Abstract
Bark and exudates are widely commercialized non-timber forest products. However, the ecological impacts of the harvesting of these products have seldom been studied. The aim of this study is to investigate the relationship of tree resilience to harvesting intensity in Himatanthus drasticus, a tree that is highly exploited in the Brazilian savanna (Cerrado) for its medicinal latex. Although the traded product is the latex, the traditional harvesting systems involve the removal of the bark of the trees to allow exploitation. A 3-year experiment was conducted in two different Cerrado ecosystems (open savanna and savanna woodland). Trees were debarked at four debarking intensities to simulate the effects of traditional management systems. Measurements of bark growth were taken every 6 months, and quantitative and qualitative indexes of bark regeneration were obtained. The mortality of the debarked trees was low and could not be related to the intensity of harvesting. No signs of attack by fungi or insects were recorded. Compared with other species exploited for bark, H. drasticus is very resilient to harvesting; however, bark regeneration is relatively slow. In both analyzed ecosystems, the regeneration indexes showed higher values in the controls than in the treatments, indicating that 3 years is not sufficient for total recovery of the rhytidome. Bark regeneration occurred primarily by sheet growth and was more rapid in open savanna than in savanna woodland. No differences in the rate of bark recovery were found among management treatments. Based on the results, sustainable harvesting guidelines are suggested for the species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Humans have gathered useful plant species from diverse ecosystems from the earliest times (Schippmann et al. 2006; Laird et al. 2010). Among the potential uses of plants, those related to medicine play a central role because they are essential to human survival (Toledo et al. 2009). Approximately 80 % of the global population depends on medicinal plants (MPs) for healthcare (WHO 2001). In developing countries, especially in rural areas, the use of MPs is particularly relevant due to the difficulty of access to modern medicine (Rani and Khullar 2004; Haq 2011), the prohibitive cost of pharmaceutical products (Shanley and Luz 2003; Malviya et al. 2012) or as a result of cultural preferences (Cocks and Dold 2006; Cocks et al. 2011; Suleman and Alemu 2012).
Currently, phytotherapy is experiencing a revival in the developed countries (Efferth and Greten 2012). One of the reasons for this popularity is that natural products are preferred to products of industrial origin (Di Stasi 1996; Efferth and Greten 2012). The growing demand for herbal products has led to a significant increase in the volume of plant materials traded within and between countries (Pandey et al. 2010; Freitas et al. 2012). Most MP products are derived from natural populations; relatively few species are cultivated (Leaman 2004; Geldenhuys and Mitchel 2006). For this reason, there is an increasing concern about the sustainable management of MPs because many species are sensitive to high levels of harvest or to ecosystem changes (Diederichs et al. 2006; Pandey et al. 2010).
Despite all the valuable efforts to establish ecological and socioeconomic criteria for the certification of non-timber forest products (Shanley et al. 2002) or specific guidelines for desirable field collection practices for MPs (WHO 1993; 2002; 2003; Kathe 2006), a lack of knowledge about sustainable harvest rates and practices remains one of the major challenges to the development of sustainable wild collection (Schippmann et al. 2006; Ticktin and Shackleton 2011). Therefore, there is an urgent need to obtain ecological data on species that are currently under exploitation, primarily MPs for which the market demand is high.
In the Brazilian savanna (Cerrado), the demand for marketable MPs is producing a decrease in their natural populations (Felfili and Silva Junior 1988; Borges-Filho and Felfili 2003; Zardo and Henriques 2011). Himatanthus drasticus (Apocynaceae), commonly known as janaguba, is one of the most commonly harvested tree species in the Cerrado biome. The medicinal value of its latex for the treatment of cancer was recently recognized based on pharmacological studies (Souza et al. 2010; Mousinho et al. 2011), and this finding has caused the trade in the latex to increase, with potential negative impacts on the natural populations of the species. Although the traded product is the latex, the traditional harvesting systems involve the removal of the bark of the trees to allow exploitation, and ringbarking of the tree is common.
In species harvested for bark, the definition of a maximum sustainable harvesting limit for the bark is necessary to ensure the persistence of individuals and populations (Delvaux et al. 2010). Overall, it is important to consider the response of bark regeneration not only under several intensities of debarking but also in different environments. Few studies, however, have investigated how different environments or ecosystems affect harvest impacts in non-timber forest products (Gaoue and Ticktin 2007).
To evaluate the impacts of current harvesting activities on natural populations of H. drasticus as well as to suggest limits for the sustainable harvesting of the species, we addressed the following specific questions:
-
1.
Does harvesting cause an increase in mortality in H. drasticus?
-
2.
What are the effects of different debarking intensities on the time needed for bark regrowth?
-
3.
Do the bark regrowth rates depend on the type of ecosystem?
-
4.
What is the most appropriate management regime for H. drasticus in each studied ecosystem?
Materials and methods
Study area and species
The study area is located in the Araripe mesoregion, in the center of northeastern Brazil. This region is characterized by a particular territorial pattern, including socially and economically underdeveloped areas as well as relatively developed areas supporting activities that integrate the social and economic dynamics of the country (Cardoso 2010; MDA 2010).
