Abstract
Three biosurfactant-producing strains designated as BS-1, BS-3, and BS-4 were screened out from crude oil-contaminated soil using a combination of surface tension measurement and oil spreading method. Thin layer chromatography and infrared analysis indicated that the biosurfactants produced by the three strains were lipopeptide, glycolipid, and phospholipid. The enhancement of solubilization and biodegradation of petroleum hydrocarbons in groundwater employing biosurfactant-producing strains was investigated. The three strain mixtures led to more solubilization of petroleum hydrocarbons in groundwater, and the solubilization rate was 10.5 mg l−1. The combination of biosurfactant-producing strains and petroleum-degrading strains exhibited a higher biodegradation efficiency of 85.4 % than the petroleum-degrading strains (71.2 %). Biodegradation was enhanced the greatest with biosurfactant-producing strains and petroleum-degrading strains in a ratio of 1:1. Fluorescence microscopy images illustrate that the oil dispersed into smaller droplets and emulsified in the presence of biosurfactant-producing strains, which attached to the oil. Thus, the biodegradation of petroleum hydrocarbons in groundwater was enhanced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A great amount of petroleum hydrocarbons inevitably enter the environment as a result of accidents and improper handling in the exploitation, transport, and refinery of crude oil. The petroleum hydrocarbons then migrate into the groundwater system in various ways, such as atmospheric precipitation and soil leaching, thereby leading to groundwater contamination in many countries and regions (Lesage et al. 1997; Vazquez and Mansoori 2000; Wang et al. 2002).
Various existing cleanup technologies for petroleum hydrocarbon treatment can be categorized into three general schemes: chemical, physical, and biological methods (Kermanshahi et al. 2005). Bioremediation has gained much attention and has been applied to groundwater contamination as a potential technology for its numerous advantages, such as high efficiency, relatively low cost, cleanness, and simple application (Holliger 1995; Baveye et al. 1998; Langwaldt and Puhakka 2000). The presence of degrading microorganisms in bioremediation is a key factor for the efficiency of the process (Gallego et al. 2001; Bundy et al. 2002; Kaplan and Kitts 2004). If the degrading microorganism is available, the solubility and bioavailability of petroleum hydrocarbons are other limiting factors (Francy et al. 1991). Existing investigations reported that only 1 to 5 % of contaminants are dissolved in the water phase, 62 % is present as the free nonaqueous phase [free-product or nonaqueous-phase liquid (NAPL)], and 33 % is adsorbed on the aquifer medium or wrapped in the medium pore because of the capillary force in petroleum-contaminated groundwater (Mackay and Cherry 1989). Surfactants can be used to increase the solubility of NAPL constituents in groundwater (Fountain et al. 1991).
Recent interest in biosurfactants is fueled by their characteristics, such as low toxicity and effectiveness, to reduce interfacial tension between oil and water phases (Zhang and Xiang 2010; Ibrahim et al. 2013). Biosurfactants have been evaluated in the laboratory for their ability to enhance heavy metal ion solubilization, fat-soluble organic contaminant solubilization, and bioavailability by several authors (Mulligan and Eftekhari 2003; Mulligan and Wang 2006; Urum et al. 2003; Banat 1995). Enhanced bioremediation of petroleum hydrocarbon-contaminated soil using biosurfactants has been demonstrated at laboratory scale (Lai et al. 2009). However, little data are available on biosurfactants in enhancing the bioremediation of petroleum hydrocarbon-contaminated groundwater, and enhancing petroleum hydrocarbon biodegradation of groundwater in the presence of biosurfactant-producing strains which can metabolize the biosurfactant in situ has not been previously reported.
In this study, biosurfactant-producing strains instead of biosurfactants were added to groundwater. The addition of such strains will ensure the long-term release of biosurfactants and low-cost biosurfactant production because a large amount of organic solvents will be consumed in the extraction of the biosurfactant. Biosurfactant-producing strains were screened and isolated, and the biosurfactants produced by the strains were identified. Petroleum hydrocarbon solubilization and biodegradation in the presence of the biosurfactant-producing strains were also evaluated.
