Abstract
Free-living marine nematode diversity was analyzed between Avicennia marina and Rhizophora mucronata mangrove covers of the Vellar Estuary (southeast coast of India). A total of 4,976 specimens of free-living marine nematodes were collected in 56 species. Comparatively, a higher species richness was obtained for A. marina (52 species) than for R. mucronata (44 species), whereas 40 species commonly existed in both mangrove covers. A higher density of nematodes was found in sediments of sandy nature, whereas there was lower total organic carbon compared to silt/clay composition; epigrowth feeders were dominant over the other feeding groups based on organic enrichment in surface sediments. Principal component analysis clearly explained the relationship between the environmental parameters of various months. Higher R values of analysis of similarities revealed significant differences in nematode assemblages between months, and it was quite evident by non-metric multidimensional scaling. Diversity indices showed higher values in the dry months. RELATE analysis explained serial changes in nematode species composition between months, and a relationship between biotic and abiotic variables was clarified using the BIO-ENV procedure. Viscosia spp., Metachromadora spp., Theristus spp., and Sphaerolaimus spp. were candidate species of A. marina leaf interaction by observation.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangroves are one of the most productive ecosystems in the world, an important ecological asset, and economic resource of the coastal environment (Kathiresan et al. 1996). They protect coasts from erosion and provide food and shelter for a large number of commercially valuable fin and shellfishes (Kathiresan and Bingham 2001). They not only help in the production of detritus/organic matter and recycling of nutrients thereby enriching the coastal waters to support the benthic production of the environment (Krishnamurthy and Jayaseelan 1983), but mangrove sediments also generally support higher densities of benthic organisms than other non-vegetated sediments of the estuarine environment (Edgar 1990; Sasekumar and Chong 1998; Kathiresan and Bingham 2001).
Through several studies on macrofauna, meiofauna especially of the free-living marine nematode assemblage of the mangrove environment has been studied in different parts of the world (e.g., Alongi 1990; Nicholas et al. 1991; Vanhove et al. 1992; Olafsson 1992, 1995; Somerfield et al. 1998; Olafsson et al. 2000); in India, it has also been carried out (Krishnamurthy et al. 1984; Rao 1986; Kondalarao and Ramanamurthy 1988; Ansari and Parulekar 1993; Sarma and Welsanand 1994; Goldin et al. 1996; Sulthan Ali et al. 1998; Chinnadurai and Fernando 2007; Anila Kumary 2008). The present study area is an artificially developed mangrove environment established in 1991 by Prof. Kathiresan (Samiduari et al. 2012), and investigations on meiofauna especially on free-living marine nematodes were scarce (Chinnadurai and Fernando 2007), but meiofauna was comparatively well studied near the Pichavaram mangrove forest (Krishnamurthy et al. 1984; Kondalarao and Ramanamurthy 1988; Sulthan Ali et al. 1998).
They remain poorly understood despite the fact that they are extremely abundant and diverse, often numbering in millions per square meter of sediments, and exist in more habitats than any other metazoan group (e.g., Platt and Warwick 1980; Heip et al. 1985). As a result, nematodes have been better studied than any other members of the mangrove meiofauna (Olafsson 1995). Free-living marine nematodes are usually the most abundant metazoans inhabiting marine benthic ecosystems, often representing more than 60–90 % of the benthic meiofauna (Sajan et al. 2010). Their significance in terms of energy flexes in the food chain through ways of degradation and mineralization of organic matter is high. No information is available to date regarding the distribution and diversity of free-living marine nematodes from an artificially developed mangrove ecosystem especially in different plant covers.
The main objective of this quantitative study was to investigate (1) the distribution and diversity of free-living marine nematode assemblage in the artificially developed mangrove ecosystem, (2) the interaction between free-living nematode species and mangrove covers, and (3) which physicochemical variables determine the distribution and diversity of free-living marine nematode assemblages in the artificially developed mangrove ecosystem.
Materials and methods
Study area
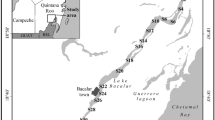
In the present investigation, free-living marine nematodes were collected throughout the year 2010 from two different mangrove covers (Avicennia marina and Rhizophora mucronata) in an artificially developed mangrove environment located in the Vellar Estuary (Fig. 1), southeast coast of India (latitude, 11°29′N; longitude, 79°46′E). Nearly 1.5 km upstream from the mouth at the tidal zone and the northern bank of the Vellar Estuary, an artificial mangrove plantation covering an area of 10 ha was established in 1991 (Ajmal Khan et al. 2005; Samiduari et al. 2012).
Sampling strategy
Nematode samples were collected using a PVC corer (with an internal diameter of 2.5 cm and a length of 15 cm) in the artificially developed mangrove environment (Vincx 1996) each month (January to December 2010). Each mangrove covers a total of five stations that were fixed, and triple corer sediment samples were collected in each station every month to reduce sampling variation. At each corer, the upper 5 cm of samples was analyzed for nematodes. These samples were fixed in buffered formalin at a concentration of 4 %. The replicate core samples were processed separately for downstream analyses. Observations were made on the physicochemical characteristics of the estuarine bottom water (temperature by centigrade thermometer, dissolved oxygen by Winkler's method following Strickland and Parsons (1972), salinity by refractometer, and pH by Elico pH meter). Sediment granulometry was done using the pipette method as proposed by Krumbein and Pettijohn (1938). Total organic carbon content was estimated using chromic acid oxidation method followed by titration with ammonium ferrous sulfate (Walkley–Black method) as modified by Gaudette et al. (1974).