The Chapada do Araripe is a plateau in the Araripe mesoregion. The highest areas of this plateau support savanna vegetation (Cerrado); humid and dry forest and caatinga dry and moist forests occur on the slopes. This region is an area of extremely high priority for the conservation of the Cerrado (Oliveira and Marquis 2002) and Caatinga biomes (MMA 2007). The Araripe National Forest (FLONA-Araripe, Fig. 1), in the Araripe mesoregion, is the first National Forest in Brazil, established in 1946. This protected area includes parts of five municipalities of Ceará State (Barbalha, Crato, Jardim, Missão Velha, and Santana do Cariri).
FLONA-Araripe is considered a habitat island, with higher rainfall and lower temperature than the surrounding Caatinga biome (semi-arid forest; Costa 2004). The soil is a red-yellow dystrophic latosoil (Cavalcanti and Lopes 1994), and the climate is Tropical wet and dry or Savanna climate (Aw) (Köppen 1948). The biodiversity of this area is high and includes an endemic and critically endangered bird, the Araripe manakin (Antilophia bookermanni). Several species with socioeconomic importance are found in FLONA-Araripe, including pequi (Caryocar coriaceum), faveira (Dimorphandra mollis), and janaguba (H. drasticus).
H. drasticus is a tree species endemic to Brazil. Its height ranges from 3 to 7 m. Young branches are dark brown with light brown spots; older branches are pale brown (Spina 2004). This species occurs in the Cerrado and Caatinga (semi-arid forest) biomes, and its distribution includes all of northeastern Brazil (Spina 2004). Mixed with water, the latex of H. drasticus (“leite de janaguba”) is popularly used to treat gastritis, hemorrhoids, anemia, inflammations, and many types of cancer (Lucetti et al. 2010).
Experimental design, data collection, and analyses
For study, we selected two ecosystems of Araripe National Forest: “cerrado sensu stricto” (hereafter cerrado) and “cerradão.” Cerrado is defined as “a vegetation dominated by 3–8-m tall trees and shrubs with more than 30 % crown cover but with still a fair amount of herbaceous vegetation between them”, whereas “cerradão” is described as “an almost closed woodland with crown cover of 50 % to 90 %, made up of 8–12-m-tall trees casting considerable shade so that the ground layer is much reduced” (Oliveira and Marquis 2002).
The experiments involved the removal of strips 2.0 m in length. Four levels of debarking were used. The approximate percentage of the circumference at breast height subjected to debarking was identified as follows: approximately 25 %—“one side”, 50 %—“two sides”, 75 %—“three sides,” and 100 %—“four sides.” These treatments were used to represent the management systems employed by the harvesters in the study area. These values are approximate because bark is removed with a scythe or machete. Even in the “four sides” treatment, a narrow strip of bark remained on the individual tree. These remaining strips were used as a control and compared with the values obtained for regeneration.
Each treatment was applied to 15 individuals, with a total of 120 sampled trees (60 in each ecosystem). All trees were exploited by the same harvester to eliminate the additional variation that could result from differences among harvesters. The latex was extracted after the bark was removed. The exposed area was measured on the next day, after the drained latex had dried.
The term “bark” is somewhat generic, and the consideration of bark as a single unit may obscure important aspects of the biochemistry, physiology, ecology, and evolutionary biology of the species of interest (Romero 2006). Thus, a more detailed definition would recognize the “inner bark” as a product of the vascular cambium composed of live phloem, whereas the “outer bark,” or rhytidome, would be a complex of tissues including the products of phellogen, epidermis, cortex, primary and secondary phloem, as well as all tissues external to the phellogen dead at maturity (Romero 2006). In this experiment, the rhytidome was removed to simulate the traditional management systems. The depth of the bark removed was variable, with higher values in trees with greater diameters because their rhytidome was thicker.
Evaluations were made every 6 months for 3 years. The mortality in each area/treatment was also recorded. In the first evaluation, it was observed that regeneration occurs through sheet growth (regrowth on the surface of the wound). For this reason, a bark gauge was used to obtain data on the thickness of the recovered bark (TRB). Three measurements were performed on each tree, and the average of these measurements (centimeters) was used in the analyses. A two-way ANOVA was performed to compare TRB values between ecosystems and treatments. The average TRB values were compared with the controls, and confidence intervals were obtained from 10,000 bootstrap runs.
A qualitative evaluation of bark regeneration was also conducted. For this purpose, the bark regeneration of each individual was classified using an increasing scale that ranged from zero to five, zero indicating the absence of regeneration and five indicating complete recovery of the bark, including the rhytidome (Fig. 2). This measure was termed the “bark regeneration index” (BRI). The BRI scores were determined by one researcher and one harvester. The BRI values were compared among treatments and between areas with a Scheirer–Ray–Hare test (Dytham 2011).
