Abstract
This paper deals with the spatial distribution and diversity of macrobenthos and their relationships between physico-chemical parameters of the water and sediment in different mangrove habitats of Tamil Nadu, India during different seasons (2008). Among the different ecosystems of mangrove benthic faunal assemblages, macrofauna species number, density, richness, and Shannon–Wiener index were the highest and the Simpson dominance index was medial at riverine mangrove community. However, the Pielou Evenness index of riverine mangrove community was slightly lower than other communities. The similarities among the macrobenthic communities at different sampling sites were determined using Bray–Curtis similarity coefficient and ordinations of non-metric multidimensional scaling (MDS). Thirty-one species were recorded in developing (16 polychaetes, six bivalves, seven gastropods, and two crustaceans), 35 species were recorded in riverine (20 polychaetes, six bivalves, five gastropods, and four crustaceans) and 31 species were recorded in island mangrove ecosystem (19 polychaetes, four bivalves, five gastropods, and three crustaceans). Among the three ecosystems, a total of 46 benthic macrofauna consisting of 27 species of polychaetes, eight species of gastropods, seven species of bivalves, and four species of crustaceans were recorded. However, there were obvious differences among the community structures in the three mangrove habitats. This result implied that the different mangrove ecosystem had different effects on the macrofauna communities and shed light on the macrofauna adaptation capability to specific habitats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangroves form a habitat for a wide variety of species, some occurring in high densities and provide food and shelter for a large number of commercially valuable fin and shellfishes. They are productive habitats and support coastal fisheries (Manson et al. 2005). Mangrove forests are extremely important coastal resources, which are vital for socio-economic development of the region. As a detritus-based ecosystem, leaf litter from the mangroves provides the basis for adjacent aquatic and terrestrial food webs. It also serves as breeding, feeding, and nursery grounds for most of the commercially important fin and shellfishes, on which thousands of coastal people depend for their livelihood. It is considered to have physical, chemical, and biological processes which promote the adaptation of inhabiting organisms to tolerate greater amplitude of environmental characters both morphologically and physiologically. Krom and Berner (1980) have reported that the decomposition of organic matter consists of nutrients such as nitrogen and phosphorus, which play a vital role in the establishment of healthy mangroves. However, sediment where the animals inhabit often acts as buffer either as a source or sink of nutrients especially phosphorus by adsorption–desorption reactions (Krom and Berner 1980). Hence, the sediment plays a crucial role on benthic faunal diversity in the mangrove ecosystem. Benthic organisms constitute an important component that influences the productivity of the habitat to a greater extent. Benthos helps in the recycling of nutrients, which in turn promotes primary productivity. A detailed and complete knowledge of the bottom fauna is not only important for the determination of productivity (Raveenthiranath Nehru 1990) but is also helpful in understanding the diversity of the habitat. Macrofauna are the most widely studied benthic organisms which are retained on 0.5-mm sieve. They reside beneath the sediment surface in burrows and tubes. Thus, seemingly, the bottom of the mangrove substratum habitats forms a wide array of macrobenthic organisms of various size and taxonomic categories. Indian mangrove ecosystems are known to have a total of 3,985 biological species that include 919 floral species and 3,066 faunal species. Of the biological species, the faunal species occupy about 77%, and the floral species 23%. Thus, the faunal species component is about three times greater than the floral component of the mangrove ecosystem (Kathiresan 2000; Kathiresan and Quasim 2005). Of these, polychaetes, molluscs, and crustaceans are found to be the major macrobenthic organisms in mangrove environment. Most of the macrobenthos assist in the breakdown of particulate organic material by exposing them to microbes and their waste materials contain rich nutrients forming the food for other consumers. Thus, the macrobenthos play a major ecological role in the mangrove ecosystem (Warren and Underwood 1986). Mangroves are inhabited by a variety of macrobenthic invertebrates, such as brachyuran crabs, gastropods, bivalves, hermit crabs, barnacles, sponges, tunicates, polychaetes, and sipunculids. Mangrove invertebrates often exhibit marked zonation patterns and colonize a variety of specific micro-environments (Smith et al. 1991). While some species dwell on the sediment surface or reside in burrows, others live on pneumatophores and lower tree trunks or prop-roots, burrow in decaying wood, or can even be found in the tree canopies (Sasekumar 1974; Ashton 1999). They form important links between the primary detritus at the base of the food web and consumers of higher trophic levels (Macintosh 1984), and because of their large abundance and biomass (secondary production), the energy assimilated by the macrofauna plays a significant role in nutrient recycling in the mangrove ecosystem. The macrobenthic fauna serve as the food for a variety of demersal organisms of higher trophic level. Under these conditions, studying macrobenthic community of different mangrove types and correlating them with the prevailing physico-chemical properties would provide valuable information on the factor influencing the distribution of macrobenthos in the mangrove ecosystem. Thus, the studies on benthic faunal diversity will be an immediate concern to understand the status of mangrove ecosystem. An assessment of the health of a particular ecosystem can be achieved only through a careful analysis of benthic fauna.