Nematode extraction
In the laboratory, sediment samples were washed through a set of 0.5- and 0.053-mm sieves. The sediment retained in the 0.053-mm sieve was decanted to extract meiofauna following the methods of Pfannkuche and Thiel (1988). Sorting of meiofauna from the sediment was based on flotation technique which has an efficiency of around 95 % (Armenteros et al. 2008). The meiofaunal organisms were stained with Rose Bengal prior to extraction and sorting. All of the nematodes enumerated under a stereomicroscope (Meiji, Japan) were mounted in glass slides using the formalin–ethanol–glycerol technique of Seinhorst (1959) and were subsequently identified to the highest taxonomic level under a compound microscope (Olympus CX 41) based on standard pictorial keys (e.g., Platt and Warwick 1983, 1988; Warwick et al. 1998) and the NeMys Database (Steyaert et al. 2005).
Feeding types
Each nematode species was assigned to one of the four functional feeding groups as per Wieser (1953) classification based on buccal cavity morphology:
-
1.
Selective deposit feeders (1A). They are species without a buccal cavity or with only a narrow tubular buccal cavity.
-
2.
Non-selective deposit feeders (1B). They are species with a large buccal cavity that is not armed with teeth.
-
3.
Epigrowth feeders (2A). They are species having a buccal cavity armed with small or moderate-sized teeth.
-
4.
Predators or omnivores (2B). They are species with large teeth or jaws.
Although various authors have later revised and modified this classification (e.g., Romeyn and Bouwman 1983; Jensen 1987; Moens and Vincx 1997; Moens et al. 2004), in this study, nematode genera were assigned to feeding guilds of Wieser (1953) since this is the only classification which takes into consideration feeding habits of free-living marine nematodes from a variety of habitats.
Statistical analysis
Univariate and multivariate analyses of the nematode community structure were conducted using the PRIMER v6.0.2 software package (Clarke and Gorley 2006). Univariate methods (Shannon–Wiener diversity H′ log e, Margalef's species richness d, Pielou's evenness J′, Simpson's dominance index Lambda′, taxonomic diversity Delta, and total phylogenetic diversity index sPhi+) and multivariate analysis data were fourth-root-transformed prior to construction of the Bray–Curtis similarity matrix (Clarke et al. 2006), and two-dimensional ordinations of assemblages were subsequently created using non-metric multidimensional scaling (nMDS). The significance of differences in community structure across the scales of investigation was assessed using a series of one-way analysis of similarities (ANOSIM). The contribution of individual species to the differences observed was calculated using similarity percentages (SIMPER) routine. Relationships between multivariate biotic patterns and environmental variables were assessed by calculating the Spearman rank correlation (ρ) between a similarity matrix derived from biotic data and metrics derived from environmental data (BIO-ENV procedure). Relationships between nematode density and environmental parameters were assessed using principal component analysis (PCA). They were also assessed based on environmental parameters (sediment temperature, salinity, pH, dissolved oxygen, sand, silt, clay, and TOM) and nematode species distribution. Other simple statistics (correlation, descriptive statistics, and two-way ANOVA) were made using MS Excel.
Results
Hydrography
Hydrographical parameters like surface sediment temperature, salinity, dissolved oxygen concentration, and pH were observed at their maximum during the month of May and minimum during the months of December (temperature and salinity) and November (dissolved oxygen and pH) in both mangrove covers. Surface sediment waters were characterized by high values for temperature (22.42–31.32 °C at the A. marina cover and 22.68–31.20 °C at the R. mucronata cover) and salinity (21.98–30.32 practical salinity units (psu) at the A. marina cover and 22.49–32.02 psu at the R. mucronata cover). Dissolved oxygen concentration in normoxic levels was observed (6.64–7.62 ml/l at the A. marina cover and 6.96–7.88 ml/l at the R. mucronata cover), and acidic pH values varied (6.86–7.50 at the A. marina cover and 6.92–7.63 at the R. mucronata cover). Hydrographical parameters between the mangrove covers were compared, temperature was at its maximum at the A. marina cover, and other parameters were high at the R. mucronata cover (Table 1).
Sedimentology
Sediments of sandy nature were mostly observed in both mangrove covers than sediments of silt/clay composition. Sand content varied from 51.97 to 60.19 % at the A. marina cover and 55.17 to 61.23 % at the R. mucronata cover, whereas silt/clay composition ranged between 39.81 and 48.03 % at the A. marina cover and between 38.77 and 44.83 % at the R. mucronata cover. The lowest concentration of total organic carbon was recorded in the months of July (12.03 mg/g at the A. marina cover) and May (11.83 ± 0.38 mg/g at the R. mucronata cover), whereas the highest concentration was recorded in the months of November (15.38 mg/g at the A. marina cover) and October (14.76 mg/g at the R. mucronata cover) (Table 1). Interrelationship between environmental parameters was assessed using PCA; the first axis represented 59.6 %, and the second axis explained 27.7 % with the total of 87.3 % in variability. The first axis clearly separated all the months sampled. It evidently demonstrated higher values of total organic carbon with silt/clay composition in the months of the monsoon season (October, November, and December), whereas there was more sand content in the pre-monsoon season (July, August, and September); other hydrographical parameters were at their maximum in the months of the summer season (April, May, and June) (Fig. 2).