Materials and methods
Strains
Biosurfactant-producing strains and petroleum-degrading strains were screened from crude oil-contaminated soil. The degrading strains were mixed strains acclimatized in low temperature (10 °C) and conserved at 4 °C. These strains were identified as Acinetobacter sp. and Yarrowia sp.
Culture media
Enrichment medium is composed of the following, in g l−1 distilled water: (NH4)2SO4 1, KH2PO4 3.4, K2HPO4 4.4, NaCl 1.1, KCl 1.1, MgSO4 · 7H2O 0.2, and yeast extract 0.5. 2 ml of trace element, and 2 ml of 0# diesel oil were supplemented. Fermentation medium composition is composed of the following, in g l−1 distilled water: (NH4)2SO4 1, Na2HPO4 · 12H2O 1.5, KH2PO4 1.5, NaCl 1, MgSO4 · 7H2O 0.2, EDTA 1, and yeast extract 0.5. 2 ml of trace element and 10 ml of 0# diesel oil were supplemented. Mineral salt medium (MSM) is composed of the following, in g l−1: (NH4)2SO4 2, NH4NO3 1.2, K2HPO4 1.55, and Na2HPO4 · 12H2O 0.58. Trace element solution is composed of the following, in g l−1 distilled water: ZnSO4 · 7H2O 2.0, CaCl2 · 2H2O 1.0, FeSO4 · 7H2O 5.0, NaMoO4 · 2H2O 0.2, CuSO4 · 5H2O 0.2, CoCl2 · 6H2O 0.4, and MnCl2 · 2H2O 1.0. The pH values of the media were adjusted to 7, and the media were autoclaved at 121 °C for 20 min.
Isolation and screening of biosurfactant-producing strains
Approximately 5 g of oil-contaminated soil sample was added to the enrichment medium and incubated in a rotary shaker at 30 °C and 140 r min−1 for 7 days. Approximately 10 ml of liquid supernatant was transferred to the enrichment medium containing 10 ml of 0# diesel oil and incubated under the same condition for another 7 days. After the third subculture, the culture broth was coated on crude oil agar plates. The oil plates were visually inspected for emulsification circles around the colonies, which were indicative of biosurfactant production. The diameter of the emulsification circle depends on the biosurfactant concentration.
The strains were isolated and screened using the modified method of Zhang et al. (2012). In brief, filter papers were uniformly soaked with crude oil and placed on enrichment medium agar plates without carbon source under sterile conditions. Approximately 0.4 ml of the enrichment culture was coated on oil agar plates and cultured for 7 days at 30 °C. The plates were visually inspected for zones of clear oil-dissolved circles, indicating that the biosurfactant was produced. Colonies with large and clear circles were selected, inoculated in fermentation medium, and incubated at 30 °C and 180 r min−1 for 4 days. Strains with efficient biosurfactant-producing capacity were screened out using surface tension measurement and oil spreading method. The surface tension was measured with a JYW-200 surface tension instrument at room temperature (25 °C). The oil-spreading test was done as described by Chandankere et al. (2013). Morphologies of the strains after 24 h of cultivation were observed under a microscope.
Characterization of biosurfactants
To determine the chemical characteristics of the biosurfactants, they were qualitatively analyzed by thin layer chromatography (TLC). Approximately 0.2 ml of the supernatant of the biosurfactant-producing fermentation broth was dissolved in l ml of chloroform and subjected to TLC analysis with 65:15:2 (v:v:v) chloroform/methanol/water solution as a developing solvent. The chromogenic reagents were as follows (Zhang et al. 2010): (a) phenol–sulfuric acid reagent, in which 3 g of phenol and 5 ml of sulfuric acid were dissolved in 95 ml of ethanol, and a brown dot blot will appear if glycolipid is present; (b) bromine thyme phenol reagent, in which 40 mg of bromine thyme phenol was dissolved in 100 ml of 0.01 N NaOH, and a blue dot blot will appear if phospholipid is detected; and (c) ninhydrin acetone reagent, in which 0.5 g of ninhydrin was dissolved in 100 ml of acetone, and a red dot blot will appear in the presence of lipopeptide.