In this study, the spatial and temporal distribution, and diversity of macrobenthos and their relationships among the physico-chemical parameters of the water and sediment samples were studied in the different mangrove habitats.
Materials and methods
Research region overview
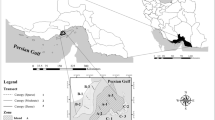
Station I (developing mangrove ecosystem) is located at 11°29′N 79°46′E. This is one of the best-studied estuaries in India comparable to the world conditions. It is highly productive and rich in floral and faunal resources. This located estuary remains open with the Bay of Bengal as it is a “true estuary” without complete closure of the mouth. Along the course of this estuary, in an area of 10 ha, a mangrove forest was developed by Dr. K. Kathiresan and his team, CAS in Marine Biology, Parangipettai since the year 1991 onwards. It is grown near the shore of Vellar estuary and is very good in supporting biodiversity. Hence, this site was selected as a developing mangrove ecosystem.
Station II (riverine mangrove ecosystem) is located at 11°27′N 79°47′E and it is situated on the southeast coast of India. It is one of the typical mangrove swamps of India, with high productivity of about 8 t of organic plant detritus/ha/year (Kathiresan 2000). It consists of small and large islets covering an area of 1,100 ha. In this station, 50% is covered by forest, 40% by water way, and the remaining 10% by sand and mud flats.
Station III (island mangrove ecosystem) consists of three different islands of Gulf of Mannar, namely Kurusadai, Poomarchan, and Manauli. It is located between 9°14′N 79°12′E and 9°12′N 79°7′E, along the south east coast of India. These islands are well known for their mangrove species composition and have lagoon pools and open mud flats (Fig. 1).
Water sampling and analysis of physico-chemical parameters
Four seasonal collections were made from January 2008 to December 2008. Samples were collected from each station (four seasons × three stations × six replicates). Rainfall data were collected from the Metrological Department office at CAS in Marine Biology, Annamalai University, Parangipettai for stations I and II and at Pamban for station III. Parangipettai (Meteorological Department, Government of India) data were used for both stations I and II as it is only 10 km away from Pichavaram. Water samples were collected in pre-cleaned polypropylene containers, just below the water surface separately from the sampling sites. After collection, all the samples were cooled and then brought to the laboratory in an insulated thermocool box. Soon after returning to the laboratory, the water samples were filtered through a Whatman GF/C filter paper for nutrient analysis. Water temperature was measured using a mercury centigrade thermometer with 0.5°C accuracy. The pH of samples was measured by using a calibrated pH pen (Phep; Hanna instruments Mauritius Ltd., Portugal) with an accuracy of ±0.1. The pH in the solution was measured using a pH meter, calibrated with standard buffer solution prior to use. The salinity of samples was measured by using a hand refractometer (Atago, Japan). Water samples were transferred carefully to BOD bottles. The modified Winkler’s method described by Strickland and Parsons (1972) was adopted for the estimation of dissolved oxygen fixed. The nitrate, nitrite, inorganic phosphate, and reactive silicate content of water samples were analyzed by the method of Strickland and Parsons (1972).
Sediment sampling and analysis of physico-chemical parameters
Van veen grab (0.1 m2) was used to collect sediment samples. The soil temperature was measured using a standard centigrade thermometer by directly inserting in the sediment. The soil pH was determined by adopting the method of Jackson (1958). Water was added to air-dried samples in the ratio of 1:1 and stirred in a mechanical shaker for an hour, and pH was measured in this solution. A known amount of sediment samples was moisturized with double distilled water up to the moisture saturation of the sediment. Then double the volume of saturation level of water was added, mechanically shaken for 15 min, and the water with salt was filtered through a Whatman No. 1 filter paper and the salinity was measured using a hand refractometer. Redox potential was measured using a millivoltmeter with platinum electrode (pH 315i/SET; Wissenschaftlich Technische Werkstatten, Germany). Soil samples were brought to the laboratory in clean polythene bags, air dried, and stored for further analysis. The percentage composition of sand, silt, and clay in the sediment samples were determined by the sieving method of Krumbein and Pettijohn (1938). Macronutrients in the sediment samples were analyzed in the Sugarcane Breeding Institute, Cuddalore (Subbiah and Asija 1956; Olsen et al. 1954).
Macrofauna sampling and identification
The samplings covered all the tidal levels and were done by using a line transect method. Van veen grab (0.1 m2) was used for unit sampling. Six replicates for each station were maintained. Soon after retrieval, samples were gently sieved through a 0.5-mm sieve. The organisms retained by the sieve were preserved in 5% formalin and brought to the laboratory for further identification. The sorted organisms were first segregated into different groups and then identified to specific, genetic, or other higher levels to the greatest extent possible with the help of standard taxonomic references (Polychaeta—Fauvel 1953; Day 1967; Mollusca—Subba Rao et al. 1991). The organisms were counted under a stereoscopic microscope, and abundance was expressed as individuals per square meter. In the present study, the qualitative and quantitative assessments of benthic macrofauna were noted only to polychaetes, bivalves, and gastropods (molluscs), and isopods and amphipods (crustaceans).