To test the effect of tree size on the bark recovery rates, the trees were assigned to three classes according to their diameter at ground level (DGL): class 1 (7.0 ≤ DGL < 10.0), class 2 (10.0 ≤ DGL < 13.0), and class 3 (≥13.0). The indexes obtained were subjected to an ANOVA and a Tukey test. To verify the possible influence of light on the bark regeneration process, the crown illumination index (CII) (Clark and Clark 1992) was used. This index is a visual estimate of the proportion of the canopy of a tree that receives light, ranging from one (completely shaded canopy) to five (fully exposed canopy). A Spearman correlation coefficient (r s) was subsequently calculated to associate the TRB and BRI values with the CII. All analyses were performed in the R environment (R Development Core Team 2012).
Results
Regeneration patterns
Only two individuals initiated bark regrowth from the center of the exposed area. The regeneration was most likely triggered by living cells remaining on the xylem at the time of debarking. A single individual showed edge growth. These exceptions excluded, all individuals showed sheet growth, characterized by the simultaneous regeneration of tissues throughout the surface of the exposed area. The details of the regeneration process of the inner bark are still unclear, but it apparently occurs through growth in all directions from the thin layer of inner bark that is left by the harvesters when collecting the latex.
In most individuals, a significant structural modification of the internal bark, indicating tissue regeneration, occurred only in the second year of the experiment. These results contrast with the information provided by many harvesters, who consider 1 year sufficient for bark regeneration (Baldauf and Santos 2013). However, this issue is controversial among the harvesters because some of them believe that more than 18 months is needed for bark regrowth.
Over the 3-year study period, 9 of the 120 harvested trees died (7 in the cerradão and 2 in the cerrado). In terms of the degree of debarking of the dead trees, one tree had 25 % debarking, two had 50 %, four had 75 %, and only one had 100 % debarking.
Effects of the environment and the management system
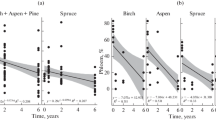
The values of bark recovery (thickness) ranged from 0.15 to 0.65 cm (mean = 0.32; sd = 0.09). Bark regrowth varied between ecosystems, with higher average values in the cerrado than in the cerradão (Table 1 and Fig. 3). However, the bark recovery values obtained in each management treatment did not differ (Table 1, Fig. 3a). The same pattern was found for the BRI (Table 2, Fig. 3b).
In both analyzed ecosystems, a comparison of the TRB values showed higher values in the controls than in the treatments, indicating that 3 years is not sufficient for total recovery of the rhytidome (Table 3). A recovery of 60.2 and 46.0 % of the rhytidome was observed in the cerrado and cerradão ecosystems, respectively.
No association was found between bark regeneration and the diameter of the harvested trees in the cerrado. However, the regeneration in the cerradão was higher in diameter class 3 than in the smaller trees, diameter class 1 (F = 4.485; p = 0.015 and F = 6.991; p = 0.002 for BRI and TRB, respectively). No differences were found in the other contrasts.
Finally, a positive correlation was found between the TRB and the CII (r s = 0.43; df = 109; p < 0.01), as well as between the BRI and the CII (r s = 0.57, df = 109; p < 0.01), indicating greater regeneration in the trees that received more sunlight.
Discussion
Regeneration patterns and mortality
Bark regeneration can result from edge growth and/or sheet growth. Regeneration from the edges is the most common pattern. In this case, regeneration originates from the bark of living tissues (vascular cambium, phloem, and phellogen) along the edge of the exploited area toward the center (Mariot 2008). In sheet growth, the living tissue regenerates simultaneously over the entire exposed surface area from the damaged xylem. Certain species may also show regeneration from the center of the wound. However, this pattern is relatively uncommon in trees (Mariot 2008).
A wide variety of tissues may be involved in the regeneration of the bark. These tissues include the phloem parenchyma, xylem parenchyma, immature xylem zone, and cambium (Delvaux 2009). The details of the regeneration process in H. drasticus and the tissues involved are still unknown. Throughout the experiment, regrowth occurred as sheet growth, and only three individuals showed a different pattern. In these three individuals, however, the harvester accidentally penetrated the bark during exploitation of the latex, reaching the heartwood. Thus, the pattern of regeneration from the edges or center appears to be a response to deeper damage and thus represents an exception to the commonly observed pattern of sheet growth.
Responses to damage in the bark and, consequently, the formulation of criteria for sustainable management depend on a number of factors, such as the type of damaged tissue and the extent of damage, the morphology and anatomy of bark, and the presence of exudates (Guariguata and Gilbert 1996; Schoonenberg et al. 2003; Romero and Bolker 2008; Pandey et al. 2012). Because of these multiple factors, the effect of bark removal and the sustainability of harvesting practices can be considered species-specific (Chungu et al. 2007; Delvaux et al. 2009).
Romero and Bolker (2008) studied the effects of debarking in seven species in the Bolivian Amazon. Their study found that species that produce some type of exudate showed more efficient bark recovery and that wound healing occurs more readily in species with thick bark that in species with thin bark. These findings are important in the case of H. drasticus because it produces exudates and has relatively thick bark. These characteristics indicate that H. drasticus is potentially capable of showing efficient bark regeneration and, consequently, that it is potentially suitable for sustainable harvesting.