Infrared analysis of the purified biosurfactant was conducted followings the methods described by Chandran and Das (2010).
Batch solubility experiments
Experiments were conducted for single-, two-, and three-strain mixtures of BS-1, BS-3, and BS-4. The single-, two-, and three-strain mixtures referred to the single cell suspensions of the three, mixtures of two cell suspensions (1:1, v:v) of the three, and mixtures of the three cell suspensions (1:1:1, v:v:v), respectively. The optical density (OD) of the aforementioned mixtures was 0.4. Approximately 1 ml of each of the single-, two-, and three-strain mixtures was added to 50 ml of MSM supplemented with 10 μl of 0# diesel. The samples were shaken on a rotary shaker at 10 °C and 120 r min−1 for 7 days. The samples were allowed to stand for 2 h. Then, 10 ml of the lower aqueous phase was drawn using an injector and extracted with 2 ml of hexane. Total petroleum hydrocarbons (TPHs) were analyzed by gas chromatography (GC). The culture solution (0.5 ml) was collected and observed under a microscope before and after 4 days of cultivation.
The MSM in this study was prepared with shallow groundwater obtained from Chaoyang district, Changchun. The analytical determination of groundwater was pH 7.3, and electrical conductivity was 320 μs cm−1. Common ions and heavy metals in groundwater are given in Table 1. Common ions were analyzed using ion chromatograph (Metrohm, Switzerland). Water samples for heavy metals determination were preserved in nitric acid (5 %) within 24 h and then analyzed using ICP-MS (Agilent 7500c, America).
Petroleum hydrocarbons biodegradation
Two sets of biodegradation experiments were performed. In set 1, 100 ml of MSM and 20 μl of 0# diesel were mixed with 1 ml of cell suspension of degrading strains (OD 0.4). In set 2, 100 ml of MSM and 20 μl of 0# diesel were mixed with 1 ml of cell suspension of degrading and biosurfactant-producing strains (1:1, v:v; OD 0.4). Both sets were cultivated in a rotary shaker incubator at 10 °C and 120 r min−1 for 7 days. The cultivation solution (10 ml) was collected every day and extracted using 2 ml of hexane. The analysis of TPH was done as Thavasi et al. (2011) and the biodegradation efficiency was calculated as given below.
Biodegradation efficiency (%) = \( \left(1-\frac{c_{\mathrm{t}}}{c_0}\right)\times 100\% \)
where c t and c 0 are concentrations of TPH after and before cultivation.
Biodegradation experiments with degrading and biosurfactant-producing strains at various ratios were carried out by adding 1 ml of cell suspensions (OD 0.4) of biosurfactant-producing and degrading strains (2:1, 1:1, 2:3, and 1:2, v:v) to 100 ml of MSM supplemented with 20 μl of 0# diesel. These samples were cultivated at 10 °C and 120 r min−1 for 7 days. The cultivation solution (10 ml) was collected after 7 days and extracted using 2 ml of hexane. TPHs were analyzed by GC, and the biodegradation rate was calculated.
Biosurfactant-producing strains in enhancing biodegradation
To examine biosurfactant-producing strains in enhancing biodegradation, 900 μl of the upper layer of the fermentation broth containing petroleum was collected after 24 h of cultivation in fermentation medium, blended with 90 μl of phosphate buffer (pH 8.0) and 10 μl of 50 mM EDTA, and added with CFDA dyestuff to obtain a final concentration of 10 μM. The dyed samples were cultivated in the dark at 35 °C for 30 min to eliminate unnecessary natural background. Approximately 0.3 ml of the dyed samples was observed under fluorescence microscopy.
