Abstract
Antibiotics consumption has increased worldwide, and their residues are frequently reported in aquatic environments. It is believed that antibiotics reach aquatic water bodies through sewage. Medicine consumed for healthcare practices are often released into sewage, and after sewage treatment plant, it reaches the receiving water bodies of lakes or rivers. In the present study, we determined the fate of some commonly used antibiotics in a sewage treatment plant (STP) located in Delhi and the environmental concentration of these antibiotics in the Yamuna River, which receives the sewage and industrial effluent of Delhi. There are many reports on antibiotics occurrences in STP and river water worldwide, but monitoring data from the Indian subcontinent is sparse. Samples were taken from a STP and from six sampling sites on the Yamuna River. Several antibiotics were tested for using offline solid-phase extraction followed by high-performance liquid chromatography equipped with photodiode array analysis. Recoveries varied from 25.5–108.8 %. Ampicillin had the maximum concentration in wastewater influents (104.2 ± 98.11 μg l−1) and effluents (12.68 ± 8.38 μg l−1). The fluoroquinolones and cephalosporins had the lower concentrations. Treatment efficiencies varied between 55 and 99 %. Significant amounts of antibiotics were discharged in effluents and were detected in the receiving water body. The concentration of antibiotics in the Yamuna River varied from not detected to 13.75 μg l−1 (ampicillin) for the compounds investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sewage treatment effluent quality is important because often, water is used, treated, and released for reuse by other communities. At every use–release cycle, water may be drawn for use with little or no treatment. In the last few years, a new category of pollutants (known as emerging contaminants, ECs) have been detected in effluents and receiving waters at trace levels. Antibiotics are among ECs detected in aquatic matrices. Antibiotics have important uses in both human and veterinary medicine for their antibacterial properties and as growth promoters. As their consumption increases, they are being detected in all the sectors of the environment. The total production of major antibiotics in India was more than 2,332 Mt in 2006–2007, with a growth rate of 10 %. Nearly 85 % of production is consumed in domestic markets (IDMA 2009). After administration to humans and animals, up to 90 % of the antibiotics can be excreted unchanged via urine and/or feces (Hirsch et al. 1998). These substances are only partially eliminated during sewage treatment (Ternes et al. 2003; Berset et al. 2004). Antibiotic residues entering in sewage are directly proportional to the amount of antibiotics used for human and other uses in particular area. In India, self-prescription rates are very high (more than 64 % of patients buy medicines over the counter without a prescription) (Greenhalgh 1987), and so residues may be higher than where human use is controlled by doctors.
Sewage treatment plants (STPs) are not designed to remove antibiotics. Antibiotic residues have been detected in different water matrices, including hospital wastewaters (Kummerer 2001; Lindberg et al. 2004), STP effluents (Batt et al. 2006; Watkinson et al. 2007; Mutiyar and Mittal 2013), STP biosolids (Kinney et al. 2006a), soil (Kinney et al. 2006b), surface waters (Kolpin et al. 2002, 2004; Batt et al. 2006), groundwater (Hirsch et al. 1999; Lindsey et al. 2001), sediments (Kerry et al. 1996; Kim and Carlson 2006), and drinking water (Zuccato et al. 2000, 2005). The first reported case of surface water contamination by antibiotics was in England in 1980, when Watts et al. (1982) detected at least one compound from the macrolide, sulfonamide, and tetracycline group of antibiotics in river water at concentrations of 1 μg l−1. Table 1 summarizes the literature reported antibiotic residue levels in different water and wastewater matrices. The occurrence of antibacterial agents in the aquatic environment has led to increasing concern for potential environmental risks. Hernando et al. (2006) showed adverse effects of pharmaceutical residues on bacteria, invertebrates, and algal populations, although Carlsson et al. (2006) did not support those findings. Kim et al. (2008) examined the acute aquatic toxicity on a marine bacterium (Vibrio fischeri), a freshwater invertebrate (Daphnia magna), and the Japanese Medaka fish (Oryzias latipes), and reported the LC50 for these organisms at two orders of magnitude higher than environmental concentrations. An overview of effect data for antibiotics residue on living beings is presented in Table 2. The effect data presented here are for acute toxicity, but chronic effects are possible from low-dose long-term exposures. In addition, the cocktail of antibiotics present in wastewater could result in synergistic or antagonistic effects. Discharge of antibiotics to the environment from the wastewater has been linked to the development of various resistant bacterial strains (Kummerer 2004). Therefore, the fate of pharmaceutical compounds in wastewater and other water systems should be properly investigated, including monitoring ambient concentrations in STPs and receiving water bodies. Previously, wastewater treatment plant (WWTP) effluents of Patancheru, Hyderabad, India reported to have the highest levels of ciprofloxacin (CIP) antibiotics residues (up to 31,000 μg L−1) (Larsson et al. 2007). Thus, STPs are potentially important point source for these substances and present opportunities for applying centralized removal processes. Water bodies receiving WWTP effluents often have high pharmaceutical residues as peak high concentration of antibiotics residues (up to 14,000 μg L−1) from surface, groundwater, and drinking water of Hyderabad area (Fick et al. 2009). Similarly, Tamiraparani River, Kaveri River, and Vellar River in southern part of India receiving treated sewage and industrial effluents detected high concentration of pharmaceuticals residues (Ramaswamy et al. 2011). Pharmaceutical substances in India are classified as a point of great concern. Wide range of pharmaceutical formulation is manufactured and used in India, which could lead to the release of more pharmaceuticals substances in the environment (Kurunthachalam 2012). Thus, the present study was carried out to investigate the antibiotics residues levels in Delhi’s sewage and its receiving water body, Yamuna River. The objectives of this research were to establish contamination profiles of water matrices in Delhi and to determine environmental loadings of selected antibiotics from a STP. The selected antibiotics were ampicillin (AMP), ciprofloxacin (CIP), gatifloxacin (GAT), sparfloxacin (SPA), and cefuroxime (CEF) (Table 3). The antibiotics were selected to cover different groups of antibiotics (β-lactum, fluoroquinolones, and cephalosporins) and sales volume (IDMA 2009).
