Abstract
The detection of antibiotics in water systems has instigated great environmental concern due to the toxicological effects associated with these compounds. Their discharge into the environment results from the ubiquity of use in medical, veterinary, and agricultural practices. Some of the effects of antibiotics include development of antibiotic-resistant bacteria, making it difficult to treat diseases, variation in natural microbial communities, and enzyme activities. In this study, the first comprehensive survey of some frequently used antibiotics namely ampicillin (AMP), amoxicillin (AMX), sulfamethoxazole (SMX), chloramphenicol (CAP), and ciprofloxacin (CPF) within Lake Victoria Basin of Kenya is presented. Sludge and wastewater samples were collected from wastewater treatment plants (WWTPs) and hospital lagoons within the study area. Samples were extracted and cleaned by solid-phase extraction, and analysis was carried out using high-performance liquid chromatography (HPLC). All wastewater samples and sludge collected contained quantifiable levels of the selected antibiotics. The highest concentrations were recorded for AMP with WWTPs and hospitals having 0.36 ± 0.04 and 0.79 ± 0.07 μg/L, respectively. In sludge samples, SMX recorded the highest concentrations of 276 ± 12 ng/g. The high levels in sludge indicate the preferential partition of antibiotics onto solid phase, posing great danger to consumers of crops grown in biosolid-amended soils. The daily discharge loads of antibiotics from nine WWTPs ranged between 80.75 and 3044.9 mg day−1 with a total discharge of 6395.85 mg day−1, signifying a high potential of water resource pollution within the region. This report will aid in the assessment of the risks posed by antibiotics released into the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
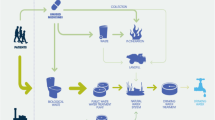
Antibiotics are getting attention as a new class of emerging pollutants in aquatic environment. Their toxic effects on human, animal, and ecosystem health are not well documented (Yang et al. 2011; Cizmas et al. 2015). These compounds are discharged into the environment as a result of the ever-increasing and often indiscriminate use of antibiotics in medical, veterinary, and agricultural practices (He et al. 2014). After administration, antibiotics can be transformed into more polar and soluble forms as metabolites or conjugates of glucuronic and sulfuric acids (Heberer et al. 2002). Antibiotics and their metabolites are readily excreted through urine and feces. They eventually enter into wastewater treatment plants, providing a potential route of antibiotics loading into aquatic environment (Straub 2015). Dispersion of antibiotics into different environmental compartments may result from various sources such as municipal sewage, hospital wastewater, and effluent of wastewater treatment plants (Bottoni and Caroli 2015; Gurke et al. 2015). Recent studies have reported the accumulation of some antibiotics in sewage sludge, posing great danger to consumers of crops in sludge-amended soils (Jelic et al. 2011). In addition, other studies have shown the presence of these compounds in public drinking water wells where septic systems are prevalent (Ternes et al. 2001; Dinsdale et al. 2009). This raises a lot of concerns due to the recalcitrance of these chemicals to conventional wastewater treatment technologies and their toxicities (Clara et al. 2005; Li 2014). In the environment, pharmaceuticals are reported to have adverse effects on aquatic ecosystem, wildlife, and human health (Lemus et al. 2008; Gao et al. 2012; Helba et al. 2016). For example, carbamazepine has been reported to induce oxidative effects on cells of non-target species, such as mussels, hence affecting their health status (Tsiaka et al. 2013). Other pharmaceuticals are reported to have adverse effects on the renal system, resulting in kidney failure (Godoy et al. 2015). In addition, a study by Matozzo (2014) cited antibiotics causing variation of immune responses in aquatic invertebrates. In addition, some antibiotics are reported to induce pathogen resistance, posing great challenges in treatment of diseases (Gracia-Lor et al. 2012).