Results and discussion
Isolation and screening of biosurfactant-producing strains
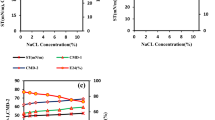
As a microorganism can be considered a promising biosurfactant producer when it is able to reduce the surface tension below 40 mN m−1 (Olivera et al. 2009), only those isolates lowering culture medium surface tension below this limit were preliminarily screened out (BS-1, BS-2, BS-3, BS-4, BS-5, and BS-6). Surface tension and oil-spreading diameter of fermentation broth produced by the six strains were determined. All the surface tensions were lowered from 69.0 ± 0.2 mN m−1 to less than 40 mN m−1 and the oil expelling circles (ranging from 3.5 to 5.2 cm) were notable and stable (Table 2). Among them, three strains designated as BS-1, BS-3, and BS-4 which were identified as Bacillus sp., Pseudomonas sp. and Micrococcus sp. exhibited significant effect on surface tension reduced to 27.8, 27.3, and 26.6 mN m−1, respectively. Based on this analysis, the three strains were selected for subsequent study. Morphologies of BS-1, BS-3, and BS-4 are shown in Fig. 1.
Chromatography
Thin layer chorography (TLC)
The chemical characteristics of the biosurfactants were evaluated using TLC. The results of TLC analysis (Fig. 2) show that red, brown, and blue dot blots for strains BS-1, BS-3, and BS-4, respectively, were observed after being sprayed with different reagents. According to literature (Bao et al. 2003), this suggests that biosurfactants produced by the three strains were types of lipopeptide, glycolipid, and phospholipid respectively.
Infrared analysis
As shown in Fig. 3, the absorbance values for each specific band included hydroxyl (OH–) stretching bands at 2,929 cm−1. Stretching bands at 2,928, 2,856, and 1,451 cm−1 confirmed the presence of methylene (−CH2–). The adsorption peaks at 1,658 and 1,657 cm−1 were attributed to unsaturated double bonds (C = C). The stretching bands at 1,870 to 1,600 cm−1 were attributed to carboxylic acids (C = O), whereas those at 1,340 and 1,361 cm−1 were attributed to cyclic lactone and glycosidic bonds (C–O–C). The adsorption peaks at 1,645 and 1,630 to 1,605 cm−1 were carbonyl. These results suggest that all the three biosurfactants were unsaturated fats and aromatic compounds. The stretching bands of BS-1 at 650 to 900 cm−1 were attributed to the presence of ammonium (N-H), which indicates that the biosurfactant was a type of lipopeptide. The ketone and carbonyl stretching bands of BS-3 at 1,735 cm−1 suggest that the biosurfactant was a type of glycolipid. The stretching bands of BS-4 at 1,260 to 1,285 cm−1 reveal characteristics of phosphorus, which confirms that the biosurfactant was a type of phospholipid.
Solubilization of petroleum hydrocarbons in groundwater
The solubilization of petroleum hydrocarbons in the presence of biosurfactant-producing strains was determined. The results in Fig. 4 suggest that the two-strain mixtures of BS-1 and BS-3 and BS-3 and BS-4, as well as the three-strain mixtures of BS-1, BS-3, and BS-4, exhibited more solubility than the single-strain mixtures. Among them, the three-strain mixture was the best enhancer in terms of TPH solubilization (10.5 mg l−1). This result might be due to biosurfactant production by these strain mixtures.
The figures (Fig. 5) before and after 4 days of cultivation illustrate that oil dispersed into smaller droplets, which were more accessible and available for microbes. Thus, the biodegradation of petroleum hydrocarbons in groundwater was enhanced by adding biosurfactant-producing strains. This result was investigated in the following section.
Biodegradation of petroleum hydrocarbons in groundwater
The biodegradation efficiency of petroleum hydrocarbons in groundwater with and without the presence of biosurfactant-producing strains are shown in Fig. 6. The data indicate that biosurfactant-producing strains had a significant influence on TPH biodegradation. The TPH biodegradation efficiency was relatively low in preliminary cultivation, but increased after 4 to 5 days of cultivation, followed by a slow increase. Biosurfactant-producing strains combined with petroleum-degrading strains exhibited a higher biodegradation efficiency of 85.4 % after 7 days of cultivation at 10 °C than the petroleum-degrading strains (71.2 %) because of biosurfactant production. This result confirms that the biosurfactant-producing strains could enhance TPH biodegradation in groundwater.