Data analysis
Macrofauna taxa collected from the beds were identified and listed. Pearson correlation coefficient was employed for the better understanding of relationship between the concentration of various nutrients, sediment composition, pH, and Eh by using statistical package (SPSS11.5). Their settlement was analyzed using several indices: univariate measures such as Margalef’s species richness (d), Shannon–Wiener diversity (H′ log) and Pielou’s evenness (J′), graphical tools like k-dominance curve and Ellipse plots, and multivariate tool such as Bray–Curtis similarity after suitable transformation of sample abundance data, classification (hierarchical agglomerative clustering using group-average linking), and ordination (multidimensional scaling, MDS) were used for treating the data and were calculated using of computer software of PRIMER (Plymouth Routines In Multivariate Ecological Research ver. 5.2).
Results
Environmental parameters
The physical parameters of water and sediment were similar in all stations (I, II, and III) throughout the experimental period, indicating the well-mixed nature of this ecosystem. The value of rainfall ranged from 17.37 to 5,400 mm in all stations. Temperature, salinity, and pH of both water and sediments ranged from 25.4°C to 33.9°C and 23.2°C to 33.6°C, 18.4 to 35 ppt and 20.5 to 37.4 ppt, and 7.3 to 8.9 and 6.9 to 8.3, respectively. The values of dissolved oxygen ranged from 3 to 5.45 ml/l. Nutrients in water such as nitrite, nitrate, inorganic phosphate, and reactive silicate ranged from 0.42 to 2.536 μg 1−1, 1.526 to 6.522 μg 1−1, 0.426 and 1.528 μg 1−1, and 12.835 to 28.536 μg 1−1, respectively. The sediment redox potential ranged from −321.5 to −40.86. In sediments, macronutrients such as nitrogen, phosphorus, and potassium were recorded, and these are varied from 26.52 to 54.1 g m−2, 0.79 to 2.96 g m−2, and 3.41 to 15.5 g m−2, respectively (Fig. 2). Sediment texture in terms of sand, clay, and silt (%) were 3.0–67.56, 19.23–43.52, and 8.2–64.15 in all the three stations (Fig. 3).
Univariate measures for macro-benthic macrofauna of study area (season-wise). a Species diversity. b Number of organisms. c Pielou’s evenness (J′). d Shannon–Wiener diversity index H′. Data presented as mean (squares), ±1 SE (boxes), and ±1 SD (whiskers). Upside-down triangles: minimum; right-side-up triangles: maximum
Species composition of macrofauna
A total of 46 macrobenthic faunal species represented by four diverse groups were encountered, of which polychaetes, gastropods, bivalves, and crustaceans were the most important groups. Polychaetes are dominated in the macrobenthic fauna (27 species) and contributed numerically up to 58.69% of the population. Gastropods consist of eight species and contribute to 17.39% of the total fauna production. Bivalves consist of seven species and contribute to 15.21% of the total fauna production. Also, crustaceans include four species and contribute to 8.69 of the total fauna production (Fig. 4).
The 31 species (16 polychaetes, six bivalves, seven gastropods, and two crustaceans) were recorded in station I, and the percentage composition was calculated and shown in Fig. 5. The 35 species (20 polychaetes, six bivalves, five gastropods, and four crustaceans) and 31 species (19 polychaetes, four bivalves, five gastropods, and three crustaceans) were recorded in stations II and III, respectively. The percentage composition was calculated for both stations and presented in Figs. 6 and 7. The species belonging to all groups are presented in Table 1. Bivalves and crustaceans were dominant in faunal biomass. The highest number of species was recorded in station II than in others. The benthic macrofaunal density (ind/m−2) was calculated and ranged from 416 to 1,223, from 375 to 328, and from 423 to 1,629 in stations I, II, and III, respectively. The highest benthic macrofaunal density was recorded in the early pre-monsoon season at station III.
Classification analyses (using Bray–Curtis similarity) followed by an ordination through MDS on benthos abundance data (numbers/0.1 m−2) independently for fauna (46 species) were undertaken. The 12 investigation stations (four seasons × three stations) have been divided into three groups: S1Pm, S2Sm, S3PrM, S4Mn; S5Pm, S6Sm, S7PrM, S8Mn; and S9Pm, S10Sm, S11PrM, S12Mn corresponding to Parangipettai (station I), Pichavaram (station II), and the Gulf of Mannar (station III) communities, respectively (Table 2). Figures 8 and 9 display results of MDS ordination and hierarchical clustering, respectively, on species abundance data representing the three stations during four seasons (post-monsoon, summer, pre-monsoon, and monsoon). Cluster analysis showed that the macrofauna communities at each of the mangrove communities were relatively most similar (Fig. 9). The 2D stress value (0.11) indicated that the results are credible. The station I and III communities, which are very similar in the result of the cluster analysis, were clearly separated. In comparison, among the three sampling stations, station II communities were the shortest, implying that the structure of macrofauna communities at this community was the most similar (Fig. 9).