Material and methods
Site description
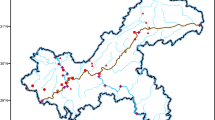
The National Capital Territory (NCT) is a part of the Indo-Gangetic Alluvial Plains and covers 1,483 km2; more than 60 % is urbanized with a population of 16.8 million (Census 2011). Yamuna River, a perennial river, originate from the Himalayan glaciers and passes through the NCT. The river is a major source of potable water to Delhi and is also the receiving water body for untreated and partially treated sewage from the city. Two percent of its total length of the river is in the city, but it receives 79 % of its total pollutant loading there, primarily through sewage and industrial discharges. Water quality in the Delhi, reaches of the Yamuna River, is characterized by high biological oxygen demand (BOD), chemical oxygen demand (COD), nutrients, total coliforms and fecal coliforms, and low dissolved oxygen (CPCB Report 2006). The water quality category of the river between Wazirabad and Okhla Barrage is “E”; only suitable for irrigation and industrial cooling. The water quality of the Yamuna River in this stretch is so bad that the photosynthesis processes are absent. BOD removal takes place mainly by the settling of organic matter. Samples were taken from Okhla STP and six different locations on the 22-km long stretch of the river passing through Delhi. The first sampling site was upstream of Wazirabad barrage near cremation ground. Sampling at this location reflects the water quality before receiving the wastewater discharges from Delhi; drinking water for Delhi is drawn from here. The other five locations were selected to reflect the impact of wastewater discharge from various sources. Raw and treated sewage samples were taken from Okhla STP, which has the capacity to process 636 million liters per day (MLD). The plant operates to 75 % of its design capacity, with BOD, COD, and turbidity removal of 92.0, 92.9, and 95.2 %, respectively (Jamwal and Mittal 2010). The plant has several different units, with capacities ranging from 55 to 236 MLD. The study was carried out at 110 MLD old unit. Figure 1 shows the sampling points.
Sample collection
Two types of samples were collected. Type 1was wastewater samples; type 2 was environmental samples from Yamuna River (Fig. 1). The sewage samples were taken from 110 MLD of the Okhla STP. The STP uses the activated sludge process. Samples were collected from influents (S1, Fig. 2) and effluents (S2, Fig. 2) of the STP. Type 1 samples were taken five times, and type 2 samples were taken thrice in winter, summer, and monsoon seasons from the six environmental sampling locations on the river. Samples were collected in 5 L amber colored, food grade, unused plastic bottles. The bottles were washed with tap water and properly rinsed with distilled water. Before sampling, the bottles were rinsed twice with the sample water. All samples were collected as per APHA, AWWA 2000 (Page No 6-2, 6010 B). The collected sample bottles were kept in airtight iced plastic containers and were transported to the laboratory within 2–4 h of their collection. Samples were acidified to pH 3 in the field using formic acid. Collected samples were preserved at 4 °C until further analysis.
Analytical procedure
Extraction of antibiotics residues
The samples were vacuum filtered through 0.7 μm glass fiber filters to remove suspended matter. The extraction was performed in the same day to avoid any sample degradation. A solid-phase extraction (SPE) procedure was applied to the wastewater samples using commercial Oasis HLB (divinylbenzene/N-vinylpyrrolidone copolymer) cartridges (200 mg, 6 cm3) from Waters (Milford, MA, USA). A vacuum manifold assembly (Milford, MA, USA) fitted with an external pump was used for this purpose. The SPE cartridges were preconditioned with 5 ml of methanol and 5 ml of Milli-Q LC-grade water (pH 3) at a flow rate of 3–5 ml min−1. Samples (500 ml) were loaded at a flow rate of 5–8 ml min−1 followed by a washing step with 5 ml of water (pH 7). After that, the cartridges were dried by nitrogen stream for approximately 15 min and finally eluted with 4 × 2 ml of methanol at 1 ml min−1. The extracts were initially concentrated in Rotavapor® (Buchi, Switzerland) and finally evaporated to dryness by a gentle nitrogen stream. The residues were redissolved in 1 ml of mobile phase (1:1, 0.1 % aqueous TFA and ACN) for HPLC analysis.
Chromatographic conditions
Chromatographic separation of antibiotics were performed on Waters Spherisorb® ODS-2 (250 mm × 4.6 mm, 5 μm) HPLC column. Analyses were performed at a flow rate of 1.0 ml min−1 at the ambient temperature. A gradient flow programming with binary pumps was used, containing solvent A (0.1 % aqueous TFA) and solvent B (ACN) as mobile phase during the analysis. The details of flow programming are given in Table 4. The injection volume was fixed to 20 μl by using standard volume loop. All the compounds were eluted within 20 min, thus a chromatographic run was programmed for 30 min. The PDA detector was used for detection, and the chromatograms were extracted at two different wavelengths of 215 and 280 nm. The chromatograms were extracted at 215 nm for ampicillin, as lambda-max of ampicillin is around 215 nm, and there was no absorbance at 280 nm shown in Figs. 3 and 4, which represents the UV absorbance by ampicillin at these two different wavelengths. The rest of the compounds show high UV absorbance at 280 nm than 215 nm (Fig. 4). The chromatographic and integrated data were recorded and processed by using Empower Software (Waters, USA). Quantification was performed using external calibration and peak area measurement. The details of antibiotic class, retention time, and wavelength of extraction are given in Table 5.
Limit of quantification and linearity
For concentration to be accepted as limit of quantification, the percent deviation from the nominal concentration (accuracy) must be ±20 %, and the relative standard deviation should be less than 20 %. Linearity was tested in the concentration range of 10–1,000 μg l−1 (10, 50, 100, and 500 μg and 1 mg l−1). All the compounds showed good correlation with coefficients, r2 = 0.98–0.99 (Table 5). The detection limits (DL) and quantification limits (QL) were calculated to be 10 and 30 ng ml−1, respectively, considering signal to noise (S:N) ratios. A S:N ratio of 3 was taken as DL, while a S:N ratio of 10 was taken as QL. The relative recoveries of antibiotics were calculated by comparing the peak areas for extracted antibiotics from spiked water and a standard solution of the antibiotic in deionized water.
