). Adsorption of tetracycline on soil and sediment: effects of pH and the presence of Cu(II). Journal of Hazardous Materials, 190, 856–862.
Zhao, L. Y., Schulin, R., & Nowack, B. (2009). Cu and Zn mobilisation in soil columns percolated by different irrigation solutions. Environmental Pollution, 157, 823–833.
Zhao, L., Dong, Y. H., & Wang, H. (2010). Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. The Science of the Total Environment, 408, 1069–1075.
Zhu, Y., Johnson, T. A., Su, J., Qiao, M., Guo, G., Steadtfeld, R. D., Hashsham, S. A., & Tiedje, J. M. (2013). Diverse and abundant antibiotic resistant genes in Chinese swine farms. PNAS, 110, 3435–3440.
Acknowledgments
We thank Mr. Hou Yong for his help during field work, the filling in of the questionnaires during the farmers’ survey and their translation. Furthermore, we want to express our gratitude to Manfred Grote and his group, who carried out first orienting tetracycline and fluoroquinolone extractions and measurements of manure samples. Also, we thank Martin Krauss for analysing the concentrations of various sulfonamides in preliminary experiments. The study was funded by the German Federal Ministry of Education and Research (BMBF) (FKZ: 0330847D) and the Chinese Ministry of Science and Technology (MOST) (grant no. 2009DFA32710).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(PDF 81 kb)
Online Resource 2
(PDF 84 kb)
Rights and permissions
About this article
Cite this article
Ostermann, A., Gao, J., Welp, G. et al. Identification of soil contamination hotspots with veterinary antibiotics using heavy metal concentrations and leaching data—a field study in China. Environ Monit Assess 186, 7693–7707 (2014). https://doi.org/10.1007/s10661-014-3960-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3960-x