Effects of environment and management systems
Several experiments have shown that the most important factor for successful recovery of the bark is the humidity at the exposed layer (Neely 1988; Stobbe et al. 2002; Juan 2006), which may be related to the occurrence of a rainy season. Moreover, seasonality can influence the regeneration of the bark due to variations in tissue water content and in the activity of the cambium (Puritch and Mullick 1975; Dujesiefken and Liese 1990).
Mariot et al. (2008) tested the effect of different harvest seasons on the regenerative ability of the species Drimys brasiliensis (Winteraceae) in an Atlantic Forest area and found no seasonal differences in the speed of biomass regeneration. In Myracrodruon urundeuva (Anacardiaceae), a medicinal species whose bark is exploited in the Brazilian dry forest (Caatinga), no significant correlation was found between the monthly percent regeneration and the average monthly precipitation (Monteiro et al. 2011). However, Delvaux et al. (2010), assessing the bark regrowth patterns of 12 species in Benin, found that the rate of regeneration was higher during the spring. Other species in which bark recovery is influenced by the season are Terminalia arjuna (Combretaceae) and Litsea glutinosa (Lauraceae), a highly exploited Indian medicinal tree (Pandey and Mandal 2012).
Vermeulen and Geldenhuys (2004) studied the effects of bark harvesting in three tree species in South Africa and observed that debarking in the winter (dry season and low temperatures) favored edge growth, whereas exploiting the same species in the summer (rainy season and high temperatures) promoted sheet growth. Because bark growth in H. drasticus occurs through sheet growth, it is possible that the rainy season is favorable for harvesting. Thus, seasonality is an important variable to be tested in future studies on the bark regeneration potential of the species.
The results showed that the regeneration of the bark was favored by open environments because the correlation between TRB/BRI and light was significant and positive and the regeneration of bark was more rapid in the cerrado than in the cerradão. These data apparently contradict the trend cited before, in which humidity is considered the most important factor for the regeneration of the bark through laminar growth, because the cerrado has lower levels of humidity than the cerradão. However, it is possible that the latex that remains in the trees after extraction serves to provide the necessary humidity to trigger the cellular division process that will culminate in the recovery of injured xylem and phloem. Plants of certain families, such as Apocynaceae, Euphorbiaceae, Moraceae, and Canellaceae, show great resilience after debarking, in part because the cambium is protected by exudates after the bark is removed (Cunningham 2001).
The absence of differences among groups with different percentages of debarking and the lack of signs of morbidity in the trees with approximately 100 % removal of the bark represents a contrast with the results of several previous studies on bark harvesting. In many species studied to date, overexploited individuals died shortly following the exploitation, most likely due to the disruption of water and/or nutrient flows (Cunningham and Mbenkum 1993; Borges Filho and Felfili 2003; Geldenhuys et al. 2007; Guedje et al. 2003, 2007; Delvaux et al. 2010).
Species in which individuals can survive ringbarking are rare. Walburgia salutaris is an African medicinal plant that strongly and intensely regenerates bark after ringbarking (Cunningham and Mbenkum 1993; Botha et al. 2004). Other species that can recover after complete debarking are Prunus africana (Cunningham and Mbenkum 1993) and Adansonia digitata (Fasola and Egunyomi 2005). In A. digitata, the wood parenchyma immediately below the exposed surface dedifferentiates and regenerates the xylem and phloem (Fisher 1981); however, the bark regrowth is considered to occur slowly (Fasola and Egunyomi 2005).
A possible explanation for the resilience of H. drasticus is that most of the harvesters remove only the rhytidome. This type of bark removal does not impede the flow of water and photosynthates. Vermeulen and Geldenhuys (2004) found a high rate of regeneration if a thin layer of bark and cambium was left on the exploited trees, whereas little or no regeneration was observed if the bark was completely removed in three species. This trend was also verified by Delvaux et al. (2009), who detected greater sheet regeneration if a layer of bark was left on harvested trees than if all the bark was removed. In P. africana, trees that were harvested without disrupting the vascular cambium showed an increased probability of survival (Stewart 2009).
Debarking may also favor attack by diseases caused by insects and fungi, especially if a large amount of bark is removed (Fasola and Egunyomi 2005; Mariot et al. 2008; Chungu et al. 2007). Nevertheless, the current study found no evidence of attacks by insects or fungi, even in plants with approximately 100 % of the bark removed. The absence of pathogen attacks might be related to the secondary metabolites with antimicrobial activity found in Himatanthus bark and latex (Silva et al. 1998; Souza et al. 2004). Furthermore, no evidence of crown senescence was observed in the harvested trees of H. drasticus. Crown health has been used as an indicator of tree health after bark harvesting because the crowns of the exploited trees frequently display signs of senescence (Sunderland and Tako 1999; Hall et al. 2000; Stewart 2009; Uniyal 2013).