Reagents and chemicals
Analytical grade (Merck, Darmstadt Germany) chemicals were used throughout the study without any further purification. The solvent used were of HPLC grade only. Milli-Q water was used to prepare all the reagents and calibration standards. The glassware was washed with dilute nitric acid (1.15) followed by several portions of distilled water. Standards of antibiotics were procured from Sigma-Aldrich Chemicals Private Ltd., Bangalore (India) and Dr. Ehrenstrofer GmbH, Augsburg (Germany). The working standards of antibiotics were prepared by dissolving the suitable amount of antibiotics in mobile phase. All solutions prepared for HPLC were passed through 0.2–0.6 μm polypropylene filters (Millipore, EMD Millipore Corp., Billerica, MA, USA) before HPLC analysis.
Results and discussion
Residues recovery
The test compounds are drawn from three groups of antibiotics, β-lactum, fluoroquinolones, and cephalosporins. These compounds exist as cationic species in acidic pH, zwitterionic species at neutral pH, and anionic species at basic pH. Rao et al. (2008) reported the highest recoveries for antibiotics with Oasis HLB as compared to Supelco C18, Lichrolut EN, and Isolute ENV at pH 3 for these groups. Thus, Oasis HLB cartridges were used for solid-phase extraction, retaining the compounds as cationic species. The relative analytical recoveries from present method were checked by spiking different known concentration of antibiotics in the double-distilled water at acidic pH (pH 3). Five hundred milliliter of double-distilled water was spiked with 1, 10, 50, and 100 μg l−1 concentrations and recovery was checked. The resulting recovery efficiencies (REs) (Table 5) categorize the antibiotics into low, medium, and high recoveries. RE for AMP was 25.5, relatively lower than previous reports. Rao et al. (2008) reported RE of 80–103 %, Jones-Lepp (2006) reported 77 ± 22 % recoveries, while Logananthan et al. (2009) reported recoveries of 50 % for antibiotics. The lower RE could be due to analyzing 500-ml samples, as antibiotic RE decreases with increasing sample volumes. Rao et al. (2008) reported reduction in RE of up to half when sample volume increased from 100 to 500 ml.
Analysis of samples from STP
All targeted antibiotics were detected in the wastewater samples from Okhla STP. The antibiotic residues were less in the treated effluents than untreated influents (Fig. 2). The minimum and maximum concentrations detected in influents were for GAT and AMP, respectively. AMP and CIP were detected in each influent sample (N = 5), SPA was detected in four samples, while GAT and CEF were detected in three samples. The mean residues concentration was 104.2 μg l−1 for AMP, 20.1 μg l−1 for CIP, 2.7 μg l−1 for GAT, 22.5 μg l−1 for SPA, and 3.4 μg l−1 for CEF (Table 6). For effluents, the mean antibiotics residues concentration was 12.68 μg l−1 for AMP, 8 μg l−1 for CIP, 1.22 μg l−1 for GAT, 0.14 μg l−1 for SPA, and 0.22 μg l−1 for CEF (Table 6). These are higher levels of antibiotics in wastewater than previously reported by some others (e.g. Yang et al. 2005; Xu et al. 2007; Heidler and Halden 2008; Logananthan et al. 2009) from different parts of the world, but lesser than those reported by Li et al. (2008) (see Table 1). Xu et al. (2007) detected residues up to 2.1 μg l−1, while Heidler and Halden (2008) reported similar levels of antibiotics to those reported here (i.e., 10.8 μg l−1 in treated wastewater). Yang et al. (2005) reported concentrations of pharmaceutical residues around 1.1 μg l−1. Li et al. (2008) reported 389 mg l−1 of penilloic acid, a degradation product of penicillin G, with 153 μg l−1 of the parent compound also present. Residue levels in wastewater is a function of several factors, such as consumption rates, number of medical care units in the watershed, the general civic sensibility regarding the medicine use, and disposal of unused and out of date medicines.
Antibiotics removal and loading estimations from STP
Mass balance estimates were used to investigate how well the STP removed pharmaceuticals from the wastewater, assuming the detected concentrations in the influents and effluents are representative of inflow and outflow masses of antibiotics from the system. The mass balance for each compound was obtained using the following equation:
With removal efficiency expressed as percent
- Ci :
-
Mean influent concentration
- Ce :
-
Mean effluent concentration
- VSTP :
-
Volume of STP (110 MLD)
The influent loadings varied from 301 to 11,462 g/day for the different antibiotics, and 15.4–1,395 g/day were released in the effluents (Fig. 5). Removal efficiencies are presented in Table 6 and varied from 55 to 99 %. Golet et al. (2003) suggested that sorption to particulates and subsequent removal in sewage sludge is the primary mechanism to reduce fluoroquinolone concentrations during secondary wastewater treatment. Castiglioni et al. (2006) found that the removal of pharmaceuticals in surface waters depended on their behavior in the particulate phase, supporting the removal by sorption. In a field study at a full-scale municipal wastewater treatment plant in Switzerland, Golet et al. (2003) reported a 49–60 % reduction in dissolved fluoroquinolone (ciprofloxacin and norfloxacin) concentrations during biological treatment. Partial elimination of macrolides in effluents from wastewater treatment plants has been reported by McArdell et al. (2003). Lin et al. (2009) reported varied removal efficiencies among WWTPs; high removal rates (72–100 %) were generally for non-steroidal anti-inflammatory drugs, estrogens, and caffeine, but some antibiotic groups (macrolides, penicillin, and imidazole) were not removed at all. Bendz et al. (2005) reported lesser removal efficiencies (<50 %) for antibiotics in STPs. Removal efficiencies for antibiotics appears to vary with plants, affected by their operations, geographic locations, and environmental factors. The estimated loadings were 1,395 g/day for AMP, 880 g/day for CIP, 134 g/day for GAT, 15.4 g/day for SPA, and 24.2 g/day for CEF, and the total loading from these compounds is 2,428 g/day. This is a rough estimate, and needs to be used with caution because suspended particulates of wastewater samples were not analyzed (we accounted only for antibiotics occurring in the dissolved fraction), and the samples are only from one unit of one STP. The mass balance for these five compounds suggests that effluent may contain hundreds of such compounds, as treatment does not seem to remove all of the influent concentrations, with grab samples with activated sludge process as treatment process.