Among the frequently detected antibiotics in water resources worldwide are sulfonamides, tetracyclines, β-lactams, and macroclides (Liang et al. 2013; Li 2014; Matozzo 2014). In Kenya, some of the widely used antibiotics include but not limited to ampicillin, amoxicillin, sulfamethoxazole, chloramphenicol, and ciprofloxacin, which have been detected in water systems within Nairobi River (K’oreje et al. 2012). Another study by K’oreje et al. (2016) reported high levels of antibiotics and antiretroviral residues in water systems in Nairobi and Kisumu City, Kenya. Currently, there is limited literature on the levels of antibiotic residues in tropical systems especially the Lake Victoria Basin (LVB) of Kenya. The rapidly growing population and rapid urbanization within Lake Victoria catchment are causing strain to the limited waste disposal facilities available. Trace micropollutants such as perfluorinated surfactants, polyaromatic hydrocarbons, and pesticides have been detected within the LVB, indicating general pollution of the region (Chirikona et al. 2015; Lisouza et al. 2011; Werimo et al. 2009; Orata et al. 2009). Therefore, there is an urgent need to document the concentrations of antibiotics in order to assess the potential risks to aquatic ecosystem and human health. This study therefore reports, for the first time, the levels and discharge loads of five most commonly used antibiotics (ampicillin, amoxicillin, sulfamethoxazole, chloramphenicol, and ciprofloxacin) within LVB.
Materials and methods
Chemicals, standards and reagents
High-purity standards for chloramphenicol, ampicillin, sulfamethoxazole, ciprofloxacin, and amoxicillin were purchased from Sigma-Aldrich, Germany. Analytical-grade and high-performance liquid chromatography (HPLC)-grade water, methanol, and acetonitrile for extraction and analysis were purchased from Sigma-Aldrich through Kobian Kenya Limited. Solid-phase extraction cartridges and nylon microfilters were obtained from Estec Kenya Limited. All stock solutions were prepared using HPLC-grade methanol.
Study area and sampling
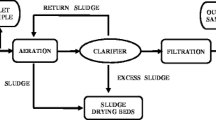
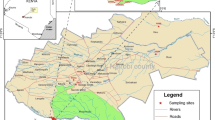
The study was conducted in major towns namely Kisii, Homabay, Kisumu, Kakamega, Mumias, Webuye, Bungoma, and Eldoret within LVB in Kenya (Fig. 1). Sludge and wastewater were collected from hospital lagoons and wastewater treatment plants (WWTPs) between October 2014 and September 2015. The selected WWTPs are used to treat wastewaters originating from domestic, hospital, and industrial areas, while their characteristics are presented in Table 1. During sampling, it was noted that effluents from the WWTPs were being discharged into rivers, which are tributaries of Lake Victoria. Wastewater samples were collected in 2-L amber glass bottles and taken to the laboratory in an ice box. The samples were then refrigerated at −4 °C prior to extraction within 48 h. Sludge samples were also collected, air-dried, ground, and passed through 200-mm sieve prior to extraction.
Extraction of samples for residue analysis
Wastewater samples were extracted by passing 300 ± 3.0 mL aliquots through a Phenomenex C18 solid-phase extraction (SPE) cartridge that had been preconditioned with 10 mL of methanol and distilled water, successively. Elution was carried out using 10 mL of HPLC-grade methanol, which was then evaporated to dryness under vacuum. The residue was redissolved to 2 mL with HPLC-grade methanol (Van der Ven et al. 2004). All samples were filtered using 0.45-μm nylon microfilters prior to injection to LC instrument. Sludge samples were extracted by weighing 0.5 ± 0.05 g of dry sample into a conical flask and adding 15 mL of HPLC-grade methanol. The samples were shaken for 8 h in an orbital shaker at 100 rpm, after which, the supernatant was filtered through glass wool that had been preconditioned with 10 mL of HPLC-grade methanol. The filtrate was evaporated to dryness and sample re-dissolved in 100 mL distilled water. Cleanup of samples was performed by SPE following the same procedure for wastewater samples (Nguyen et al. 2015).
Analysis by HPLC
A Shimadzu LC-20AD fitted with a SIL-20A (HT) autosampler and a SPD-20A prominence ultraviolet-visible (UV) detector was used for HPLC analysis. A reverse-phase Phenomenex (C18) column (4.6-mm i.d. × 250 mm, 5-μm particle) was used for separation of the analytes (Van der Ven et al. 2004). Gradient elution was carried out using a mobile phase consisting of (50: 50 v/v) HPLC-grade acetonitrile/methanol (A) and water with 0.1 % formic acid (B) at a flow rate of 0.30 mL/min. The gradient program was set as follows: (0–3 min), A = 90 %; 8 min, A = 65 %; 17 min, A = 50 %; 20 min, A = 0 %; and 30 min, A = 90 %. Quantification was done using a UV detector at 250-nm wavelength (Park and Choung 2007). Analyte identification was based on comparison of chromatograms of unknowns with those of standards. Standards and blanks were measured periodically throughout the analysis for quality assurance. Quantitative analysis of antibiotics was achieved through the integration of selected HPLC chromatograms. All analyses were carried out in triplicate.