Taxonomic composition of nematodes
In the artificial mangrove environment in the southeast coast of India, a total of 4,976 free-living marine nematode specimens were examined, and 56 species belonging to 27 genera and 18 families were identified based on taxonomic characters. Among these, 2,564 specimens were examined and 52 species belonging to 27 genera were identified at the A. marina cover and 2,412 specimens and 44 species belonging to 25 genera were identified at the R. mucronata cover. Among these, 40 species of free-living marine nematodes were associated with both mangrove covers. Overall, the R. mucronata cover had the lowest number of nematode abundances (198.83 ± 95.72), whereas the A. marina cover (211.33 ± 109.7) had the most number of nematode abundances. An ANOSIM revealed significant differences in the nematode assemblages between months (R = 0.858, p = 0.001 at the A. marina cover; R = 0.824, p = 0.001 at the R. mucronata cover), which was also confirmed by nMDS. Four main groups were highlighted by the nMDS analysis in both mangrove covers: the first group was composed of the monsoon season (October, November, and December), the second group was composed of the post-monsoon season (January, February, and March), the third group was composed of the pre-monsoon season (July, August, and September) and the forth group was composed of the summer season (April, May, and June). Nematode assemblage and species composition clearly explain the seasonal variations, and they were confirmed by nMDS analysis (Fig. 3a, b).
a nMDS plot of the free-living marine nematode assemblages (square root transformed) at the A. marina cover. The sampling grouping was based on Bray–Curtis clustering. b nMDS plot of the free-living marine nematode assemblages (square root transformed) at the R. mucronata cover. The sampling grouping was based on Bray–Curtis clustering
Feeding types
As far as the trophic groups were concerned, there was dominance of the epistrate feeders (2A), followed by the non-selective deposit feeders (1B), then the selective deposit feeders (1A), and finally the predators (2B). Only the epigrowth feeders were significantly different from the comparison of the mangrove covers (ANOVA, p < 0.01). Epigrowth feeders were significantly more abundant in all of the seasons except in the monsoon season where there was contributed dominance of non-selective deposit feeders (Tukey's test p < 0.05) in both mangrove covers.
Diversity
Diversity indices clearly explained the significant changes in nematode assemblages between seasons in both mangrove covers. Shannon–Wiener index (H′ log e) ranged from 3.52 to 2.89 (A. marina) and from 3.42 to 2.72 (R. mucronata), while Pielou's evenness index (J′) ranged from 0.95 to 0.92 (A. marina) and from 0.96 to 0.92 (R. mucronata). Shannon–Wiener diversity values were significantly lower in the months of the monsoon season (ANOVA, p < 0.05; Tukey's test p < 0.05). Margalef's richness (d) values were in the range 6.68–4.28 (A. marina) and 6.48–4.12 (R. mucronata), while Simpson's dominance indices (Lambda′) were in the range 0.06–0.03 (A. marina) and 0.07–0.04 (R. mucronata). Calculating the taxonomic diversity (Delta) and total phylogenetic diversity index (sPhi+) did not vary as much in both mangrove covers. In the A. marina cover, Delta values varied from 55.57 to 56.31, while in the R. mucronata cover, Delta values varied from 55.47 to 56.15. Total phylogenetic diversity values ranged between 1,450 and 883.3 in the A. marina cover, while it ranged between 1,350 and 850 in the R. mucronata cover (Table 2).
Discussion
The integration of our results provided a quantitative description of the distribution and diversity of free-living marine nematodes in different mangrove covers of artificially developed mangrove ecosystems in the southeast coast of India.
Mangrove forests are among the world's most productive ecosystems (Kathiresan et al. 1996). They enrich coastal waters, yield commercial forest products, protect coastlines, and support coastal fisheries. However, mangroves exist under conditions of high salinity, extreme tides, strong winds, high temperatures, and muddy, anaerobic soils (Kathiresan and Bingham 2001). In general, information on the physicochemical parameter studies is essential for nematode ecological studies (Armenteros et al. 2009). In the present investigation, the hydrographical parameters, namely temperature, salinity, pH, and dissolved oxygen, showed a significant positive correlation (p < 0.001) with nematode abundance. In seasonal variations, all of the physicochemical parameters were at their maximum during the summer season (April, May, and June) and were at their minimum during the monsoon season (October, November, and December). Similar findings were observed in Cochin backwater mangroves by Anila Kumary (2008). Dissolved oxygen is an index to investigate the productivity of an environment. It mainly depends on the nature and abundance of the planktonic organism, monsoonal downpour, salinity, etc. (Ansari et al. 1980). Carpenter (1996) noticed that the solubility of oxygen in water is a function of temperature and salinity. This present investigation noted the same trend: dissolved oxygen positively correlated with temperature and salinity (p < 0.01).
Studies on sediment composition are of paramount importance in benthic ecology. Sediment grain size is one factor that determines the distribution and composition of nematode assemblages (Wieser 1960; Ferris and Ferris 1979; Heip et al. 1985; Ansari and Parulekar 1998). Sediment interstitial space determines the density and diversity of free-living marine nematodes (Heip et al. 1985; Armenteros et al. 2009). In the present investigation, the sandy nature of sediments was dominant throughout the period of investigation, and the abundance of nematodes was also on the higher side. Sand content showed a significant positive correlation (p < 0.05) with nematode density in both mangrove covers, while silt content negatively correlated (p < 0.05) with nematode density in both mangrove covers. Silt/clay content did not show any correlation with nematode density. Similar findings were noted by various authors in mangrove environments (Rao 1986; Alongi 1987; Sarma and Welsanand 1994; Nicholas et al. 1991; Anila Kumary 2008). An important feature of nematode populations is the presence of a large number of species present in a single habitat (Ingole and Singh 2010). Differences in food availability both quantitative and qualitative appear to be an important factor in controlling the total abundance of free-living marine nematodes at the taxonomic level (Vincx et al. 1994; Soltwedel 1997; Ingole and Singh 2010; Sajan et al. 2010). Physicochemical factors and competition, both among individuals within a species as well as among the species, can also play a major role in limiting nematode abundance and distribution (Soltwedel 2000; Sajan and Damodaran 2007).