Antibiotics levels in the Yamuna River
The Yamuna River receives effluents from 17 STPs and also carries sewage and industrial discharges from 17 stormwater drains. Stormwater drains in Delhi are highly polluted by discharge of domestic and industrial discharge. The river water samples taken across six different sites showed the presence of all targeted antibiotic residues in different seasons (Fig. 1). Antibiotics residue levels were lowest in monsoon, followed by summers, and maximum in winters, both in terms of frequency and concentrations. The concentration varied from 0.2 to 13.75 μg l−1 for AMP, ND-1.44 μg l−1 for CIP, ND-0.48 μg l−1 for GAT, ND-2.09 μg l−1 for SPA, and ND-1.7 μg l−1 for CEF in winters. In winters, in river, AMP was found in all the six sampling sites, while GAT was found at three sites only. CIP, SPA, and CEF were present in samples from five sites. In summers, CIP was found in all the six sampling sites, while AMP was found at five sites only. SPA and CEF were present in samples from four and three sites, respectively, while GAT was not detected at any of the sampling site (Fig. 1). Antibiotics residue levels and occurrence frequency were reduced in monsoon. This may be observed due to the fact that monsoon brings huge freshwater to Yamuna River, especially in Delhi stretch which has very less freshwater flow throughout the year. Antibiotics levels in the Yamuna River are found at higher concentrations than reported for other rivers in Italy and Serbia (Table 1), while similar levels (lower micrograms per liter, up to 5.2 μg l−1) of pharmaceutical residue (triclosan) have been reported in river water from the southern part of India (Ramaswamy et al. 2011). Higher concentrations levels of antibiotics have been reported in literature as follows: 11.92 μg l−1 of sulfamethoxazole in the Llobregat River in NE Spain (Munoz et al. 2009), 10 μg l−1 of CIP in the Arc River in France (Feitosa-Felizzola and Chiron 2009), 1.3 μg l−1 of CIP in the Brisbane River Australia (Watkinson et al. 2009), and 80 μg l−1 of ERY in the Duhan River in Taiwan (Lin and Tsai 2009). Higher levels of antibiotics in Yamuna River water could be expected as the sampled section of the Yamuna River is one of the most contaminated river stretches in India. It receives around 3,000 MLD of sewage from various drains. Generally, wastewater treatment is not universal and the disposal rate of unused medicine is high across India. Though along the Delhi stretch of Yamuna, no specific trend in pharmaceutical residues was observed, but data revealed that one of the sites (YMN-2) was having the maximum concentration of these pharmaceuticals. There was a sharp increase in antibiotic residues levels from sites YMN-1 to YMN-2. This may be due to the fact that site YMN-1 is located upstream of a city where no major drain meets the river. Site 1 represents the water quality of the river before it receives the wastewater discharges from Delhi. Highest concentration and maximum number of antibiotics detected at this site (YMN-2; Fig. 1) could be justified on the basis of huge load of mixed sewage brought by Najafgarh drain (the largest drain in Delhi) at this site. In sites YMN-3 to YMN-6, changes were marginal in antibiotic residues levels. Marginal changes in antibiotic residues levels (YMN-3 to YMN-6) may be due to reduction by adsorption, degradation, and photolysis of antibiotic residues in the river and addition of more sewage (treated and untreated) by various drains to the river.
The detection of antibiotics residues (even at sub-nanogram per liter) is alarming for ecosystem sustainability. These compounds are specially engineered to show their effect at trace levels. There are no reports of direct effect on human beings from contaminated water but effects on other organisms have been documented. Kummerer (2004) reported the development of various resistant bacterial strains associated with discharges of antibiotics. Kim et al. (2008) and Oetken et al. (2004) reported death and decline in reproduction of standard test organisms like V. fischeri, D. magna, M. macrocopa, O. latipes, and some invertebrates. Alighardashi et al. (2009) reported acute sensitivity of sludge bacteria to erythromycin causing floc disintegration and breakage of filaments. Various aquatic toxicity data were summarized in Table 2. Antibiotic residues levels in untreated sewage were enough to show acute toxic effect to multiple test organisms (Tables 2 and 6), and as untreated sewage also finds its ways to various drains and river Yamuna in Delhi, the river water could possibly have harmful ecotoxicological effects to aquatic organisms. Antibiotic residues levels detected in river Yamuna were in the similar ranges to show acute toxicity. Ciprofloxacin concentration in winters at site YMN-2 and YMN-3 (1.44 and 1.19 μg l−1) were in close proximity to cause growth inhibition to algal species Microcystis aeruginosa (Table 2, Halling-Sorensen et al. 2000). Algal species are very sensitive to antibiotics, and, as algae are the basis of the food chain, even slight decreases in algal populations could affect the nutrient cycle and food supply to higher trophic levels. Also note that the effects reported in the test organisms during toxicity tests are carried out under single-drug exposure. The observed effect concentration (EC) decreases when organisms are exposed to mixture of drugs. DeLorenzo and Fleming (2008) reported a 75 % reduction in EC50 in Dunaliella tertiolecta for mixture of drugs when compared to the same level of single drugs. Aquatic lives in the water bodies receiving treated sewage are exposed to a mixture of drugs residues, not just single compounds. Similarly, developments of antibiotic-resistance bacteria (Middleton and Salierno 2012; Shah et al. 2012) and risk to aquatic organisms (Zhang et al. 2012) have been correlated with pharmaceutical residues presence in wastewater from inadequate wastewater treatment systems.