Suggestions for sustainable management
The results obtained for H. drasticus indicate that the species is extremely tolerant to debarking. However, bark regeneration is relatively slow. Regardless of the ecosystem or management system, the bark of the exploited trees had not recovered completely by the end of the 3-year experimental period.
The recovery of the inner bark in H. drasticus progressed to an advanced stage or was complete by the end of the experimental period. However, the formation of rhytidome appears to be a slower process (personal observation). Rhytidome plays an important role because it serves as a physical barrier to protect against attacks by herbivores, insects, fungi, and fire (Catry et al. 2012). Thus, even if the inner bark recovers, the exploited individuals in cerrado areas are more vulnerable because the thickness of the bark is an important determinant of survival probabilities if fires occur (Hare 1965; Prance and Prance 1993). In Quercus suber, bark thickness and bark harvesting are the major factors limiting resistance to fire (Catry et al. 2012).
In this context, despite the resilience of H. drasticus to latex exploitation, a balance must be reached between product demand and the recoverability of the bark. This is especially the case in the cerradão, where the regeneration of the bark is slower than in the cerrado. Another factor that should be considered is that bark regeneration is slower in the smaller plants in the cerradão. This result is surprising because the literature has reported faster regeneration in younger and middle-aged trees (Delvaux et al. 2010; Pandey and Mandal 2012).
The results obtained in the cerrado and cerradão show that specific management regimes are needed for each distinct ecosystem. To maintain protection against fire, the removal of a maximum of 50 % of the bark (two sides) is suggested. It is recommended that the exploitation of individuals less than 10 cm in DGL (class 1) in cerradão areas be avoided because bark regeneration is slower in these individuals.
Under the assumption that the regeneration of the bark is constant in other size classes, the optimal interval between harvesting events (to allow the bark to recover completely) would be 5 years in cerrado areas and 6.5 years in cerradão areas. This suggestion is based on the assumption that complete recuperation of the bark is needed for sustainable management. This assumption may not be true, but there is no doubt that partial recovery is essential for plant protection and survival if fires occur. Therefore, the intervals suggested can serve as reference values for the process of developing participatory management plans for H. drasticus. The opinions and knowledge of the multiple stakeholders must be considered in this process so that the socioeconomic and cultural dimensions of sustainability can also be addressed.
Conclusions
Compared with other species exploited for bark, H. drasticus is very resilient to harvesting. No relationships between harvesting intensity and mortality or bark recovery speed were found in this species by the current study. However, bark regeneration is relatively slow and ecosystem dependent. It is more rapid in the open areas than in the woodlands. The establishment of criteria for the harvesting of this species is of the utmost importance because the demand for the product has been increasing substantially. These increasing demands can potentially cause overharvesting. In this context, the results obtained in this study are key elements for the development of a sustainable management plan for the species.
References
Baldauf, C., & Santos, F. A. M. (2013). Ethnobotany, traditional knowledge, and diachronic changes in non-timber forest products management: the case study of Himatanthus drasticus (Apocynaceae) in the Brazilian Savanna. Economic Botany, 67(2), 110–120.
Borges Filho, H. C., & Felfili, J. M. (2003). Avaliação dos níveis de extrativismo da casca de barbatimão [Stryphnodendron adstringens (Mart.) Coville] no Distrito Federal, Brasil. Revista Árvore, 27(5), 735–745.
Botha, J., Witkowski, E., & Shackleton, C. (2004). The impact of commercial harvesting on Warburgia salutaris (‘pepper-bark tree’) in Mpumalanga, South Africa. Biodiversity and Conservation, 13(9), 1675–1698.
Cardoso, M. R. C. (2010). Desenvolvimento rural e sustentabilidade-o caso da mesorregião Chapada do Araripe. Brasília: Universidade de Brasílila.
Catry, F. X., Moreira, F., Pausas, J. G., Fernandes, P. M., Rego, F., Cardillo, E., et al. (2012). Cork oak vulnerability to fire: the role of bark harvesting, tree characteristics and abiotic factors. PLoS ONE, 7(6), e39810.
Cavalcanti, A. C., & Lopes, O. F. (1994). Condições edafoclimáticas da Chapada do Araripe e viabilidade de produção sustentável de culturas. Brasília: EMBRAPA-SPI.
Chungu, D., Muimba-Kankolongo, A., Roux, J., & Malambo, F. (2007). Bark removal for medicinal use predisposes indigenous forest trees to wood degradation in Zambia. Southern Hemisphere Forestry Journal, 69(3), 157–163.
Clark, D. A., & Clark, D. B. (1992). Life history diversity of canopy and emergent trees in a neotropical rain forest. Ecological Monographs, 62(3), 315–344.
Cocks, M., López, C., & Dold, T. (2011). Cultural importance of non-timber forest products: opportunities they pose for bio-cultural diversity in dynamic societies. In S. Shackleton, C. Shackleton, & P. Shanley (Eds.), Non-timber forest products (pp. 107–128). Berlin: Springer.
Cocks, M. L., & Dold, A. P. (2006). Cultural significance of biodiversity: the role of medicinal plants in urban African cultural practices in the Eastern Cape, South Africa. Journal of Ethnobiology, 26(1), 60–81.