Mangrove ecosystems are known to be highly productive ecosystems, with a net primary productivity estimated at 149 mol C m−2 year−1 (Bouillon et al. 2008). In addition, they can store large amounts of organic carbon in their substrate up to several meters in depth (Lallier-Verges et al. 1998). Quantitatively, the most important source of organic carbon in the mangrove environment is litter from mangrove trees deposited at the sediment surface and subsurface (Alongi 1998). Sediments of artificially developed mangrove environment in the southeast coast of India are highly enriched in total organic carbon compared to the open or non-vegetated sediments, with maximum values of up to 15.38 ± 0.20 mg/g at the A. marina cover and 14.76 ± 0.32 mg/g at the R. mucronata cover compared to the open environmental sediments of 2.05 mg/g at 5 m in depth (e.g., Mondal et al. 2010). The highest values of total organic carbon were obtained at the A. marina cover over the R. mucronata cover. This result agreed with the investigation of Alongi et al. (1998) on Malaysian mangroves. This can be the result of a more developed root system of R. mucronata (e.g., Hesse 1961) compared to the radial cable root system of A. marina that developed only in the subsurface (e.g., deGranville 1974). In the present study, total organic carbon values significantly and positively correlated with nematode assemblages in both mangrove covers (p < 0.05 A. marina; p < 0.01 R. mucronata). The dominance of epigrowth and deposit feeders also significantly correlated with organic enrichment in both mangrove covers (Tukey's test p < 0.01).
The density of free-living marine nematodes has a direct relationship with various environmental parameters such as temperature, salinity, sediment texture, and organic matter content in the sediment (Ansari et al. 1980; Grove et al. 2006; Armenteros et al. 2009; Ajmal Khan et al. 2012). In the present investigation, the abundance of free-living marine nematodes was found to be in the range of 106 ± 1.58 number of individuals (no. of ind.) (October) to 463.4 ± 2.41 no. of ind. (May) in the A. marina cover and 99.8 ± 1.14 no. of ind. (October) to 400.6 ± 1.14 no. of ind. (May) in the R. mucronata cover. This is comparable with the observations made by Sulthan Ali et al. (1983); Kondalarao and Ramanamurthy (1988); Goldin et al. (1996); Chinnadurai and Fernando (2006), and Anila Kumary (2008) in Indian mangroves. The range observed presently also agrees with those of numerous authors from the Australian mangroves (Hodda and Nicholas 1986; Alongi 1987, 1990; Nicholas et al. 1991), African mangroves (Dye 1983; Vanhove et al. 1992; Olafsson 1995; Olafsson et al. 2000), and Malaysian mangroves (Somerfield et al. 1998). In the present investigation, artificial mangroves showed that nematode abundance and species composition are more or less similar in both mangrove covers. In the natural mangrove environment, generally A. marina showed maximum abundance over other mangroves (Nicholas et al. 1991; Goldin et al. 1996; Somerfield et al. 1998; Olafsson et al. 2000).
In the present investigation, as many as 56 species of free-living nematodes belonging to 27 genera were recorded from the artificial mangrove environment. So far, around 225 species of free-living marine nematodes have been reported in various regions, including estuaries, backwaters, lagoons, mangroves, and coastal environments of Indian waters (Timm 1961, 1967a, b; Gerlach 1962; Rao and Ganapati 1968; Sulthan Ali et al. 1998; Chinnadurai and Fernando 2003, 2006, 2007; Nanajkar and Ingole 2007; Sajan and Damodaran 2007; Sajan et al. 2010; Ansari et al. 2012a, b, c).
The differences between the structures of the nematode assemblages found in these artificial mangrove covers were clearly shown in the nMDS plot (Fig. 3a, b). Here, the sampling months were grouped into four separate clusters within the seasons based on the species composition. Therefore, serial changes in nematode species composition were calculated using RELATE analysis (Clarke and Warwick 2001). The serial changes in species composition with different mangrove covers were clearly explained. The Spearman rank correlation (Rho) values obtained were 0.416 (A. marina) and 0.72 (R. mucronata) having the sample statistic of 0.4 and 0.1 %, respectively, indicating significant changes in species composition with different mangrove covers. Interrelations between the biotic and abiotic variables were derived by calculating the correlation matrix using BEST-BIOENV procedure, and it gave a higher Spearman rank correlation value of 0.728 at the A. marina cover with the combination of temperature, salinity, dissolved oxygen, silt/clay composition, and total organic carbon, whereas a correlation value of 0.721 was exposed at the R. mucronata cover with the combination of temperature, salinity, dissolved oxygen, sand content, and total organic carbon.
Multiple k-dominance plots (Clarke and Warwick 2001) facilitated the discrimination of nematodes according to the species' relative contribution to standard stock. When the k-dominance was plotted in both mangrove covers (Fig. 4), it did not show much variation (40 species in both mangrove covers); however, the curve for the A. marina cover was low, indicating the highest diversity (52 species), whereas the curve for the R. mucronata, which showed the lowest diversity (44 species), was high. This index has been defined as the opposite to equitability, and whenever dominance index is maximum, the evenness index is the least and vice versa. The dominance index is a useful tool to finding out the influential species within the habitats (Pearson and Rosenberg 1978). In the present study, it is clearly explained that the highest diversity in the A. marina cover is influenced by species such as Viscosia abyssorum (Allgén, 1933), Viscosia glabra (Bastian, 1865), Metachromadora remanei Gerlach, 1951, Metachromadora sp.1, Metachromadora sp.2, Theristus flevensis Stekhoven, 1935, Theristus longus Platt, 1973, Sphaerolaimus gracilis De Man, 1884, and Sphaerolaimus islandicus Ditlevsen, 1926. These species were also obtained by SIMPER analysis.