Conclusions
Pharmaceutical compounds are used in human medicine and animal treatment. They can reach the aquatic environment via sewage treatment plants. Too little is known about the occurrence and fate of antibiotics in the environment and the potential risk they pose to aquatic ecosystems. Fundamental data on the occurrence and levels of antimicrobials in different sections of the environment are needed. LC/MS or LC/MS/MS with SPE are often used for antibiotic determination. The advantages of LC/MS or LC/MS/MS over HPLC-PDA are better detection, lower detection limit and quantification limit, reduced matrix effect etc., but very high cost of the LC/MS or LC/MS/MS is a major hurdle in getting access to these types of instruments at the laboratories of developing countries. HPLC-PDA is relatively cheap and widely available at most of the research institutes, and, thus, researchers can select among both the methods (LC/MS or HPLC-PDA) depending upon the facility available. Since STPs acts as point source discharges for these compounds, they are useful monitoring points to calculate loadings. This screening study represents the first measurement of antibiotics residues in aqueous environments in Delhi, India, and one of the few ever conducted in India. We evaluated the levels of antibiotics in influents wastewater at a STP, determined the effect of treatment (removable efficiencies ranged from 55 to 99 %), and measured high concentrations in the receiving water body, the Yamuna River, using the optimized HPLC-PDA method. The potential for ecotoxicological impact from these antibiotics makes it a priority to monitor these compounds more widely. A detailed study is needed to evaluate the total load of pharmaceutical compounds released from STPs, along with investigation of the physical, chemical, and biological processes in the receiving aquatic system such as adsorption on particulates, colloids, and organic matter in order to better assess the possible ecotoxicological risks. Significant gaps still exists in the understanding of the interaction between antibiotic residues, metabolites, and resistance promotion, and synergistic effect from organism exposure to multiple compounds in receiving water bodies.
References
Alighardashi, A., Pandolfi, D., Potier, O., & Pons, M. N. (2009). Acute sensitivity of activated sludge bacteria to erythromycin. Journal of Hazardous Materials, 172, 685–692.
APHA, AWWA. (2000). Standard methods for examination of water and wastewater investigations. Washington: APHA, AWWA.
Backhaus, T., Scholze, M., & Grimme, L. H. (2000). The single substance and mixture toxicity of quinolones to the bioluminescent bacterium Vibrio fisheri. Aquatic Toxicology, 49, 49–61.
Batt, A. L., Bruce, I. B., & Aga, D. S. (2006). Evaluating the vulnerability of surface waters to antibiotic contamination from varying wastewater treatment plant discharges. Environmental Pollution, 142, 295–302.
Bendz, D., Paxeus, N. A., Ginn, T. R., & Loge, F. J. (2005). Occurrence and fate of pharmaceutically active compounds in the environment, a case study: Hoje River in Sweden. Journal of Hazardous Materials, 122, 195–204.
Berset, J. D., Kupper, T., Etter, R., & Tarradellas, J. (2004). Consideration about the enantioselectivity transformation of polycyclic musks in wastewater, treated wastewater, and sewage sludge and analysis of their fate in a sequencing batch reactor plant. Chemosphere, 57, 987–996.
Berto, J., Rochenbach, G. C., Barreiros, M. A. B., Correa, A. X. R., Peluso-Silva, S., & Radetski, C. M. (2009). Physicochemical, microbiological, and ecotoxicological evaluation of a septic tank/Fenton reaction combination for the treatment of hospital wastewaters. Ecotoxicology and Environmental Safety, 72, 1076–1081.
Boxall, A. B. A. (2002). The environmental side effects of medication: how are human and veterinary medicines in soils and water bodies affecting human and environmental health? EMBO Reports, 5, 1110–1116.
Brain, R. A., Johnson, D. J., & Richards, M. (2004). Effects of 25 pharmaceutical compounds on Lemna gibba using a 7-day static renewal test. Environmental Toxicology and Chemistry, 23, 371–382.
Brain, R. A., Hanson, M. L., Solomon, K. R., & Brooks, B. W. (2008). Aquatic plants exposed to pharmaceuticals: effects and risks. Reviews of Environmental Contamination and Toxicology, 192, 67–115.
Brambilla, G., Civitarcale, C., & Migliore, L. (1994). Experimental toxicity and analysis of bacitracin, flumequine, and sulfadimethoxine in terrestrial and aquatic organisms as a predictive model for ecosystem damage. Science of the Total Environment, 13, 114–118.
Calamari, D., Zuccato, E., Castiglioni, S., Bagnati, R., & Fanelli, R. (2003). A strategic survey of therapeutic drugs in the rivers Po and Lambro in northen Italy. Environmental Science and Technology, 37, 1241–1248.
Carlsson, C., Johansson, A. K., Alvan, G., Bergman, G., & Kuhler, T. (2006). Are pharmaceuticals potent environmental pollutants? Part I: environmental risk assessments of selected active pharmaceutical ingredients. Science of Total Environment, 364, 67–87.
Castiglioni, S., Fanelli, R., Calamari, D., Bagnati, R., & Zuccato, E. (2004). Methodological approaches for studying pharmaceuticals in the environment by comparing predicted and measured concentrations in River Po, Italy. Regulatory Toxicology and Pharmacology, 39, 25–32.
Castiglioni, S., Bagnati, R., Fanelli, R., Pomati, F., Calamari, D., & Zuccato, E. (2006). Removal of pharmaceuticals in sewage treatment plants in Italy. Environmental Science and Technology, 40, 357–363.
Census (2011). http://censusindia.gov.in. Accessed 24 Sep 2012.