Discussions
The levels of selected antibiotics in sludge and sediment samples from hospitals, WWTPs, rivers, and streams within LVB, Kenya
All sludge samples contained quantifiable levels of the selected antibiotics. The mean concentrations in sludge and sediment samples from ten WWTPs and two hospitals, rivers, and streams within LVB are presented in Tables 2 and 3.
The average antibiotic concentrations in the sludge and sediment samples varied for individual compounds and amongst samples from different sources (Tables 2 and 3). The mean residue levels for all the five antibiotics in sludge were found to range from <50 to 276 ± 12 ng/g, while sediment samples recorded lower levels ranging from <50 to 94 ± 3 ng/g. This may be attributed to dilution in the river environment (Table 2). The maximum amount of antibiotics recorded was 276 ± 12 ng/g sulfamethoxazole (SMX) in sludge discharged from Mumias Hospital general ward (Table 2). The high levels of SMX in the sludge samples may be due to prescription patterns and the intrinsic properties of the compound. The low octanol-water partition of SMX (log Kow = 0.89) makes it preferentially adsorb onto sludge (Liang et al. 2013). Notably, amoxicillin levels were generally lower compared to the other antibiotics. This may be due to the unstable β-lactam ring in amoxicillin, which can be degraded by chemical hydrolysis and β-lactamase-producing bacteria present in WWTPs (Chagas et al. 2011; Kihampa 2014).
Antibiotic residues in river water and effluents from hospital lagoons and WWTPs within LVB, Kenya
Antibiotics were widely detected in effluents from wastewater treatment plants within the study region as shown in Table 4. To the best of our knowledge, this is the first survey on antibiotic residues within the LVB of Kenya.
Antibiotic levels in hospital effluents were high ranging from <0.05 to 0.79 ± 0.07 μg/L. Eldoret Hospital recorded the highest concentrations (ampicillin (AMP) = 0.79 ± 0.07 μg/L). This shows that hospitals are hot spots for antibiotic discharge into WWTPs. The mean antibiotic concentrations in effluents from the ten WWTPs studied ranged between <0.05 and 0.36 ± 0.04 μg/L. The highest levels in WWTPs were recorded in Nyalenda Kisumu with AMP levels of 0.36 ± 0.04 μg/L. Kisumu is the largest among the towns within the region with the highest population as shown in Table 1. The mean concentrations of antibiotics in rivers and streams within the region ranged from <0.05 to 0.29 ± 0.02 μg/L. Although the antibiotic residues were lower in rivers and streams compared to WWTPs, they may pose long-term effects to aquatic ecosystem. The presence of higher levels of antibiotic residues downstream of the WWTP indicates the transfer of these compounds from WWTP effluents to rivers (Table 5). During sampling, it was observed that effluents from these WWTPs are discharged directly into rivers and streams, which are tributaries of the Lake Vitoria. This poses great health concerns due to the possible ecotoxicological effects of these compounds on aquatic ecosystem and human health (Liu et al. 2009).
Discharge loads of antibiotics from WWTPs
The daily discharge load from WWTPs was estimated based on the assumption that the concentrations of antibiotics in wastewater and sludge recorded were constant throughout the day (Nakata et al. 2005; Chirikona et al. 2015). This was done using Eq. 1:
where
DdDaily discharge of antibiotics (mg day−1),
C wConcentration of individual antibiotic in wastewater (μg L−1) (obtained from Table 3),
C WWTPCapacity of WWTP (M3 day−1) (obtained from Table 1),
1000Conversion factor from cubic meters to liters,
10−3Conversion from nanogram to milligram.