Similarly, in the present study, the confirmation of species richness in the mangrove covers to find out the sufficiency of the sample size was calculated using the rarefaction method (expected number of individuals). The rarefaction curves clearly explained that the number of samples collected from the mangrove covers is quite sufficient to record all of the 56 species. For the comparison of sample size, it required the recording of all species from the A. marina cover (2,620 individuals) and the recording of all species from the R. mucronata cover (2,475 individuals) (Fig. 5). The rarefaction results were quite evident in the collected samples of the entire study (A. marina 2,564 individuals; R. mucronata 2,412 individuals).
Based on feeding types, in general, epistrate feeders are present more commonly in sediments of sandy nature, while deposit feeders (selective and non-selective) are predominant in finer sediments; predators could be the main representatives in medium and fine sediments (Heip et al. 1985; Sinha and Choudhury 1987). In the present investigation, throughout the study period, sediments had a sandy substrate and comparatively silt/clay composition. However, the nematode assemblages were distinct in relation to mangrove covers. Both mangrove covers were dominated by epistrate feeders, closely followed by deposit feeders, and then predators. Similar findings were observed by various authors in the mangrove environment (Dye 1983; Sulthan Ali et al. 1983; Alongi 1987, 1990; Alongi and Christofferen 1992; Vanhove et al. 1992; Ansari and Parulekar 1993; Olafsson 1995; Goldin et al. 1996; Chinnadurai and Fernando 2006).
Species diversity is a simple and useful measure of a biological system (Redding and Cory 1975). In the present investigation, diversity indices were maximum in the summer and minimum in the monsoon. Similar findings were made by Hodda and Nicholas (1986), Nicholas et al. (1991); Ansari and Parulekar (1993); Somerfield et al. (1998); Olafsson et al. (2000), and Anila Kumary (2008). Species richness of the meiofauna varies much among different habitats. However, they are postulated to play an important role in litter degradation in high detritus systems (Tietjen 1980; Reiper-Kirchner 1989). Anila Kumary (2008) reported higher diversity and richness values in sandy substratum. The present study is in agreement with the above study.
Conclusion
Mangrove forests are extremely important coastal resources, which are vital to our socioeconomic development. Generally, mangrove sediments support higher densities of nematode assemblages than those of other estuarine communities (Sasekumar and Chong 1998; Kathiresan and Bingham 2001). The studied artificially developed mangrove environment was differently exposed to the different species of mangroves, and consequently, they were subject to similar hydrodynamic conditions as confirmed by the hydrographic and sedimentology analyses. Higher number of species and density of free-living marine nematodes at the A. marina cover may be due to organic enrichment and more silt/clay composition than the R. mucronata cover. Organic enrichment may be due to the degradation of leaf litter which is higher at the A. marina cover than at the R. mucronata cover. In particular, nematode species like V. abyssorum, V. glabra, M. remanei, Metachromadora sp.1, Metachromadora sp.2, T. flevensis, T. longus, S. gracilis, and S. islandicus were found only at the A. marina cover. These species make the A. marina leaf a better candidate for interaction than the R. mucronata leaf. In this consequence, further studies such as nutrient and other biochemical compositions of mangrove leaves will be useful to detect the indicator nematode species from the mangrove environments particularly organic enrichment.
References
Ajmal Khan, S., Raffi, S. M., & Lyla, P. S. (2005). Brachyuran crab diversity in natural (Pichavaram) and artificially developed mangroves (Vellar estuary). Current Science, 88, 1316–1324.
Ajmal Khan, S., Ansari, K. G. M. T., & Lyla, P. S. (2012). Organic matter content of sediments in continental shelf area of southeast coast of India. Environmental Monitoring and Assessment, 184, 7247–7256.
Alongi, D. M. (1987). Inter-estuary variation and intertidal zonation of free-living nematode communities in tropical mangrove systems. Marine Ecological Progress Series, 40, 103–114.
Alongi, D. M. (1990). Community dynamics of free-living nematodes in some tropical mangrove and sand-flat habitats. Bulletin of Marine Science, 46, 358–373.
Alongi, D. M., & Christofferen, P. (1992). Benthic infauna and organism-sediment relations in a shallow tropical coastal area: influence of out welled mangrove detritus and physical disturbance. Marine Ecological Progress Series, 81, 229–245.
Alongi, D. M. (1998). Coastal ecosystem processes. Boca Raton: CRC Press.
Alongi, D. M., Sasekumar, A., Tirendi, F., & Dixon, P. (1998). The influence of stand age on benthic decomposition and recycling of organic matter in managed mangrove forests of Malaysia. Journal of Experimental Marine Biology and Ecology, 225, 197–218.
Anila Kumary, K. S. (2008). Diversity of meiobenthic nematodes in the Poonthura estuary (Southwest coast of India). Journal of the Marine Biological Association of India, 50, 23–28.
Ansari, Z. A., Parulekar, A. H., & Jagtap, T. G. (1980). Distribution of sub-littoral meiobenthos off Goa coast, India. Hydrobiologia, 74, 209–214.
Ansari, Z. A., & Parulekar, A. H. (1993). Distribution, abundance and ecology of the meiofauna, in a tropical estuary along the west coast of India. Hydrobiologia, 262, 115–126.