CPCB Report (2006). Water quality status of River Yamuna (1999–2005). ADSORBS/41/2006-07 Central Pollution Control Board, Govt. of India. Available via http://www.cpcb.nic.in/newitems/11.pdf. Accessed 24 Sep 2012.
DeLorenzo, M. E., & Fleming, J. (2008). Individual and mixture effects of selected pharmaceuticals and personal care products on the marine phytoplankton species Dunaliella tertiolecta. Archives of Environmental Contamination and Toxicology, 54, 203–210.
Diwan, V., Tamhankar, A. J., Khandal, R. K., Sen, S., Aggarwal, M., Marothi, Y., et al. (2010). Antibiotics and antibiotic-resistant bacteria in waters associated with a hospital in Ujjain, India. BMC Public Health, 10, 414–422.
Duong, H. A., Pham, N. H., Nguyen, H. T., Hoang, T. T., Pham, H. V., Pham, V. C., et al. (2008). Occurrence, fate, and antibiotic resistance of fluoroquinolone antibacterials in hospital wastewaters in Hanoi Vietnam. Chemosphere, 72, 968–973.
Feitosa-Felizzola, J., & Chiron, S. (2009). Occurrence and distribution of selected antibiotics in a small Mediterranean stream (Arc River, Southern France). Journal of Hydrology, 364, 50–57.
Ferrari, B., Paxeus, N., Giudice, R. L., Pollio, A., & Garric, J. (2003). Ecotoxicological impact of pharmaceuticals found in treated wastewaters: study of carbamazepine, clofibric acid, and diclofenac. Ecotoxicology and Environmental Safety, 5, 359–370.
Fick, J., Soderstrom, H., Linderberg, R. H., Phan, C., Tysklind, M., & Larsson, D. G. J. (2009). Contamination of surface, ground, and drinking water from pharmaceutical production. Environmental Toxicology and Chemistry, 28, 2522–2527.
Golet, E. M., Xifra, I., Siegrist, H., Alder, A. C., & Giger, W. (2003). Environmental exposure assessment of fluoroquinolone antibacterial agents from sewage to soil. Environmental Science and Technology, 37, 3243–3249.
Greenhalgh, T. (1987). Drug prescription and self-medication in India: an exploratory survey. Social Science and Medicine, 25, 307–318.
Grujic, S., Vasiljevic, T., & Lausevic, M. (2009). Determination of multiple pharmaceutical classes in surface and ground waters by liquid chromatography–ion trap–tandem mass spectrometry. Journal of Chromatography A, 1216, 4989–5000.
Halling-Sorensen, B., Lutzhoft, H. H. C., Andersen, H. R., & Ingerslev, F. (2000). Environmental risk assessment of antibiotics: comparison of mecillinam, trimethoprim, and ciprofloxacin. Journal of Antimicrobial Chemotherapy, 46, 53–58.
Harrass, M. C., Kindig, A. C., & Taub, F. B. (1985). Responses of blue-green and green algae to streptomycin in unialgal and paired culture. Aquatic Toxicology, 6, 1–12.
Hartmann, A., Alder, A. C., Koller, T., & Widmer, R. M. (1988). Identification of fluoroquinolone antibiotics as the main source of umuC genotoxicity in native hospital wastewater. Environmental Toxicology and Chemistry, 17, 377–382.
Heidler, J., & Halden, R. (2008). Meta-analysis of mass balances examining chemical fate during wastewater treatment. Environmental Science and Technology, 42, 6324–6332.
Hernando, M. D., Mezcua, M., Fernandez-Alba, A. R., & Barcelo, D. (2006). Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters, and sediments. Talanta, 69, 334–342.
Hirsch, R., Ternes, T. A., Haberer, K., Mehlich, A., Ballwanz, F., & Kratz, K. L. (1998). Determination of antibiotics in different water compartments via liquid chromatography-electrospray tandem mass spectrometry. Journal of Chromatography, A815, 213–223.
Hirsch, R., Ternes, T. A., Haberer, K., & Kratz, K. L. (1999). Occurrence of antibiotics in the aquatic environment. Science of the Total Environment, 225, 109–118.
India’s quality affordable generics: for global healthcare 47th Annual Publication IDMA 2009.
Isidori, M., Lavorgna, M., Nardelli, A., Pascarella, L., & Parrella, A. (2005). Toxic and genotoxic evaluation of six antibiotics on nontarget organisms. Science of the Total Environment, 345, 87–98.
Jamwal, P., & Mittal, A. K. (2010). Reuse of treated sewage in Delhi city: microbial evaluation of STPs and reuse option. Resource Conservation and Recycling, 54, 211–221.
Jones-Lepp, T. (2006). Chemical markers of human waste contamination: analysis of urobilin and pharmaceuticals in source waters. Journal of Environmental Monitoring, 8, 472–478.
Kerry, J., Coyne, R., Gilroy, D., Hiney, M., & Smith, P. (1996). Spatial distribution of oxytetracycline and elevated frequencies of oxytetracycline resistance in sediments beneath a marine salmon farm following oxytetracycline therapy. Aquaculture, 145, 31–39.
Kim, S. C., & Carlson, K. (2006). Occurrence of ionophore antibiotics in water and sediments of a mixed-landscape watershed. Water Research, 40, 2549–2560.
Kim, Y., Jung, J., Oh, S. R., & Choi, K. (2008). Aquatic toxicity of cartap and cypermethrin to different life stages of Daphnia magna and Oryzias latipes. Journal of Environmental Science and Health. Part. B, 43, 56–64.
Kinney, C. A., Furlong, E. T., Zaugg, S. D., Burkhardt, M. D., Werner, S. L., Cahill, J. D., et al. (2006a). Survey of organic wastewater contaminants in biosolids destined for land application. Environmental Science and Technology, 40, 7207–7215.
Kinney, C. A., Furlong, E. T., Werner, S. L., & Cahill, J. D. (2006b). Presence and distribution of wastewater-derived pharmaceuticals in soil irrigated with reclaimed water. Environmental Toxicology and Chemistry, 25, 317–326.