From the resulting dendrogram (Fig. 8), it is possible to classify the results according to stations and also for seasons. Station III is separated from the others. In the MDS plot (Fig. 9), it is found that all season samples are separated conforming to the dendrogram. The two-dimensional plot, otherwise referred to as Ellipse, supports the MDS ordination, and it shows that the monsoonal samples occur outside the contour (Fig. 10). The trend with respect to falling of delta+ and lambda+ values in 95% confidence funnel is very much evident here also (Fig. 11). The individual species count (Fig. 12a) was maximum (35) in station II during summer and pre-monsoon and minimum (31) in stations I and III during summer. The maximum number of organisms (Fig. 12b) is 1,629 animals/0.1 m2 (station III, pre-monsoon) and the minimum is 375 animals/0.1 m2 (station II, monsoon). The Shannon–Wiener index (Fig. 12d) ranges between 1.75 (station III, monsoon) and 3.363 (station I, monsoon). The evenness component (J′) (Fig. 12c) varied from 0.96 (station III, monsoon) to 0.515 (station I, monsoon). It is low during the monsoon season and gradually increases during the post-monsoon and summer seasons.
Univariate measures for macro-benthic macrofauna of study area (season-wise). a Species diversity. b Number of organisms. c Pielou’s evenness (J′). d Shannon–Wiener diversity index H′. Data presented as mean (squares), ±1 SE (boxes), and ±1 SD (whiskers). Upside-down triangles: minimum; right-side-up triangles: maximum
Multiple k-dominance plots facilitate the discrimination of benthos according to species-relative contribution to standard stock. The k-dominance curves obtained for different stations show higher diversity except S10Sm. The stations S6Sm and S7PrM show maximum diversity as the curves for all three stations are lying lower than others whereas the curve of the S7PrM is lying in the top and has a stiff elevation indicating the lowest diversity (Fig. 13). The k-dominance plot is plotted according to station (Fig. 14); it shows the plot for pooled data, i.e., it shows a perfect S curve indicating the high diversity of macrofauna in station II without disturbance, when the curves were drawn separately for the three stations among the seasons. The k-dominance plot is also plotted for all the seasons, and the curve drawn inputting all the stations and all the seasons are shown (Fig. 15). The curve representing during the summer season lies at the top indicating lower diversity and curve represented during the pre-monsoon season at the bottom indicating a higher diversity. Other two seasons fall in between these two seasons; the S shape of the graph is clear evidence that there is no disturbance to these resources. The relationship between the number of species of each density, diversity, richness, and evenness of macrofauna communities and physico-chemical parameters (water and sediments) and also sediment compositions of each mangrove communities are discussed.
Discussion
One of the main goals of benthic ecology has been to understand the mechanisms regulating relationships between physico-chemical parameter and organisms (Gray 1974; Rhoads 1974; Snelgrove and Butman 1994; Aller et al. 2001). The present study shows that the macrofaunal communities of three mangrove ecosystems exhibit distinct variations. Benthic macrofaunal community is characterized by temporal and spatial changes in its population. Macrofaunal distribution pattern seems to be fully governed by the physico-chemical and hydrobiological characteristics of the environment. Intertidal fauna at the study area have to cope with harsh environmental conditions marked by high salinity, increased evaporation, wide seasonal temperature fluctuations, and different degrees of tidal amplitudes. These unique physicochemical factors exert a strong influence on faunal assemblages, which are withstanding the situation. Owing to the heterogeneous nature of estuarine water, the relatively stationary benthic animals on the bottom have to endure a wide range of environmental changes when the circulation carries different kinds of water over the site or borrow (Stone and Reish 1965). Mangroves also possess some positive advantages of benthic animals, compared to the open coast. Estuaries are relatively sheltered against wind waves and ocean swell; most estuaries are also rich in food provided by river input, input from mangroves, and high primary production (Postma 1954; Schelske and Odum 1961).
This study has shown that there is difference in macrobenthic fauna at different mangrove types like developing, riverine, and island mangroves. The structure of benthic macrofauna communities is characterized by a low abundance and a very low diversity. Richer communities have been found in station II. The macrobenthic faunal density ranges from 375 to 1,629 ind/m2 in all the stations. This density is higher than the macrobenthic faunal densities as reported by Parulekar and Waugh (1975) for Zuari estuary (50 to 1,437 ind/m2), by Parulekar and Ansari (1981) for Andaman seas (80 to 998 ind/m2), and comparable with that of Harikantra et al. (1980) who record the density range of macrobenthos from 50 to 3,715 ind/m2 in the shelf region along the west coast of India. However, this value is lower than the reported density of 1,253 to 5,723 ind/m2 in northwestern Arabian Sea shelf by Parulekar and Waugh (1975) in northern sea. The difference in the benthic macrofaunal densities of different aquatic systems could be attributed mainly to variations in salinity, substratum and sediment organic carbon level, currents, and predation.