Ansari, Z. A., & Parulekar, A. H. (1998). Community structure of meiobenthos from a tropical estuary. Indian Journal of Marine Sciences, 27, 362–366.
Ansari, K. G. M. T., Manokaran, S., Raja, S., Ajmal Khan, S., & Lyla, P. S. (2012a). Checklist of nematodes (Nematoda: Adenophorea) from Southeast Continental Shelf of India. Check List, 8, 414–420.
Ansari, K. G. M. T., Lyla, P. S., & Ajmal Khan, S. (2012b). Faunal composition of metazoan meiofauna from the southeast continental shelf of India. Indian Journal of Geo-Marine Sciences, 41, 457–467.
Ansari, K. G. M. T., Lyla, P. S., & Ajmal Khan, S. (2012c). New record of free-living marine nematodes (Nematoda: Enoplida) from Indian waters. Journal of the Marine Biological Association of India, 54, 39–45.
Armenteros, M., Perez-Garcia, J. A., Perez-Angulo, A., & Williams, J. P. (2008). Efficiency of extraction of meiofauna from sandy and muddy marine sediments. Revista de Investigaciones Marinas, 29, 113–118.
Armenteros, M., Ruiz-Abierno, A., Fernandez-Garces, R., Perez-Garcia, J. A., Diaz-Asencio, L., Vincx, M., et al. (2009). Biodiversity patterns of free-living marine nematodes in a tropical bay: Cienfuegos, Caribbean Sea. Estuarine, Coastal and Shelf Science, 85, 179–189.
Bouillon, S., Borges, A. V., Castaneda-Maya, E., Diele, K., Dittmar, T., Duke, N. C., et al. (2008). Mangrove production and carbon sinks: A revision of global budget estimates. Global Biogeochemistry Cycling, 22, GB 2013.
Carpenter, J. H. (1996). New measurements of oxygen solubility in pure and natural water. Limnology and Oceanography, 11, 264–277.
Chinnadurai, G., & Fernando, O. J. (2003). Meiofauna of Pichavaram mangroves along the southeast coast of India. Journal of the Marine Biological Association of India, 45, 158–165.
Chinnadurai, G., & Fernando, O. J. (2006). Meiobenthos of Cochin mangroves (Southwest coast of India) with emphasis of free-living marine nematode assemblages. Russian Journal of Nematology, 14, 127–137.
Chinnadurai, G., & Fernando, O. J. (2007). Meiofauna of mangroves of the southeast coast of India with special reference to the free-living marine nematode assemblage. Estuarine, Coastal and Shelf Science, 72, 329–336.
Clarke, K. R., & Warwick, R. M. (2001). Change in marine communities: An approach to statistical analysis and interpretation. Plymouth: PRIMER-E.
Clarke, K. R., & Gorley, R. N. (2006). PRIMER v6: User manual/tutorial. Plymouth: PRIMER-E.
Clarke, K. R., Somerfield, P. J., Airoldi, L., & Warwick, R. M. (2006). Exploring interactions by second-stage community analyses. Journal of Experimental Marine Biology and Ecology, 338, 179–192.
deGranville, J. J. (1974). Apercu sur la structure des pneumatophores de deux especes des sols hydromorphes en Guyane. Cahiers ORSTOM Series BD, 23, 3–22.
Dye, A. H. (1983). Composition and seasonal fluctuation of meiofauna in a southern African mangrove estuary. Marine Biology, 73, 165–170.
Edgar, G. J. (1990). The influence of plant structure on the species richness, biomass and secondary production of macrofaunal assemblages associated with western Australian sea grass beds. Journal of Experimental Marine Biology and Ecology, 137, 215–240.
Ferris, V. R., & Ferris, J. M. (1979). Thread worms (Nematoda). In C. W. Harthy & S. L. Fuller (Eds.), Pollution Ecology of Estuarine Invertebrates (pp. 1–33). New York: Academic Press.
Gaudette, H. E., Wilson, R. F., Toner, L., & David, W. F. (1974). An inexpensive titration methods for determination of organic carbon in recent sediments. Journal of Sedimentary and Petrology, 44, 249–253.
Gerlach, S. A. (1962). Freilebende Meeresnematoden von den Malediven. Kieler Meeresforsch, 18, 81–108.
Goldin, Q., Mishra, V., Ullal, V., Athalye, R. P., & Gokhale, K. S. (1996). Meiobenthos of mangrove mudflats from shallow region of Thane Creek, Central West coast of India. Indian Journal Marine Sciences, 25, 137–141.
Grove, S. L., Probert, P. K., Berkenbusch, K., & Nodder, S. D. (2006). Distribution of bathyal meiofauna in the region of the Subtropical Front, Chatham Rise, south-west Pacific. Journal of Experimental Marine Biology and Ecology, 330, 342–355.
Heip, C., Vincx, M., & Vranken, G. (1985). The ecology of marine nematodes. Oceanography and Marine Biological: An Annual Review, 23, 399–489.
Hesse, P. R. (1961). Some differences between the soils of Rhizophora and Avicennia mangrove swamp in Sierra Leone. Plant and Soil, 14, 335–346.
Hodda, M., & Nicholas, W. L. (1986). Nematode diversity and industrial pollution in the Hunter river estuary. Marine Pollution Bulletin, 17, 251–254.
Ingole, B., & Singh, R. (2010). Biodiversity and community structure of free-living marine nematodes from the Larsemann Ice Shelf, East Antarctica. Current Science, 99, 1413–1419.