Cunningham, A. (2001). Applied ethnobotany. People and plants conservation manual. London: Earthscan Publications Ltd.
Cunningham, A., & Mbenkum, F. (1993). Sustainability of harvesting Prunus africana bark in Cameroon. People and Plants Working Paper, 2, 28. Paris.
Delvaux, C. (2009). “Strip-trees”: the life after: responses to bark harvesting of medicinal tree species from Forêt Classée Des Monts Kouffé, Benin. University of Ghent.
Delvaux, C., Sinsin, B., Darchambeau, F., & Van Damme, P. (2009). Recovery from bark harvesting of 12 medicinal tree species in Benin, West Africa. Journal of Applied Ecology, 46(3), 703–712.
Delvaux, C., Sinsin, B., & Van Damme, P. (2010). Impact of season, stem diameter and intensity of debarking on survival and bark re-growth pattern of medicinal tree species, Benin, West Africa. Biological Conservation, 143(11), 2664–2671.
Di Stasi, L. C. (1996). Plantas medicinais: arte e ciência: um guia de estudo interdisciplinar: Ed. Estadual Paulista: UNESP-Univ.
Diederichs, N., McKean, S., & Wynberg, R. P. (2006). Conservation and trade regulations for medicinal plants. In N. Diederichs (Ed.), commercialising medicinal plants: a Southern African guide (pp. 9–19). Stellenbosh: Sun Press.
Dujesiefken, D., & Liese, W. (1990). Einfluß der Verletzungszeit auf die Wundheilung bei Buche (Fagus sylvatica L.). Holz als Roh- und Werkstoff, 48(3), 95–99.
Dytham, C. (2011). Choosing and using statistics: a biologist’s guide. Oxford: Wiley-Blackwell.
Efferth, T., & Greten, H. J. (2012). Medicinal and aromatic plant research in the 21st century. Medicinal and Aromatic Plants, 1, e110.
Fasola, T. R., & Egunyomi, A. (2005). Nigerian usage of bark in phytomedicine. Ethnobotany Research and Applications, 3, 073–077.
Felfili, J., & Junior, M. S. (1988). Distribuição dos diâmetros numa faixa de cerrado na fazenda Água Limpa em Brasília-DF. Acta Botanica Brasilica, 2(1–2), 85–104.
Fisher, J. B. (1981). Wound-healing by exposed secondary xylem in Adansonia (Bombacaceae). IAWA Bulletin, 2, 193–199.
Freitas, A. V. L., Coelho, M. F. B., Maia, S. S. S., & Azevedo, R. A. B. (2012). Plantas medicinais: um estudo etnobotânico nos quintais do Sítio Cruz, São Miguel, Rio Grande do Norte, Brasil. Revista Brasileira de Biociências, 10(1), 48.
Gaoue, O. G., & Ticktin, T. (2007). Impacts of bark and foliage harvest on Khaya senegalensis (Meliaceae) reproductive performance in Benin. Journal of Applied Ecology, 45(1), 34–40.
Geldenhuys, C. J., & Mitchell, D. (2006). Sustainable harvesting technologies. In N. Diederichs (Ed.), Commercializing medicinal plants: a Southern African guide (pp. 23–39). Stellenbosh: Sun Press.
Geldenhuys, C. J., Syampungani, S., Meke, G. S., & Vermeulen, W. J. (2007). Response of different species to bark harvesting for traditional medicine in Southern Africa. In J. J. Bester, A. H. W. Seydack, T. Vorster, I. J. Van der Merwe, & S. Dzivhani (Eds.), Multiple use management of natural forests and woodlands: policy refinement and scientific progress (pp. 55–62). Pretoria: Department of Water Affairs and Forestry.
Guariguata, M. R., & Gilbert, G. S. (1996). Interspecific variation in rates of trunk wound closure in a Panamanian lowland forest. Biotropica, 28(1), 23–29.
Guedje, N. M., Lejoly, J., Nkongmeneck, B.-A., & Jonkers, W. B. (2003). Population dynamics of Garcinia lucida (Clusiaceae) in Cameroonian Atlantic forests. Forest Ecology and Management, 177(1), 231–241.
Guedje, N. M., Zuidema, P. A., During, H., Foahom, B., & Lejoly, J. (2007). Tree bark as a non-timber forest product: the effect of bark collection on population structure and dynamics of Garcinia lucida Vesque. Forest Ecology and Management, 240(1), 1–12.
Hall, J. B., O’Brien, E. M., & Sinclair, F. L. (2000). Prunus africana: a monograph (vol. 18). Bangor: University of Wales, School of Agricultural and Forest Sciences
Haq, F., Ahmad, H., & Alam, M. (2011). Traditional uses of medicinal plants of Nandiar Khuwarr catchment (District Battagram), Pakistan. Journal of Medicinal Plants Research, 5, 39–48.
Hare, R. C. (1965). Contribution of bark to fire resistance of southern trees. Journal of Forestry, 63(4), 248–251.