The species composition of benthic macrofauna in the present observation shows the dominance of polychaete followed by molluscs and crustaceans. Similar preponderance of polychaetes has been observed earlier by Sankar (1998) in Muthupet lagoon, Sunilkumar (1995) in Cochin backwaters, Prabha Devi (1994) in Coleroon estuary, and Ansari et al. (1986) in Mandovi estuary. Athalye and Gokhale (1998) reported the dominance of polychaetes followed gastropods, bivalves, and hermit crabs in the Thane creek, Mumbai. The benthic population density shows seasonal variation in such a way that the maximum is recorded in summer and pre-monsoon and the minimum during monsoon at all the stations. The dominance of polychaetes might be due to firm substrate provided by roots and dense canopy of the mangroves which also provide protection against desiccation (Mishra and Choudhury 1985). They have more opportunistic bearing potential ability to colonize in stressed environments (Raveenthiranath Nehru 1990). The aforementioned adaptable nature of polychaetes may be a plausible reason for their dominance in the species composition and their abundance in the present investigation. In the present study, molluscs form the second dominant group followed by polychaetes. The dominance of gastropods and bivalves are also observed by Kathiresan et al. (2000) in Vellar estuary on the southeast coast of India. They report that high tolerance to different environmental situation and various estuarine conditions reveal its higher abundance. In the present study, crustaceans form the third group after polychaetes and molluscs. The present observation shows numerical dominance in the decreasing order as polychaetes, molluscs (bivalves and gastropods), and crustaceans, as observed earlier by Mohammed (1995) and Kumar (2001) in other mangrove environs of India. Irrespective of mangrove types, the mangroves show the same order of polychaetes, molluscs, and crustaceans. From this, it is evident that polychaetes form the dominant group of macrobenthos in mangroves.
Environmental factors such as temperature, sediment composition, and inundation are the main factors influencing the distribution of faunal communities in tropical mangroves. Salinity is one of the important key factors which determine the composition of biological component in the marine environment. The fluctuations in salinity affect the biological characteristics of the environment. The present study did not show characteristic relationship between salinity and macrofaunal distribution; however, soil salinity showed significant negative correlation with species richness (r = −0.976; p < 0.05) at station I (Table 3) and (r = −0.996; p < 0.01) at station III (Table 5). This means that the fluctuation of salinity in riverine and island mangroves have profound influence on the species richness. Reid (1961) remarks that the momentary salinity may be regarded as a function of the quantity and quality of inflowing and outflowing waters, rainfall, and evaporations since these factors may vary with seasons (in some instants rather drastically).
In the present investigation, dissolved oxygen was high during the monsoon season at all sites, which might be due to the cumulative effect of higher wind velocity coupled with heavy rainfall and the resultant freshwater mixing. Relatively lower values were observed during summer; this may be due to the increased surface water temperature which reduces the dissolvation of O2 in the coastal waters. It is well known that temperature and salinity affect the dissolution of oxygen (Viijayakumar et al. 2000). Hydrogen ion concentration (pH) in surface waters remained alkaline at all sites throughout the study period with the maximum value during summer seasons and the minimum during the monsoon. However, the present study did not find a characteristic relationship between pH, salinity, and temperature and macrobenthic fauna, confirming that the fauna of independent mangrove system requires specific environmental characters.
Sediment texture plays an important role in the ecology of benthic invertebrates (Sanders 1956; Maurer 1969). The pelagic larvae of macrobenthic organisms before finally settling down at the bottom have to cross many barriers, and each type of bottom deposit will attract a very limited and selected set of species (Thorson 1966). A common concept in benthic animal–sediment relation is that the feeding type of the infauna is in one way correlated to the sediments (Bloom et al. 1972). Deposit or detritus feeders constitute an important and often dominating part of macrobenthic invertebrates (Fenchel et al. 1975). Sediment character has been identified as one of the driving forces in determining the macrofaunal communities. At station I, species diversity is negatively correlated with sand (r = −0.975) while in station II and III, positive correlation is obtained between density and silt (r = 0.984 and r = 0.977) at p <0.05 level (Table 4). This indicates that availability of silty soil sustains to macrofaunal diversity and density, while sand dominance will reduce the macrofaunal population. Clayey silt substrate is always known to support epifauna (KondalaRao and Ramanamurthy 1988; Goldin et al. 1996).
Soil Eh showed negative correlation with species diversity at station III (r = −0.966; p < 0.05) which indicates that the anoxic condition is not supported for higher macrofaunal diversity in the mangrove ecosystem. Such anoxic conditions are very much prevalent in island mangroves, as these soils are rarely washed by the tidal waters. English et al. (1994) have stated that the value of redox potential may ranged from totally anoxic sediments (below −200) to a typical oxygenated sediments (above +300 mV) for a temperate East African mangrove forest. Our study has found almost anoxic soil in all the three mangrove sediments (−40.86 to −321.5). This could be one of the reasons for the low macrofaunal diversity.