Jensen, P. (1987). Feeding ecology of free-living aquatic nematodes. Marine Ecological Progress Series, 35, 187–196.
Kathiresan, K., Rajendran, N., & Thangadurai, G. (1996). Growth of mangrove scalding in the intertidal area of Vellar estuary, Southeast coast of India. Indian Journal of Marine Sciences, 25, 240–243.
Kathiresan, K., & Bingham, B. L. (2001). Biology of mangroves and mangrove ecosystems. Advances in Marine Biology, 40, 81–251.
Kondalarao, B., & Ramanamurthy, K. V. (1988). Ecology of intertidal meiofauna of the Kakinada Bay (Gautami–Godavari estuarine systems), East coast of India. Indian Journal of Marine Sciences, 17, 40–47.
Krishnamurthy, K., & Jayaseelan, M. P. J. (1983). The Pichavaram (India) mangrove ecosystems. Indian Journal of Ecology and Environmental Science, 9, 79–85.
Krishnamurthy, K., Sulthan Ali, M. A., & Jayaseelan, M. P. J. (1984). Structure and dynamics of the aquatic food web community with special reference to nematodes in mangrove ecosystems. In E. Soepadmo, A. N. Rao, & D. J. MacIntosh (Eds.), Proceeding of Asian Symposium on Mangrove Environment Research and Management (pp. 429–452). Kuala Lumpur: University of Malaya Press.
Krumbein, W. C., & Pettijohn, F. J. (1938). Manual of Sedimentary Petrography. New York: Appleton-Century-Crofts.
Lallier-Verges, E., Perrussel, B. P., Disnar, J. R., & Baltzer, F. (1998). The relationship between environmental conditions and the diagenetic evolution of organic matter derived from higher plant in a present mangrove swamp system (Guadeloupe, French West Indies). Organic Geochemistry, 29, 1663–1686.
Moens, T., & Vincx, M. (1997). Observation on the feeding ecology of estuarine nematodes. Journal of Marine Biological Association of the United Kingdom, 77, 211–227.
Moens, T., Yeates, G. W., & DeLey, P. (2004). Use of carbon and energy sources by nematodes. Nematology Monographs and Perspectives, 2, 529–545.
Mondal, N., Rajkumar, M., Sun, J., Kundu, S., Lyla, P. S., Ajmal Khan, S., et al. (2010). Biodiversity of brackishwater amphipods (Crustacean) in two estuaries, southeast coast of India. Environmental Monitoring and Assessment, 17, 471–486.
Nanajkar, M. R., & Ingole, B. S. (2007). Nematode species diversity as indicator of stressed benthic environment along the central west coast of India. In P. V. Desai & R. Roy (Eds.), Diversity and life processes from ocean and land (pp. 42–52). Taleigao: Goa University Press.
Nicholas, W. L., Elek, J. A., Stewart, A. C., & Marples, T. G. (1991). The nematode fauna of a temperate Australian mangrove mudflat, its population density, diversity and distribution. Hydrobiologia, 209, 13–27.
Olafsson, E. (1992). Small-scale spatial distribution of marine meiobenthos: The effects of decaying macrofauna. Oecologia, 90, 37–42.
Olafsson, E. (1995). Meiobenthos in mangrove areas in eastern Africa with emphasis on assemblage structure of free-living marine nematodes. Hydrobiologia, 312, 47–57.
Olafsson, E., Carlstrom, S., & Ndaro, S. G. M. (2000). Meiobenthos of hypersaline tropical mangrove sediments in relation to spring tide inundation. Hydrobiologia, 426, 57–64.
Pearson, T. H., & Rosenberg, R. (1978). Macrobenthic succession in relation to organic enrichment and pollution of marine environment. Oceanography and Marine Biology an Annual Review, 16, 229–311.
Pfannkuche, O., & Thiel, H. (1988). Sample processing. In R. P. Higgins & H. Thiel (Eds.), Introduction to the study of Meiofauna (pp. 134–145). Washington: Smithsonian Institute Press.
Platt, H. M., & Warwick, R. M. (1980). The significance of nematodes to the littoral ecosystem. In J. H. Prince, D. E. C. Irvine, & W. H. Franham (Eds.), The shore environment, ecosystems (pp. 729–759). London: Academic Press.
Platt, H. M., & Warwick, R. M. (1983). Free-living marine nematodes. Part 1. British Enoplids. In D. M. Kermack & R. S. K. Barnes (Eds.), Synopses of the British Fauna (New series no. 28) (pp. 1–307). Cambridge: Cambridge University Press.
Platt, H. M., & Warwick, R. M. (1988). Free-living marine nematodes. Part II: British Chromodorids. In D. M. Kermack & R. S. K. Barnes (Eds.), Synopses of the British Fauna (New series no. 38) (pp. 1–502). Leiden: EJ Brill.
Rao, G. C., & Ganapati, P. N. (1968). Interstitial fauna inhabiting the beach of sands of Waltair coast. Proceedings of the National Institute of Science India Series B, 34, 82–125.
Rao, G. C. (1986). Meiofauna of the mangrove sediments in South Andaman. Journal of Andaman Science Association, 2, 23–32.
Redding, J. M., & Cory, R. L. (1975). Macroscopic benthic fauna of three tidal creeks adjoining the Rodhe river, Maryland. In Water-Resources Investigations Report (pp. 39–75). Reston: U.S. Geological Survey.
Reiper-Kirchner, M. (1989). Microbial degradation of North Sea macroalgal: Field and laboratory studies. Botanica Marina, 32, 241–252.
Romeyn, K., & Bouwman, L. A. (1983). Food selection and consumption by estuarine nematodes. Hydrobiological Bulletin, 17, 103–109.