Juan, D., Hong-Li, X., De-Qiang, Z., Xin-Qiang, H., Min-Jie, W., Ying-Zhang, L., et al. (2006). Regeneration of the secondary vascular system in poplar as a novel system to investigate gene expression by a proteomic approach. Proteomics, 6(3), 881–895.
Kathe, W. (2006). Revision of the “Guidelines on the conservation of medicinal plants” by WHO, IUCN, WWF AND TRAFFIC In R. J. Bogers, L. E. Craker, & D. Lange (Eds.), Medicinal and aromatic plants (pp. 109–120): Springer.
Köppen, W. (1948). Climatologia: con un estudio de los climas de la tierra. Fondo de Cultura Econômica (pp. 479).
Laird, S. A., McLain, R. J., & Wynberg, R. P. (2010). Wild product governance: finding policies that work for non-timber forest products. London: Earthscan/James & James.
Leaman, D. J. (2004). The global strategy for plant conservation—what can it mean for medicinal plants? Newsletter of the Medicinal Plant Specialist Group, 9/10.
Lucetti, D. L., Lucetti, E. C., Bandeira M. A., Veras, H. N., Silva, A. H., Leal, L. K., et al. (2010). Anti-inflammatory effects and possible mechanism of action of lupeol acetate isolated from Himatanthus drasticus (Mart.) Plumel. Journal of Inflammation, 7, 60.
Malviya, J., Joshi, V., & Singh, K. (2012). Antimicrobial activity of some ethno-medicinal plants used by Baiga Tribes from Amarkantak, India. Advances in Life Science and Technology, 4, 19–26.
Mariot, A. (2008). Fundamentos para o manejo de populações naturais de Drimys brasiliensis Miers-Winteraceae. Florianópolis: Universidade Federal de Santa Catarina.
MDA. (2010). In M. D. Agrário (Ed.), Plano Territorial de Desenvolvimento Rural Sustentável: Território Cidadania do Cariri (p. 348). Fortaleza: Instituto Agropolos do Ceará.
MMA. (2007). Áreas prioritárias para a conservação, uso sustentável e repartição de benefícios da biodiversidade brasileira: atualização - Portaria MMA Nº 09, 23 de janeiro de 2007. (pp. 300). Brasília: MMA/SBF.
Monteiro, J. M., Lins Neto, E. M., Araújo, E. L., Amorim, E. L., & Albuquerque, U. P. (2011). Bark regeneration and tannin content in Myracrodruon urundeuva Allemão after simulation of extractive damages—implications to management. Environmental Monitoring and Assessment, 180(1), 31–39.
Mousinho, K. C., Oliveira, C. C., Ferreira, J. R. d. O., Carvalho, A. A., Magalhães, H. I. F., Bezerra, D. P., et al. (2011). Antitumor effect of laticifer proteins of Himatanthus drasticus (Mart.) Plumel–Apocynaceae. Journal of ethnopharmacology, 137(1), 421–426.
Neely, D. (1988). Wound closure rates on trees. Journal of Arboriculture, 14, 250–254.
Oliveira, P. S., & Marquis, R. J. (2002). The cerrados of Brazil: ecology and natural history of a neotropical savanna. New York: Columbia Univ Press.
Pandey, A. K., & Mandal, A. K. (2012). Sustainable harvesting of Terminalia arjuna (Roxb.) Wight & Arnot (Arjuna) and Litsea glutinosa (Lour.) Robinson (Maida) bark in Central India. Journal of Sustainable Forestry, 31(3), 294–309.
Pandey, A. K., & Yadav, S. (2010). Variation in gymnemic acid content and non-destructive harvesting of Gymnema sylvestre (Gudmar). Pharmacognosy Research, 2(5), 309.
Prance, G. T., & Prance, A. E. (1993). Bark. Portland: Timber Press.
Puritch, G. S., & Mullick, D. B. (1975). Effect of water stress on the rate of non-suberized impervious tissue formation following wounding in Abies grandis. Journal of Experimental Botany, 26(6), 903–910.
R Development Core Team (2012). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org.
Rani, P., & Khullar, N. (2004). Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multi-drug resistant Salmonella typhi. Phytotherapy Research, 18(8), 670–673.
Romero, C. (2006). Tree responses to stem damage. University of Florida.
Romero, C., & Bolker, B. M. (2008). Effects of stem anatomical and structural traits on responses to stem damage: an experimental study in the Bolivian Amazon. Canadian Journal of Forest Research, 38(3), 611–618.
Schippmann, U., Leaman, D., & Cunningham, A. B. (2006). A comparison of cultivation and wild collection of medicinal and aromatic plants under sustainability aspects. In R. J. Bogers, L. E. C. L.E., & L. D. (Eds.), Medicinal and aromatic plants (vol. 17, pp. 75–95). Dordrecht: Springer.
Schoonenberg, T., Pinard, M., & Woodward, S. (2003). Responses to mechanical wounding and fire in tree species characteristic of seasonally dry tropical forest of Bolivia. Canadian Journal of Forest Research, 33(2), 330–338.