Food supply seldom acts as a limiting factor in the seasonal abundance of macrobenthos (Qasim 1970). Organic nutrients enhance the growth of different types of algae that provide food resources for benthos (Hearld and Odum 1970). In the present study, the higher density macrobenthos is observed during pre-monsoon and summer seasons. Higher organic matter gets deposited during the post-monsoon season in the mangrove areas. It would be converted into available organic carbon by various fungal and bacterial sources, which in turn increase the macrobenthic forms especially polychaetes (Sunilkumar and Antony 1994). High organic carbon induced abundance of macrofauna in Coleroon estuary (Prabha Devi and Ayyakkannu 1989). This confirmed that the abundance of benthic fauna is highly related to organic carbon. In the present study, the macrobenthic faunal density ranged from 375 to 1,629 ind/m2. The highest abundance (1,629 ind/m2) has been recorded at station III (Gulf of Mannar) during the pre-monsoon season. The maximum abundance in station III might be due to the abundance of single species, namely, Cerithidea cingulata.
To find out a clear picture of species diversity and distribution, various univariate and multivariate analyses have been carried out and results are discussed. Individual species is a simple and useful measure of the biological system. The minimum species count (29) and the maximum (35) was recorded in stations III and II, respectively. Moreover, Pearson and Rosenberg (1978) proposed that the use of diversity indices is advantageous for the description of fauna at different stages in succession. Species individual diversity in the present study registered a wide fluctuation between 1.75 (monsoon) and 3.36 (pre-monsoon) among stations and seasons. The lower species diversity was recorded during monsoon and higher diversity values during pre-monsoon in the study area. This is in conformity with the earlier observations made in Vellar (Chandran 1987) and Coleroon estuaries (Prabha Devi 1994). In the present study, negative correlation is obtained between species diversity and richness at stations II (r = −0.976) and III (r = −0.965) at p <0.05 level.
All the nutrients (N, P, and K) are found enriched in the sediment during monsoon. These nutrients might have reached the benthic realm through food web during summer and pre-monsoon seasons. Species richness of benthic macrofauna was found maximum during the post-monsoon season (5.342). A similar observation was reported by Kumar (2001) in Cochin backwaters. The low richness was recorded during monsoon (3.786) might be due to the high freshwater inflow with low saline conditions, which in turn affect the distribution of benthos, particularly the polychaetes. Maximum diversity and richness recorded during pre-monsoon at the all sites might be due to stable and optimum environmental factor such as salinity, which plays an important role in faunal distribution and abundance. Shannon diversity was exceptionally high and it was in the range of 1.75–3.363. The minimum (1.75) and maximum (3.363) was recorded at station I during the monsoon season and at station III during the pre-monsoon season, respectively. Shannon diversity in the present study was considered to be good and the range recorded in the present study vouchsafes for the healthy nature of mangrove ecosystem. Similar seasonal pattern is evident from the view of Sourav Kundu et al. (2009).
Species area plots used to show the cumulative number of different species observed as each new sample is added. The advantage of plotting this technique is to predict the total number of stations to be sampled for getting the maximum number of species in a station. The present study revealed that 12 times sampling during various seasons is enough to get all the species in the study areas. The dendrograms derived in the present study showed clustering of stations and gradual change in species composition from the island mangrove ecosystem towards riverine mangrove ecosystem. This means that a certain level of similarity prevails in faunal diversity in developing and island mangroves than in the riverine mangrove ecosystem. Derived multidimensional scaling (MDS) ordination reveals the same grouping of stations as in the cluster analysis. The stress values found in the MDS configuration is low (0.11), indicating good representation of the interrelationship between the macrofauna of each station. Benthic population (r) is positively correlated with sand (r = 0.975) in station I. In station II, benthic population is positively correlated with water salinity (r = 0.980), soil salinity (r = 0.984), and NO2 (r = 0.971). In station III, water salinity (r = 0.967), sediment salinity (r = 0.978), silt (r = 0.977), and NO2 (r = 0.979) are positively correlated with benthic population at p <0.05 level.
Conclusion
Among the three ecosystems, riverine mangrove (station II) ecosystem is more pristine in nature than the developing and island mangrove ecosystems. Benthic macrofauna species assemblage is comparatively higher in station II than in stations I and III. Analysis of data undertaken with predictable like line Shannon diversity, Simpson richness, and recently introduced diversity indices such as taxonomic diversity index and total phylogenetic index clearly opined that healthy nature of the mangrove ecosystem. From the present study, it could be concluded that the hydrography, nutrients, and sediment texture are the major factors responsible for fluctuation in benthic macrofaunal assemblages in the study area.
References
Aller, J. Y., Woodin, S. A., & Aller, R. C. (2001). Organism–sediment interactions. Columbia: University of South Carolina Press.
Ansari, Z. A., Ingole, B. S., Banerjee, G., & Parulekar, A. H. (1986). Spatial and temporal changes in benthic macrofauna from Mandovi & Zuari estuaries of Goa, West coast of India. Indian Journal of Marine Sciences, 15, 223–229.
Ashton, E. C. (1999). Biodiversity and community ecology of mangrove plants, molluscs and crustaceans in two mangrove forests in Peninsular Malaysia in relation to management practices. Ph.D. thesis. University of York, UK.
Athalye, R. P., & Gokhale, K. S. (1998). Macrobenthos from the mudflats of Thane creek, Maharashtra, India. Journal of the Bombay Natural History Society, 95, 259–266.