Figure 7 shows that the biodegradation efficiency of TPH was enhanced the greatest with biosurfactant-producing strains and petroleum-degrading strains in the ratio of 1:1.
Biosurfactant-producing strains in enhancing biodegradation
Biosurfactant-producing strains have a great function in enhancing the biodegradation of petroleum hydrocarbons. As shown in Fig. 8, the oil dispersed into smaller droplets and was emulsified in the presence of the biosurfactant-producing strains, which was advantageous for degrading strains to access and metabolize hydrocarbons in the water phase. On one hand, the droplets greatly increased the opportunity for degrading bacterial cells to come into contact with hydrocarbons. The uptake mechanism of hydrophobic substrate occurs by the direct contact between the hydrocarbon and cell surface (Kumari et al. 2012). On the other hand, cell surface hydrophobicity and affinity between cells and hydrocarbons significantly improved. Thus, TPH biodegradation in groundwater was enhanced in the presence of biosurfactant-producing strains.
Conclusion
This study was performed to investigate biosurfactant-producing strains employed in enhancing the biodegradation of petroleum hydrocarbons in groundwater. Three strains, namely, Bacillus sp., Pseudomonas sp., and Micrococcus sp., which produced lipopeptide, glycolipid, and phospholipid biosurfactants, respectively, were screened from crude oil-contaminated soil. In the presence of the three biosurfactant-producing strains, the oil dispersed into small droplets and emulsified. Petroleum solubilization and biodegradation in groundwater were enhanced. Three-strain mixtures exhibited better effects on petroleum hydrocarbon solubilization in groundwater, and the solubilization rate was 10.5 mg l−1. Biosurfactant-producing strains combined with petroleum-degrading strains degraded 85.4 % of TPH in groundwater, whereas the degrading strains metabolized only 71.2 % after 7 days of cultivation at 10 °C. This result confirms that the biosurfactant-producing strains could enhance TPH biodegradation in groundwater.
References
Banat, I. M. (1995). Bioproduction and possible uses in microbial enhanced oil recovery and oil pollution remediation: a review. Bioresource Technology, 51, 1–12.
Bao, M., Mu, B., & Wang, X. (2003). Metabolic process of organisms used in oil recovery. Chem. Res. Appl., 15, 555–557.
Baveye, P., Vandevivere, P., Hoyle, B. L., DeLeo, P. C., Sanchez, D., & Lozada, D. (1998). Environmental impact and mechanisms of the biological clogging of saturated soils and aquifer materials. Crit Rev Environ Sci Tech, 28, 123–191.
Bundy, J. G., Paton, G. I., & Campbell, C. D. (2002). Microbial communities in different soil types do not converge after diesel contamination. Applied Microbiology, 92, 276–288.
Chandankere, R., Yao, J., Choi, M. M. F., Masakorala, K., & Chan, Y. (2013). An efficient biosurfactant-producing and crude-oil emulsifying bacterium Bacillus methylotrophicus USTBa isolated from petroleum reservoir. Biochemical Engineering Journal, 74, 46–53.
Chandran, P., & Das, N. (2010). Biosurfactant production and diesel oil degradation by yeast species Trichosporon asahii isolated from petroleum hydrocarbon contaminated soil. International Journal of Engineering, Science and Technology, 2, 6942–6953.
Fountain, J. C., Klimek, A., Beikirch, M. G., & Middleton, T. M. (1991). The use of surfactants for in situ extraction of organic pollutants from a contaminated aquifer. Haz. Mater., 28(3), 295.
Francy, D. S., Thomas, J. M., Raymond, R. L., & Ward, C. H. (1991). Emulsification of hydrocarbons by subsurface bacteria. Journal of Industrial Microbiology, 8, 237–246.
Gallego, J. R., Loredo, J., Llamas, J. F., Va’zquez, F., & Sa’nchez, J. (2001). Bioremediation of diesel-contaminated soils: evaluation of potential in situ techniques by study of bacterial degradation. Biodegradation, 12, 325–335.
Holliger, C. (1995). The anaerobic microbiology and biotreatment of chlorinated ethenes. Current Opinion in Biotechnology, 6, 347–351.