Kolpin, D. W., Furlong, E. T., Meyer, M. T., Thurman, E. M., Zaugg, S. D., Barber, L. B., et al. (2002). Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environmental Science and Technology, 36, 1202–1211.
Kolpin, D. W., Skopec, M., Meyer, M. T., Furlong, E. T., & Zaugg, S. D. (2004). Urban contribution of pharmaceuticals and other organic wastewater contaminants to streams during differing flow conditions. Science of the Total Environment, 328, 119–130.
Kummerer, K. (2001). Drugs in the environment: emission of drugs, diagnostic aids, and disinfectants into wastewater by hospitals in relation to other sources—a review. Chemosphere, 45, 957–969.
Kummerer, K. (2004). Resistance in the environment. Journal of Antimicrobial Chemotherapy, 54, 311–320.
Kurunthachalam, S. K. (2012). Pharmaceutical substances in India are a point of great concern ? Hydrology Current Research, 3, 3–5.
Larsson, D. J. G., de-Pedro, C., & Paxeus, N. (2007). Effluent from drug manufactures contains extremely high levels of pharmaceuticals. Journal of Hazardous Materials, 148, 751–755.
Laville, N., Ait-Aissa, S., & Gomez, E. (2004). Effects of human pharmaceuticals on cytotoxicity, EROD activity, and ROS production in fish hepatocytes. Toxicology, 196, 41–55.
Li, D., Yang, M., Hu, J., Zhang, Y., Chang, H., & Jin, F. (2008). Determination of penicillin G and its degradation products in a penicillin production wastewater treatment plant and the receiving river. Water Research, 42, 307–317.
Li, B., Zhang, T., Xu, Z., & Fang, H. P. P. (2009). Rapid analysis of 21 antibiotics of multiple classes in municipal wastewater using ultra-performance liquid chromatography-tandem mass spectrometry. Analytica Chimica Acta, 645, 64–72.
Lin, A. Y. C., & Tsai, Y.-T. (2009). Occurrence of pharmaceuticals in Taiwan’s surface waters: impact of waste streams from hospitals and pharmaceutical production facilities. The Science of the Total Environment, 407, 3793–3802.
Lin, A. Y. C., Yu, T. H., & Lateef, S. K. (2009). Removal of pharmaceuticals in secondary wastewater treatment processes in Taiwan. Journal of Hazardous Materials, 167, 1163–1169.
Lindberg, R., Jarnheimer, P. A., Olsen, B., Johansson, M., & Tysklind, M. (2004). Determination of antibiotic substances in hospital sewage water using solid-phase extraction and liquid chromatography/mass spectrometry and group analogue internal standards. Chemosphere, 57, 1479–1488.
Lindsey, M. E., Meyer, T. M., & Thurman, E. M. (2001). Analysis of trace levels of sulfonamide and tetracycline antimicrobials in groundwater and surface water using solid-phase extraction and liquid chromatography/mass spectrometry. Analytical Chemistry, 73, 640–4646.
Logananthan, B., Phillips, M., Mowery, H., & Jones-Lepp, T. (2009). Contamination profiles and mass loadings of macrolide antibiotics and illicit drugs from a small urban wastewater treatment plant. Chemosphere, 75, 70–77.
Lutzhoft, H. H. C., Halling-Sorensen, B., & Jorgensen, S. E. (1999). Algae toxicity of antibacterial agents applied in Danish fish farming. Archives of Environmental Contamination and Toxicology, 36, 1–6.
McArdell, C., Molnar, E., Suter, M. J. F., & Giger, W. (2003). Occurrence and fate of macrolide antibiotics in wastewater treatment plants and in the Glatt Valley Watershed Switzerland. Environmental Science and Technology, 37, 5479–5486.
Middleton, J.H., & Salierno, J.D. (2012). Antibiotic resistance in triclosan tolerant fecal coliforms isolated from surface waters near wastewater treatment plant outflows (Morris County, NJ, USA). Ecotoxicology and Environmental Safety, 1–10.
Migliore, L., Brambilla, G., Grassitellis, A., & Delupis, G. D. (1993). Toxicity and bioaccumulation of Sulfadimethoxine in Artemia (Crustacea, Anostraca). International Journal of Salt Lake Research, 2, 141–152.
Minh, T. B., Leung, H. W., Loi, I. H., Chan, W. H., So, M. K., Mao, J. Q., et al. (2009). Antibiotics in the Hong Kong metropolitan area: ubiquitous distribution and fate in Victoria Harbour. Marine Pollution Bulletin, 58, 1052–1062.
Muñoz, I., Gómez-Ramos, M. J., Agüera, A., Fernández-Alba, A. R., García-Reyes, J. F., & Molina-Díaz, A. (2009). Chemical evaluation of contaminants in wastewater effluents and the environmental risk of reusing effluents in agriculture. TrAC Trends in Analytical Chemistry, 28, 676–694.
Mutiyar, P.K., & Mittal, A.K. (2013). Occurrences and fate of an antibiotic amoxicillin in extended aeration-based sewage treatment plant in Delhi, India: a case study of emerging pollutant. Desalination and Water Treatment, doi:10.1080/19443994.2013.770199, (in press).
Nakata, H., Kannan, K., Jones, P. D., & Giesy, J. P. (2005). Determination of fluoroquinolone antibiotics in wastewater effluents by liquid chromatography–mass spectrometry and fluorescence detection. Chemosphere, 58, 759–766.
Nunes, B., Carvalho, F., & Guilhermino, L. (2005). Acute toxicity of widely used pharmaceuticals in aquatic species, Gambusia holbrooki, Artemia parthenogenetica and Tetraselmis chuii. Ecotoxicology and Environmental Safety, 61, 413–419.
Oetken, M., Bachmann, J., Schulte-Oehlmann, U., & Oehlmann, J. (2004). Evidence for endocrine disruption in invertebrates. International Review of Cytology, 236, 1–44.