Antibiotic discharge loads from the various WWTPs are shown in Table 6. The discharge loads were calculated only for WWTPs whose daily capacities are known. An estimated total amount of antibiotics discharged from the nine WWTPs was calculated using Eq. 2.
where
- DdTotal :
-
Total amount of the five antibiotics discharged from the nine WWTPs studied,
- \( {\displaystyle \sum {Dd}_{\mathrm{WWTPs}}=} \) :
-
Sum of the daily discharge loads of antibiotics in the WWTPs (mg day−1)
The daily discharge loads from the nine WWTPs ranged between 80.75 and 3025 mg day−1 (see Table 6). Nyalenda WWTP in Kisumu recorded the highest amounts of antibiotic discharge of 3025 mg day−1. This could be attributed to the high population in Kisumu city, which is the largest among the other towns studied (Table 1). The total discharge load from the nine WWTPs was 6395.85 mg day−1.
Comparison of results with data from other parts of the world
Results from this study were compared with data from other countries worldwide. The concentrations of antibiotics obtained in effluents from WWTPs in this study ranged between <0.05 and 0.36 ± 0.04 μg/L. Hospital effluents recorded levels ranging from <0.05 to 0.79 ± 0.07 μg/L. These results are comparable to results from other studies elsewhere. A study by Li and Zhang (2011) reported antibiotics ranging from 0.01 to 0.233 μg/L in WWTPs in China. Another study by Giger et al. (2003) reported antibiotic levels ranging from 0.057 to 0.330 μg/L. However, a study by Kihampa (2014) recorded higher levels of up to 37 μg/L in WWTP effluent from Dar es Salaam City in Tanzania. This may be attributed to the high population in the capital city of Tanzania (Kihampa 2014).
Antibiotic residues in the sludge samples from WWTPs in the present study ranged between <50 and 154 ± 9 ng/g. Sludge from hospital lagoons recorded antibiotic residues between <50 and 276 ± 12 ng/g. Similar levels ranging between 299 and 455 ng/g were detected in sludge samples from Spain (Radjenović et al. 2009). The daily discharge loads of antibiotics from the nine WWTPs studied ranged between 80.75 and 3025 mg day−1, which are comparable to other data elsewhere. For example, results obtained from China had daily discharge loads between 3000 and 5200 mg day−1 (Li and Zhang 2011), while in Michigan (USA), 4800 mg day−1 was reported (Nakata et al. 2005). The difference in discharge loads is largely dependent on the capacity of WWTP, population served, and the method of treatment employed (Li and Zhang 2011).
Conclusions and recommendations
In this study, the concentrations of individual antibiotics varied for different sources. The highest individual antibiotic concentrations in wastewater and sludge were detected for ampicillin (0.79 ± 0.07 μg/L) and sulfamethoxazole (276 ± 12 ng/g), respectively. Sediment and water samples from streams and rivers were recorded up to 94 ± 3 ng/g and 0.29 ± 0.02 μg/L ampicillin, respectively. Total discharge loads of the selected antibiotics in the nine WWTPs were 6333.01 mg day−1 with Nyalenda in Kisumu contributing 47.3 %. The high levels of antibiotics recorded in sludge indicate that sorption may be one of the main controlling factors in removal of these compounds in WWTPs. The use of sewage sludge and treated wastewater in agriculture could introduce antibiotics into the food chain after plant uptake, hence the need to improve wastewater treatment methods in order to minimize release of antibiotics into surface waters, sludge, and soils. Eco-friendly prescription of antibiotics with low toxicity levels and less persistence in the environment is also necessary. In addition, emerging pollutants such as antibiotics should be included in environmental regulations and standards for water monitoring.
References
Bottoni, P., & Caroli, S. (2015). Detection and quantification of residues and metabolites of medicinal products in environmental compartments, food commodities and workplaces. A review. J. Pharmac and Biomed. Anal., 106, 3–24.
Chagas, T. P. G., Seki, L. M., Cury, J. C., Oliveira, J. A. L., Dávila, A. M. R., Silva, D. M., & Asensi, M. D. (2011). Multiresistance, beta-lactamase-encoding genes and bacterial diversity in hospital wastewater in Rio de Janeiro Brazil. Journal of Applied Microbiology, 111, 572–581.
Chirikona, F., Filipovic, M., Ooko, S., & Orata, F. (2015). Perfluoroalkyl acids in selected wastewater treatment plants and their discharge load within the Lake Victoria basin in Kenya. Environ. Monit. and Ass., 187(5), 238.