Ibrahim, M. L., Ijah, U. J. J., Manga, S. B., Bilbis, L. S., & Umar, S. (2013). Production and partial characterization of biosurfactant produced by crude oil degrading bacteria. International Biodeterioration & Biodegradation, 81, 28–34.
Kaplan, C. W., & Kitts, C. L. (2004). Bacterial succession in a petroleum land treatment unit. Applied and Environmental Microbiology, 70, 1777–1786.
Kermanshahi, P. A., Karamanev, D., & Margaritis, A. (2005). Biodegradation of petroleum hydrocarbons in an immobilized cell airlift bioreactor. Water Research, 39, 3704–3714.
Kumari, B., Singh, S. N., & Singh, D. P. (2012). Characterization of two biosurfactant producing strains in crude oil degradation. Process Biochemistry, 47, 2463–2471.
Lai, C. C., Huang, Y. C., Wei, Y. H., & Chang, J. S. (2009). Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. Journal of Hazardous Materials, 167, 609–614.
Langwaldt, J. H., & Puhakka, J. A. (2000). On-site biological remediation of contaminated groundwater: a review. Environmental Pollution, 107, 187–197.
Lesage, S., Hao, X., & Kent, S. N. (1997). Distinguishing natural hydrocarbons from anthropogenic contamination in groundwater. Groundwater, 35(1), 149–160.
Mackay, D. M., & Cherry, J. A. (1989). Ground water contamination: pump-and-treat remediation. Environmental Science and Technology, 23(6), 630.
Mulligan, C. N., & Eftekhari, F. (2003). Remediation with surfactant foam of PCP contaminated soil. Engineering Geology, 70, 269–279.
Mulligan, C. N., & Wang, S. (2006). Remediation of a heavy metal contaminated soil by a rhamnolipid foam. Engineering Geology, 85, 75–81.
Olivera, N. L., Nievas, M. L., Lozada, M., Prado, G. D., Dionisi, H. M., & Sineriz, F. (2009). Isolation and characterization of biosurfactant -producing Alcanivorax strains: hydrocarbon accession strategies and alkane hydroxylase gene analysis. Research in Microbiology, 160, 19–26.
Thavasi, R., Jayalakshmi, S., & Banat, I. M. (2011). Application of biosurfactant produced from peanut oil cake by Lactobacillus delbrueckii in biodegradation of crude oil. Bioresource Technology, 102, 3366–3372.
Urum, K., Pekdemir, T., & Gopur, M. (2003). Optimum conditions for washing of crude oil contaminated soil with biosurfactant solutions. Transactions. Institute of Chemical Engineers, 81B, 203–209.
Vazquez, D., & Mansoori, G. A. (2000). Identification and measurement of petroleum precipitates. Petro Sci Engineer, 26, 49–55.
Wang, Z. D., Li, K., & Fingas, M. (2002). Characterization and source identification of hydrocarbons in water samples using multiple analytical techniques. Journal of Chromatography A, 971(1–2), 173–184.
Zhang, X., & Xiang, T. (2010). Review on microbial enhanced oil recovery technology and development in China. Int. J. Pet. Sci. Technol, 4, 61–80.
Zhang, X., Li, M., & Xiang, T. (2010). Genetic modification of MEOR bacterium Bacillus licheniformis H strain by low energy ion beam irradiation. Open Biotechnol, 4, 14–17.
Zhang, X. S., Xu, D. J., Zhu, C. Y., Lundaa, T., & Scherr, K. E. (2012). Isolation and identification of biosurfactant producing and crude oil degrading Pseudomonas aeruginosa strains. Chemical Engineering Journal, 209, 138–146.
Acknowledgments
This study was supported by the National Natural Science Foundation of China No: 41302185.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, H., Wang, H., Chen, X. et al. Biosurfactant-producing strains in enhancing solubilization and biodegradation of petroleum hydrocarbons in groundwater. Environ Monit Assess 186, 4581–4589 (2014). https://doi.org/10.1007/s10661-014-3721-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3721-x