Shanley, P., & Luz, L. (2003). Eastern Amazonian medicinals: marketing, use and implications of forest loss. BioScience, 53(6), 573–584.
Shanley, P., Pierce, A. R., Laird, S. A., & Guillén, A. (2002). Tapping the green market: certification and management of non-timber forest products. London: Earthscan/James & James.
Silva, J. R. A., Rezende, C. M., Pinto, Â. C., Pinheiro, M. L., Cordeiro, M. C., Tamborini, E., et al. (1998). Triterpenic esters from Himatanthus sucuuba (Spruce) Woodson. Quimica Nova, 21(6), 702–704.
Sousa, E. L. d., Grangeiro, A. R. S., Bastos, I. V. G. A., Rodrigues, G. C. R., Anjos, F. B. R. d., Souza, I. A. d., et al. (2010). Antitumor activity of leaves of Himatanthus drasticus (Mart.) Plumel-Apocynaceae (janaguba) in the treatment of Sarcoma 180 tumor. Brazilian Journal of Pharmaceutical Sciences, 46(2), 199–203.
Souza, W., Stinghen, A., & Santos, C. (2004). Antimicrobial activity of alkaloidal fraction from barks of Himatanthus lancifolius. Fitoterapia, 75(7), 750–753.
Spina, A. P. (2004). Estudos taxonômico, micro-morfológico e filogenético do gênero Himatanthus Willd. ex Schult. (Apocynaceae: Rauvolfioideae-Plumerieae). Universidade Estadual de Campinas.
Stewart, K. (2009). Effects of bark harvest and other human activity on populations of the African cherry (Prunus africana) on Mount Oku, Cameroon. Forest Ecology and Management, 258(7), 1121–1128.
Stobbe, H., Schmitt, U., Eckstein, D., & Dujesiefken, D. (2002). Developmental stages and fine structure of surface callus formed after debarking of living lime trees (Tilia sp.). Annals of Botany, 89, 773–782.
Suleman, S., & Alemu, T. (2012). A survey on utilization of ethnomedicinal plants in Nekemte Town, East Wellega (Oromia), Ethiopia. Journal of Herbs, Spices & Medicinal Plants, 18(1), 34–57.
Sunderland, T. C. H., & Tako, C. T. (1999). The exploitation of Prunus africana on the island of Bioko, Equatorial Guinea—a report for the People and Plants Initiative. In IUCN/SSC (Ed.), Medicinal Plant Specialist Group. Bonn.
Ticktin, T., & Shackleton, C. (2011). Harvesting non-timber forest products sustainably: opportunities and challenges. In S. Shackleton, C. Shackleton, & P. Shanley (Eds.), Non-timber forest products in the global context (pp. 149–169). Heidelberg: Springer.
Toledo, B., Galetto, L., & Colantonio, S. (2009). Ethnobotanical knowledge in rural communities of Cordoba (Argentina): the importance of cultural and biogeographical factors. Journal of Ethnobiology and Ethnomedicine, 5(1), 40.
Uniyal, S. K. (2013). Bark removal and population structure of Taxus wallichiana Zucc. in a temperate mixed conifer forest of western Himalaya. Environmental Monitoring and Assessment, 185(4), 2921–2928.
Vermeulen, W., & Geldenhuys, C. (2004). Experimental protocols and lessons learnt from strip harvesting of bark for medicinal use in the southern Cape forests. FRP-DFID Project R8305 Report (pp. 14). UK: Wild Resources Limited.
WHO (1993). Guidelines on the conservation of medicinal plants. Geneva: World Health Organization.
WHO (2001). General guidelines for methodologies on research and evaluation of traditional medicine. Geneva: World Health Organization.
WHO (2002). Traditional medicine strategy 2002–2005. Geneva: World Health Organization.
WHO (2003). WHO guidelines on Good Agricultural and Field Collection Practices (GACP) for medicinal plants. Geneva: World Health Organization.
Zardo, R. N., & Henriques, R. P. B. (2011). Growth and fruit production of the tree Caryocar brasiliense in the Cerrado of central Brazil. Agroforestry Systems, 82(1), 15–23.
Acknowledgments
The authors thank the Biodiversity Program Brazil-Italy (PBBI) for financial and logistic support; the National Council of Technological and Scientific Development (CNPq) for supporting this research with the Edital Universal (Process 472127/2008-0), a PhD fellowship granted to C.B. (Process 140813/2008-0) and a research productivity fellowship granted to F.A.M.S. (Process 308748/2010-7); the São Paulo Research Foundation (FAPESP) for a research grant (Process 2008/08737-4); the students of the “Janaguba project” and the FLONA-Araripe staff for field support; Manuel Guariguata for references and discussions on bark harvesting; and Julia Caram Sfair for her support and suggestions about the analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baldauf, C., dos Santos, F.A.M. The effect of management systems and ecosystem types on bark regeneration in Himatanthus drasticus (Apocynaceae): recommendations for sustainable harvesting. Environ Monit Assess 186, 349–359 (2014). https://doi.org/10.1007/s10661-013-3378-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3378-x