Bloom, S. A., Simon, J. L., & Hunter, V. D. (1972). Animal–sediment relations and community analysis of a Florida estuary. Marine Biology, 13, 43–56.
Chandran, R. (1987). Hydrobiological studies in the gradient zone of the Vellar estuary. IV. Benthic fauna. Mahasagar—Bulletin of the National Institute of Oceanography, 20(1), 1–13.
Day, J. H. (1967). A monograph on the polychaeta of Southern Africa, Part I (Errantia) & II (Sedentaria). London: Trustees of the British Museum (Natural History).
English, S., Wilkinson, C., & Baker, V. (l994). Survey manual for tropical marine resources (2nd edition), Australian Institute of Marine Science, 238.
Fauvel, P. (1953). The fauna of India including Pakistan, Ceylon, Burma and Malaya. Allahabad: The Indian Press.
Fenchel, T., Kofoed, L. H., & Lappalainen, A. (1975). Particle size-selection of two deposit feeders: the amphipod Corophium volututor and the prosobranch Hydrobiu ulvae. Marine Biology, 30, 119–128.
Goldin, Q., Mishra, V., Ullal, V., Athalye, A. R., & Gokhale, K. S. (1996). Meiobenthos of mangrove mudflats from shallow region of Thane Creek, Central westcoast of India. Indian Journal of Marine Sciences, 25, 137–141.
Gray, J. (1974). Animal–sediment relationships. Oceanography and Marine Biology Annual Review, 12, 223–261.
Harikantra, S. N., Nair, A., Ansari, Z. A., & Parulekar, A. H. (1980). Benthos of shelf region along the west coast of India. Indian Journal of Marine Pollution and Bulletin, 14(2), 41–46.
Hearld, C. L., & Odum, E. P. (1970). Mechanisms maintaining high productivity in Georgia estuaries. In Proceedings of the Gulf and Caribbean Fisheries Institute 14th Annual Session, 75–80.
Jackson, M. L. (1958). Soil chemical analysis. New Delhi: Prentice Hall.
Kathiresan, K. (2000). A review of studies on Pichavaram mangroves, southeast India. Hydrobiologia, 430, 185–205.
Kathiresan, K., & Quasim, S. Z. (2005). Biodiversity of mangrove ecosystems (p. 251). New Delhi: Hindustan Publishing Corporation.
Kathiresan, K., Rajendran, N., & Palaniselvam, V. (2000). Growth of Rhizophora apiculata in degraded areas of Ariyankuppam estuary along Pondicherry coastline, southeast coast of India. Indian Journal of Marine Sciences, 29, 86–88.
KondalaRao, B., & Ramanamurthy, K. V. (1988). Ecology of intertidal meiofauna of the Kakin Bay (Gautam–Godavari Estuarine System), east coast of India. Indian Journal of Marine Sciences, 17, 40–47.
Krom, M. D., & Berner, R. A. (1980). Adsorption of phosphate in anoxic marine sediments. Limnology and Oceanography, 25(5), 797–806.
Krumbein, W. C., & Pettijohn, F. J. (1938). Manual of sedimentary petrography. New York: Appleton Century Crofts.
Kumar, R. S. (2001). Macrobenthos in the mangrove ecosystem of Cochin backwaters, Kerala (southwest coast of India). Indian Journal of Marine Sciences, 24, 56–61.
Kundu, S., Mondal, N., Lyla, P. S., & Ajmal Khan, S. (2009). Biodiversity and seasonal variation of macro-benthic infaunal community in the inshore waters of Parangipettai Coast. Environmental Monitoring and Assessment. doi:10.1007/s10661-009-0817-9.
Macintosh, D. J. (1984). Ecology and productivity of Malaysian mangrove crab populations (Decapoda: Brachyura). Proceedings of the Asian Symposium on Mangrove Environmental Research and Management, 354–377.
Manson, F. J., Loneragan, N. R., Skilleter, G. A., & Phinn, S. R. (2005). An evaluation of the evidence for linkages between mangroves and fisheries: a synthesis of the literature and identification of research directions. Oceanography and Marine Biology Annual Review, 43, 483–513.
Maurer, D. (1969). Diversity of soft-bottom benthos in a tropical estuary: Gulf of Nicoya, Costa Rica. Marine Biology, 81, 97–106.
Mishra, A., & Choudhury. (1985). Polychaetes annalids from the mangrove swamps of Sundarbans, India. Proceedings on the National Symposium on Biological Utilization and Conservation of Mangroves, 448–452.
Mohammed, S. Z. (1995). Observation on the benthic macrofauna of the soft sediment on Western side of the Arabian Gulf (ROPME sea area) with respect to 1991 Gulf War oil spill. Indian Journal of Marine Sciences, 24, 147–152.
Olsen, S. R., Cole, C. V., Watanabe, F. S., & Dean, L. (1954). USDA Circ., 939. Washington DC: U.S. Govt. Printing Office.