Cizmas, L., Sharma, V. K., Gray, C. M., & Mcdonald, T. J. (2015). Pharmaceuticals and personal care products in waters : occurrence, toxicity, and risk. Environmental Chemistry Letters. doi:10.1007/s10311-015-0524-4.
Clara, M., Strenn, B., Gans, O., Martinez, E., Kreuzinger, N., & Kroiss, H. (2005). Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional WWTPs. Water Research, 39, 4797–4807.
Dinsdale, R. M., Kasprzyk-Hordern, B., & Guwy, A. J. (2009). The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving water. Water Research, 43, 363.
Gao, P., Mao, D., Luo, Y., Wang, L., Xu, B., & Xu, L. (2012). Occurrence of sulphonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment. Water Research, 46, 2355–2364.
Giger, W., Alder, A. C., Golet, E. M., Kohler, H. P. E., McArdell, C. S., Molnar, E., et al. (2003). Occurrence and fate of antibiotics as trace contaminants in wastewaters, sewage sludges, and surface waters. CHIMIA Int. J. Chem., 57(9), 485–491.
Godoy, A. A., Kummrow, F., & Pamplin, P. A. (2015). Occurrence, ecotoxicological effects and risk assessment of antihypertensive pharmaceutical residues in the aquatic environment—a review. Chemosphere, 138, 281–291.
Gracia-Lor, E., Sancho, J. V., Serrano, R., & Hernández, F. (2012). Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere, 87(5), 453–462.
Gurke, R., Rößler, M., Marx, C., Diamond, S., Schubert, S., Oertel, R., & Fauler, J. (2015). Occurrence and removal of frequently prescribed pharmaceuticals and corresponding metabolites in wastewater of a sewage treatment plant. Sci. Tot. Environ., 532, 762–770.
He, K., Soares, A. D., Adejumo, H., McDiarmid, M., Squibb, K., & Blaney, L. (2014). Detection of a wide variety of human and veterinary fluoroquinolone antibiotics in municipal wastewater and wastewater-impacted surface water. J. of Pharmac and Biomedl Analysis, 106, 136–143.
Heberer, T., Reddersen, K., & Mechlinski, A. (2002). From municipal sewage to drinking water: fate and removal of pharmaceutical residues in the aquatic environment in urban areas. Water Science and Technology, 46, 81–88.
Helba, L., Kantor, A., Petrova, J., & Kacaniova, M. (2016). Detection of ampicillin, its sodium salt and alkaline hydrolysis products by MALDI-TOF mass spectrometry. Scientific Papers: Animal Science and Biotechnologies, 49(1), 83–87.
Jelic, A., Gros, M., Ginebreda, A., Cespedes-Sánchez, R., Ventura, F., Petrovic, M., & Barceló, D. (2011). Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Research, 45, 1165–1176.
Kihampa, C. (2014). β-lactams and Fluoroquinolone Antibiotics in influents and effluents of Wastewater treatment plants, Dar es Salaam, Tanzania. Research Journal of Chemical Sciences, 4(6), 31–36.
K’oreje, K. O., Demeestere, K., Wispelaere, P., Vergeynst, L., Dewulf, J., & Langenhove, H. (2012). From multi-residue screening to target analysis of pharmaceuticals in water: development of a new approach based on magnetic sector mass spectrometry and application in the Nairobi River basin Kenya. The Science of the Total Environment, 437, 153–164.
K’oreje, K. O., Vergeynst, L., Ombaka, D., De Wispelaer, P., Okoth, M., Van Langenhove, H., & Demeestere, K. (2016). Occurrence patterns of pharmaceutical residues in wastewater, surface water and groundwater of Nairobi and Kisumu city, Kenya. Chemosphere, 149, 238–244.
Lemus, J. Á., Blanco, G., Grande, J., Arroyo, B., García-Montijano, M., & Martínez, F. (2008). Antibiotics threaten wildlife: circulating quinolone residues and disease in avian scavengers. PloS One, 3(1).
Li, W. C. (2014). Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. A review. Environ. Poll., 187, 193–201.