Ohlsen, K., Ternes, T., Werner, G., Wallner, U., Loffler, D., Ziebuhr, W., et al. (2003). Impact of antibiotics on conjugational resistance gene transfer in Staphylococcus aureus in sewage. Environmental Microbiology, 5, 711–716.
Park, S., & Choi, K. (2008). Hazard assessment of commonly used agricultural antibiotics on aquatic ecosystems. Ecotoxicology, 17, 526–538.
Pomati, F., Netting, A. G., Calamari, D., & Neilan, B. A. (2004). Effects of erythromycin, tetracycline, and ibuprofen on the growth of Synechocystis sp. and Lemna minor. Aquatic Toxicology, 67, 387–396.
Ramaswamy, B. R., Shanmugam, G., Velu, G., Rengarajan, B., & Larsson, D. G. J. (2011). GC-MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. Journal of Hazardous Materials, 186, 1586–1593.
Rao, R. N., Venkateswarlu, N., & Narsimha, R. (2008). Determination of antibiotics in aquatic environment by solid-phase extraction followed by liquid chromatography–electrospray ionization mass spectrometry. Journal of Chromatography A, 1187, 151–164.
Renew, J. E., & Huang, C. H. (2004). Simultaneous determination of fluoroquinolone, sulfonamide, and trimethoprim antibiotics in wastewater using tandem solid-phase extraction and liquid chromatography–electrospray mass spectrometry. Journal of Chromatography A, 1042, 13–121.
Robinson, A. A., Belden, J. B., & Lydy, M. J. (2005). Toxicity of fluoroquinolones antibiotics to aquatic organisms. Environmental Toxicology and Chemistry, 24, 423–430.
Rosal, R., Rodrıguez, A., Perdigon, J. A. M., Mezcua, M., Hernando, M. D., Leton, P., et al. (2010). Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Research, 44, 578–588.
Sengelov, G., Agerso, Y., Halling-Sorensen, B., Baloda, S. B., Andersen, J. S., & Jensen, L. B. (2003). Bacterial antibiotic resistance levels in Danish farmland as a result of treatment with pig manure slurry. Environmental International, 28, 587–595.
Shah, S. Q. A., Colquhoun, D. J., Nikuli, H. L., & Sørum, H. (2012). Prevalence of antibiotic resistance genes in the bacterial flora of integrated fish farming environments of Pakistan and Tanzania. Environmental Science and Technology, 46, 8672–8679.
Ternes, T. A., & Hirsch, R. (2000). Occurrence and behavior of X-ray contrast media in sewage facilities and the aquatic environment. Environmental Science and Technology, 34, 2741–2748.
Ternes, T. A., Stuuber, J., Herrmann, N., McDowell, D., Ried, A., Kampmann, M., et al. (2003). Ozonation: a tool for removal of pharmaceuticals, contrast media, and musk fragrances from wastewater? Water Research, 37, 1976–1982.
Vasconcelos, T. G., Kummerer, K., Henriques, D. M., & Martins, A. F. (2009). Ciprofloxacin in hospital effluent: degradation by ozone and photo processes. Journal of Hazardous Materials, 169, 1154–1158.
Watkinson, A. J., Murby, E. J., & Costanzo, S. D. (2007). Removal of antibiotics in conventional and advanced wastewater treatment: implications for environmental discharge and wastewater recycling. Water Research, 41, 4164–4176.
Watkinson, A. J., Murby, E. J., Kolpin, D. W., & Costanzo, S. D. (2009). The occurrence of antibiotics in an urban watershed: from wastewater to drinking water. Science of the Total Environment, 407, 2711–2723.
Watts, C. D., Craythorne, B., Fielding, M., & Killops, S. D. (1982). Nonvolatile organic compounds in treated waters. Environmental Health Perspectives, 46, 87–89.
Westergaard, K., Muller, A. K., Christensen, S., Bloem, J., & Sorenson, S. J. (2001). Effect of tylosin as a disturbance on the soil microbial community. Soil Biology and Biochemistry, 33, 2061–2071.
Wollenberger, L., Halling-Sørensen, B., & Kusk, K. O. (2000). Acute and chronic toxicity of veterinary antibiotics to Daphnia magna. Chemosphere, 40, 723–730.
Xu, W., Zhang, G., Li, X., Zou, S., Li, P., Hu, Z., et al. (2007). Occurrence and elimination of antibiotics at four sewage treatment plants in the Pearl River Delta (PRD) South China. Water Research, 41, 4526–4534.
Yang, S., Cha, J., & Carlson, K. (2005). Simultaneous extraction and analysis of 11 tetracycline and sulfonamide antibiotics in influent and effluent domestic wastewater by solid-phase extraction and liquid chromatography-electrospray ionization tandem mass spectrometry. Journal of Chromatography A, 1097, 40–53.
Zhang, R., Zhang, G., Zheng, Q., Tang, J., Chen, Y., Xu, W., et al. (2012). Occurrence and risks of antibiotics in the Laizhou Bay, China: impacts of river discharge. Ecotoxicology and Environmental Safety, 80, 208–215.
Zuccato, E., Calamari, D., Natangelo, M., & Fanelli, R. (2000). Presence of therapeutic drugs in the environment. The Lancet, 355, 1789–1790.
Zuccato, E., Castiglioni, S., & Fanelli, R. (2005). Identification of the pharmaceuticals for human use contaminating the Italian aquatic environment. Journal of Hazardous Materials, 122, 205–209.
Acknowledgments
One of the authors, PKM, is thankful to University Grant Commission (UGC), Delhi for awarding Junior Research Fellowship (JRF) for Doctoral Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mutiyar, P.K., Mittal, A.K. Occurrences and fate of selected human antibiotics in influents and effluents of sewage treatment plant and effluent-receiving river Yamuna in Delhi (India). Environ Monit Assess 186, 541–557 (2014). https://doi.org/10.1007/s10661-013-3398-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3398-6