Parulekar, A. H., & Ansari, Z. A. (1981). Spatial and temporal changes in benthic macrofauna from Mandovi & Zuari estuaries of Goa, West coast of India. Indian Journal of Marine Science, 15, 223–229.
Parulekar, A. H., & Waugh. (1975). Benthos studies in Goa estuaries III. Annual cycle of macrofaunal distributional production and trophic relations. Indian Journal of Marine Science, 1, 189–200.
Pearson, T. H., & Rosenberg, R. (1978). Macrobenthos secession in relation to organic enrichment and pollution of the marine environment. Oceanography and Marine Biology Annual Review, 16, 229–234.
Postma, H. (1954). Hydrography of the Dutch Wadden sea. Thesis Groningen. Arch. Neerland. Zoologie, X, 4e Livraison, 106.
Prabha Devi, L. (1994). Ecology of Coleroon estuary: studies on benthic fauna. Journal Marine Biology Assessment of India, 36(1–2), 260–266.
Prabha Devi, L., & Ayyakkannu, K. (1989). Ecology of benthic macrofauna in Cuddalore—Uppanar backwater, southeast coast of India. Indian Journal of Marine Science, 20, 200–203.
Qasim, S. Z. (1970). Some problems related to the food chain a tropical estuary. In J.H. Steele (Ed.), Marine food chains (pp. 45–51). Edinburgh: Oliver and Boyd.
Raveenthiranath Nehru (1990). Ecology of macrobenthos in and around Mahandrapalli region of Coleroon estuary, Southeast coast of India. Ph.D. thesis, Annamalai University, India, 231
Reid, G. K. (1961). Ecology of inland waters and estuaries. New York: Reinhold.
Rhoads, D. (1974). Organism–sediment relations on the muddy sea floor. Oceanography and Marine Biology Annual Review, 12, 263–300.
Sanders, H. L. (1956). Benthic studies in Buzzards Bay. Animal sediment relationships. Limnology and Oceanography, 3, 245–258.
Sankar, G. (1998). Studies on the hydrobiology, benthic ecology and fisheries of Muthupet lagoon. Ph.D. thesis, Annamalai University, India, 105
Sasekumar, A. (1974). Distribution of macrofauna on a Malayan mangrove shore. Journal of Animal Ecology, 43, 51–69.
Schelske, C. L., & Odum, E. P. (1961). Mechanisms maintaining high productivity in Georgia estuaries. In: Proceedings of the Gulf and Caribbean Fisheries Institute 14th Annual Session, 75–80.
Smith, T. J., Boto, K. G., Frusher, S. D., & Giddins, R. L. (1991). Keystone species and mangrove forest dynamics: the influence of burrowing by crabs on soil nutrient status and forest productivity. Estuarine, Coastal and Shelf Science, 33, 19–32.
Snelgrove, P. V. R., & Butman, C. A. (1994). Animal–sediment relationships revisited: cause versus effect. Oceanography and Marine Biology Annual Review, 32, 111–177.
Stone, A. N., & Reish, D. J. (1965). The effect of fresh water run-off on a population of estuarine polychaetous annelids. Bulletin of the Southern California Academy of Sciences, 64, 111.
Strickland, J. D. H., & Parsons, T. R. (1972). A practical handbook of seawater analysis. Fishery Research Board, Canada, 310p.
Subba Rao, N. V., Surya Rao, K. V., & Maitra, S. (1991). Marine molluscs. State Fauna Series. Fauna of Orissa (pp. 1–175). Kolkata: Zoological Survey of India, 1(3), 1–175.
Subbiah, B. V., & Asija, G. L. (1956). A rapid procedure for the determination of available nitrogen in soils. Current Science, 31, 196.
Sunilkumar, R. (1995). Macrobenthos in the mangrove ecosystem of Cochin backwaters, Kerala (Southwest coast of India). Indian Journal of Marine Sciences, 24, 56–61.
Sunilkumar, R., & Antony, A. (1994). Preliminary studies on the polychaete fauna of the mangrove areas of Cochin. In: Proceedings of The 6th Kerala Science Congress, Thiruvanthapuram, Kerala. R. Ravikumar (Eds.), State Committee on Science, Technology and Environment, 74–77.
Thorson, G. (1966). Some factors influencing the requirement and establishment of marine benthic communities. Journal of Sea Research, 3, 267–293.
Viijayakumar, S., Rajesh, K. M., Mridula, R. M., & Hariharan, V. (2000). Seasonal distribution and behaviour of nutrients with reference to tidal rhythm in the Mulki estuary, Southwest coast of India. Journal of Marine Biology Assessment of India, 42(1–2), 21–31.
Warren, J. H., & Underwood, A. J. (1986). Effects of burrowing crabs on the topography of mangroves swamps in New South Wales. Journal of Experimental Marine Biology and Ecology, 102, 223–235.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samidurai, K., Saravanakumar, A. & Kathiresan, K. Spatial and temporal distribution of macrobenthos in different mangrove ecosystems of Tamil Nadu Coast, India. Environ Monit Assess 184, 4079–4096 (2012). https://doi.org/10.1007/s10661-011-2245-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-011-2245-x