Li, B., & Zhang, T. (2011). Mass flows and removal of antibiotics in two municipal wastewater treatment plants. Chemosphere, 83(9), 1284–1289.
Liang, X., Chen, B., Nie, X., Shi, Z., Huang, X., & Li, X. (2013). The distribution and partitioning of common antibiotics in water and sediment of the Pearl River Estuary, South China. Chemosphere, 92(11), 1410–1416.
Lisouza, F. A., Owuor, P. O., & Lalah, J. O. (2011). Variation in indoor levels of polycyclic aromatic hydrocarbons from burning various biomass types in the traditional grass-roofed households in Western Kenya. J. Environ. Pol, 159, 1810–1815.
Liu, F., Ying, G. G., Tao, R., Zhao, J. L., Yang, J. F., & Zhao, L. F. (2009). Effects of six selected antibiotics on plant growth and soil microbial and enzymatic activities. Environmental Pollution, 157, 1636–1642.
Matozzo, V. (2014). Effects of pharmaceuticals on immune parameters of aquatic invertebrates; A review. Invertebrate Survival Journal, 11, 163–173.
Nakata, H., Kannan, K., Jones, P. D., & Giesy, J. P. (2005). Determination of fluoroquinolone antibiotics in wastewater effluents by liquid chromatography–mass spectrometry and fluorescence detection. Chemosphere, 58(6), 759–766.
Nguyen, C., Giang, D., Sebesvari, Z., Renaud, F., & Rosendahl, I. (2015). Occurrence and dissipation of the antibiotics trimethoprim, and enrofloxacin in the Mekong Delta. Vietnam, 1–24. doi:10.1371/journal.pone.0131855.
Orata, F., Quinete, N., & Wilken, R. (2009). Long chain perfluorinated alkyl acids derivatisation and identification in biota and abiota matrices using gas chromatography. Bul Environ Contam and Toxicol, 83, 630–635.
Park, H., & Choung, Y. (2007). Degradation of antibiotics (tetracycline, sulfathiazole, ampicillin) using enzymes of glutathion s-transferase. Int. J. Human and Ecolog. Risk assess., 13(5), 1147–1155.
Radjenović, J., Petrović, M., Barceló, D. (2009). Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Research, 43(3), 831–841.
Straub, J. O. (2015). Aquatic environmental risk assessment for human use of the old antibiotic sulfamethoxazole in Europe. Environmental Toxicology and Chemistry, 9999(9999), n/a–n/a. doi:10.1002/etc.2945.
Ternes, T., Bonerz, M., & Schmidt, T. (2001). Determination of neutral pharmaceuticals in wastewater and rivers by liquid chromatography-electrospray tandem mass spectrometry. Journal of Chromatography. A, 938, 175–185.
Tsiaka, P., Tsarpali, V., Ntaikou, I., et al. (2013). Ecotoxicology, 22, 1208. doi:10.1007/s10646-013-1108-3.
van der Ven, K., van Dongen, W., Maes, B. U. W., Esmans, E. L., Blust, R., de Coen, W. M. (2004). Determination of diazepam in aquatic samples by capillary liquid chromatography electrospray tandem mass spectrometry. Chemosphere, 57(8), 967–73.
Werimo, K., Bergwerff, A., & Seinen, W. (2009). Residue levels of organochlorines and organophosphates in water, fish and sediments from Lake Victoria-Kenyan portion. J. Aquatic Ecosys Health & Manag, 12(3), 337–341.
Yang, X., Flowers, R. C., Weinberg, H. S., & Singer, P. C. (2011). Occurrence and removal of pharmaceuticals and personal care products (PPCPs) in an advanced wastewater reclamation plant. Water Research, 45(16), 5218–5228.
Acknowledgments
The authors are grateful for support from Alexander Von Humboldt research grant (3.4-IP-1119366/s-700611-5044-001). We also acknowledge Chuka University for availing HPLC instrument for analysis. We thank Agnes Muyale for assistance in analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kimosop, S.J., Getenga, Z.M., Orata, F. et al. Residue levels and discharge loads of antibiotics in wastewater treatment plants (WWTPs), hospital lagoons, and rivers within Lake Victoria Basin, Kenya. Environ Monit Assess 188, 532 (2016). https://doi.org/10.1007/s10661-016-5534-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5534-6