Sajan, S., & Damodaran, R. (2007). Faunal composition of meiobenthos from the shelf region off west coast of India. Journal of the Marine Biological Association of India, 49, 19–26.
Sajan, S., Joydas, T. V., & Damodaran, R. (2010). Meiofauna of the western continental shelf of India, Arabian Sea. Estuarine, Coastal and Shelf Science, 86, 665–674.
Samiduari, K., Saravanakumar, A., & Kathiresan, K. (2012). Spatial and temporal distribution of macrobenthos in different mangrove ecosystems of Tamil Nadu coast, India. Environmental Monitoring and Assessment, 184, 4079–4096.
Sarma, A. L. N., & Welsanand, V. (1994). Littoral meiofauna of Bhitarkanika mangroves of river Mahanadi systems. East coast of India. Indian Journal of Marine Sciences, 23, 221–224.
Sasekumar, A., & Chong, V. C. (1998). Faunal diversity in Malaysian mangroves. Global Ecology and Biogeography Letters, 7, 57–60.
Seinhorst, J. W. (1959). A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica, 4, 67–69.
Sinha, B., & Choudhury, A. (1987). Observation on the ecology of nematodes inhabiting littoral sands of the Hugli estuary. Journal of the Marine Biological Association of India, 29, 124–133.
Soltwedel, T. (1997). Meiobenthos distribution pattern in the tropical East Atlantic: Indication for fractionated sedimentation of organic matter to the sea floor? Marine Biology, 129, 747–756.
Soltwedel, T. (2000). Metazoan meiobenthos along continental margins: A review. Progress in Oceanography, 46, 59–84.
Somerfield, P. J., Gee, J. M., & Aryuthaka, C. (1998). Meiofaunal communities in a Malaysian mangrove forest. Journal of Marine Biological Association of the United Kingdom, 78, 717–732.
Steyaert, M., Deprez, T., Raes, M., Bezerra, T., Demesel, I., Derycke, S., Desmet, G., Fonseca, G., de Assunc ão Franco M., Gheskiere, T., Hoste E., Ingels, J., Moens, T., Vanaverbeke, J., VanGaever, S., Vanhove, S., Vanreusel, A., Verschelde, D., Vincx, M. (2005). Electronic key to the free-living marine nematodes, http://nemys.ugent.be/. Accessed in 9 September 2005.
Strickland, J. D. H., & Parsons, T. R. (1972). A practical handbook of sea water analysis. Ottawa: Fisheries Research Board of Canada.
Sulthan Ali, M. A., Krishnamurthy, K., & Jayaseelan, M. P. J. (1983). Energy flow through the benthic ecosystem of the benthic ecosystem of the mangroves with special reference to nematodes. Mahasagar, 16, 317–325.
Sulthan Ali, M. A., Ajmal Khan, S., & Balasubramanian, T. (1998). Nematodes of the Pichavaram mangroves. GIS Based Information System for. Pichavaram: Government of India.
Tietjen, J. H. (1980). Microbial-meiofaunal interrelationships: A review. In Microbiology (pp. 135–138). Washington: American Society for Microbiology.
Timm, R. W. (1961). The marine nematodes of the Bay of Bengal. Proceeding of Pakistan Academy of Science, 1, 1–84.
Timm, R. W. (1967a). Some estuarine nematodes from the Sunderbans. Proceeding of Pakistan Academy of Science, 4, 1–14.
Timm, R. W. (1967b). New marine nematodes of the family Linhomoeidae from East Pakistan. Proceeding of Pakistan Academy of Science, 4, 15–22.
Vanhove, S., Vincx, M., Van Gansbeke, D., Gijselinck, W., & Schram, D. (1992). The meiobenthos of five mangrove vegetation types in Gazi Bay, Kenya. Hydrobiologia, 247, 99–108.
Vincx, M., Bett, B. J., Dinet, A., Ferrero, T., Gooday, A. J., Soltwedel, T., et al. (1994). Meiobenthos of the Deep Northeast Atlantic. Advances in Marine Biology, 30, 2–88.
Vincx, M. (1996). Meiofauna in marine and freshwater sediments. In G. S. Hall (Ed.), Methods for the examination of organismal diversity in soils and sediments (pp. 187–195). New York: CAB International IUBS UNESCO.
Warwick, R. M., Platt, H. M., & Somerfield, P. J. D. (1998). Free-living marine nematodes. Part III. Monhysterids. In D. M. Kermack, R. S. K. Barnes, & J. H. Crothers (Eds.), Synopses of the British Fauna (New Series no. 53) (pp. 1–296). Shrewsbury: Field Studies Council Press.
Wieser, W. (1953). Die Beziehung zwischen Mundho hlengestalt, Erna hrungsweise und Vorkommen bei freilebenden marinen Nematoden. Arkiv för Zoolgi, 4, 439–484.
Wieser, W. (1960). Benthic studies in Buzzards Bay. II. The meiofauna. Limnology and Oceanography, 5, 121–137.
Acknowledgments
The authors are thankful to Prof. T. Balasubramanian, Dean, for the encouragement and the University authorities for the facilities. The authors are also thankful to the Centre for Marine Living Resources and Ecology (CMLRE) of Ministry of Earth Sciences (MoES), Kochi, Government of India, for the financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ansari, K.G.M.T., Manokaran, S., Raja, S. et al. Interaction of free-living marine nematodes in the artificial mangrove environment (southeast coast of India). Environ Monit Assess 186, 293–305 (2014). https://doi.org/10.1007/s10661-013-3374-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3374-1