Abstract
Thirty-nine samples of recent bottom sediments were collected from Mabahiss Bay, north of Hurghada City, Red Sea, Egypt. The collected samples were subjected to a total digestion technique and analyzed by absorption spectrometer for metals including Pb, Zn, Cd, Ni, Co, Cu, and Mn. Concentration data were processed using correlation analysis, principal component analysis, and hierarchical cluster analysis. Multivariate statistical analysis classified heavy metals in the study area into different groups. The pollution level attributed to these metals was evaluated using geoaccumulation index and contamination factor in order to determine anthropogenically derived sediment contamination. The results of both geoaccumulation index and contamination factor results reveal that the study area is not contaminated with respect to Zn, Ni, Cu, and Mn; uncontaminated to moderately contaminate with Pb; and moderately to strongly contaminate with Cd. The high contents of Pb, Cd, and Co in the study area result from various anthropogenic activities including dredging, land filling, localized oil pollution, using of antifouling and anticorrosive paints from fishing and tourist boats, and sewage discharging from various sources within the study area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The presence of heavy metals in sediments poses a potential threat to the marine ecosystems. Heavy metals are of critical ecological significance due to their toxicity, resistance to degradation, and their consequent tendency to bioaccumulate (Diagomanolin et al. 2004). Metal toxicity and availability depend on the concentration of metal ions, pH, oxidative–reductive potential, dissolved oxygen, and organic and inorganic carbon content (Stauber and Florence 1987; Calmano et al. 1990; Wen and Allen 1999). In addition, metal ions concentrations are strongly dependent on biological processes, redox potential, pH, ionic strength activities of organic and inorganic chelators, and scavenging processes (Hoz et al. 2000). Heavy metals are among the most serious pollutants within the natural environments due to their toxicity, persistence and bioaccumulation problems (MacFarlane and Burchett 2000).
The elevation of metal levels in an aquatic environment is shown mainly by an increase in their concentrations in the recent bottom sediments. Their occurrence in the environment results primarily from anthropogenic activities. In addition, the natural processes, such as weathering of rocks and volcanic activities, play a noticeable role in enriching the water of reservoirs with heavy metals (Forstner and Wittmann 1983; Nriagu 1989; Veena et al. 1997). Sediments are normally mixtures of several components including different mineral species as well as organic debris. Sediments represent one of the ultimate sinks for heavy metals discharged into the environment (Bettinetti et al. 2003; Hollert et al. 2003).
Metal contamination in the sediments along the Red Sea coast was investigated by few researchers (Mansour 1999; Mansour et al. 2000; Radwan 2003). Assessment of heavy metal contamination in the recent bottom sediments of Mabahiss Bay has not been previously assessed or investigated, to the best of the authors’ knowledge where pollution sources in the area are wastewaters discharges, tourist, boat painting, etc. Such an investigation is required if the environmental impacts are to be understood quantitatively and qualitatively. It is noticed that land filling or dredging, includes construction of marinas, embankments, jetties, swimming pools, and recreational buildings where managing agencies have dumped large amounts of detrital rock, gravel, and fine material off their properties.
The main objectives of this study were (1) to use the geoaccumulation index (I geo) and contamination factor (CF) in order to evaluate the level of anthropogenic participation in the pollution caused by Pb, Zn, Cd, Ni, Co, Cu, and Mn in the area, and (2) to classify heavy metals into distinct groups using multivariate statistical methods such as principal component analysis (PCA), hierarchical cluster analysis (HCA), and correlation analysis (CA).
Location and geology of the study area
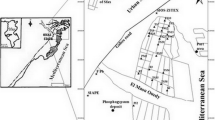
Mabahiss bay occupies an area of about 35 km2 and lies near to the southern extreme of the Gulf of Suez to the north of Hurghada City (Fig. 1). The coastal plain in this location is wide and bounded from west by granitic mountains belonging to the Eastern Desert. The surface of the coastal plain contains many dry valleys and is covered by Pleistocene reefal limestone, gravels, and sands. The offshore islands and the submerged coral system serve to dissipate wave energy during stormy conditions. The beach sediments come from weathering and erosion of the western mountain ranges comprising terrigenous quartz grains (Moussa et al. 1991). Previous studies of the Hurghada region dealt with the sedimentology of coral formation, carbonate mineralogy, and bottom topography (Crossland 1939; Badr and Crossland 1939; Shukri 1953; El Sayed 1984; El Waked et al. 1984; Moussa et al. 1991).
The reefs to the north of Hurghada City were developed along roughly parallel ridges oriented in a NNW–SSE direction (Hodgson 1983). In Mabahiss Bay, the fringing and barrier reefs are the most common type. The bay is an elongated semiclosed basin of a triangle shape and bounded by extensive growth of coral reefs (Shaabs). It is bounded by Shaab Saad and Shaab Abu Qalawa in the north, Shaab El-Fanadir in the east, and Shaab Abu Sadaf in the west and south (Fig. 1). The coral reef patches are present in two forms. The first one is attached either to the mainland or to the insular roots and forms a circular shape (fringing). The second one is independent reef and surrounded by water (Radwan 2003).
Sediments in the Hurghada area is subdivided into to two zones; northern zone of materials of more terrigenous origin than the southern zone (Beltagy et al. 1986). This subdivision is based on the chemistry of sediments from Hurghada area according to its carbonates, chlorides, sulfates, organic carbon, sodium, potassium, magnesium, strontium, and total iron contents. Increased coastal tourism leads to increased coastal urbanization. Consequently, rapid and uncontrolled tourism development has created many human infringements on the foreshore area and subsequently impact on the coastal zone. These man-made problems affect the marine and coastal environment. Moreover, the fluvial sediment discharged from surrounded mountains might have had an effect on the early morphology of the reef formations (Moussa et al. 1991).
The tide is semidiurnal having a maximum peak every 12 h with a mean tidal range of about 0.8 m. The average water salinity near the coast is 41 % and is 40 % in the deeper water. The average water temperature is 24.95 °C (Table 1). Winds mainly blow from the NW and N during most of the year. As a result, winds, waves, and tides produce coastal currents to the south and southeast. The maximum speed of the longshore current inside the zone between the reef edge and shoreline ranges from 35 to 40 cm s−1 (CRI 1994). The littoral drift along the Hurghada coast is significant, as indicated by the formation of well-developed spits.
Material and methods
Sampling and analysis
Thirty-nine representative samples of recent bottom sediments were collected from Mabahiss Bay (Fig. 1) using a grab sampler. To overcome difficulties due to the presence of coral reefs, diving was used to collect some of the samples. Then, the collected samples were poured in a plastic dishes with their associated water, to preserved the suspended fine grained sediments, and were then put to a big-labeled plastic bags. The coordinates of each sampling station were performed using Magellan 500 (NAVIGATION GPS 5000 PRO, USA).
Heavy metals contents were accomplished following by the method described by Oregioni and Aston (1984). The collected samples from the bay bottom sediments were poured in big glassy dishes and remained sufficient time until the fine grains precipitated. Then, the associated water was siphoned off, and the samples were air-dried at room temperature. During dryness, the samples were disaggregated by fingers. The 0.3 g of the dried sample put in a Teflon crucible and digested using HNO3 (65 %), HF (40 %), and HClO4 (70 %) with volume ratio of 3:2:1. The crucible was heated on a hot plate for about 2 h. Then, the samples were cooled, filtered, and transferred to volumetric flasks made up to a volume of 25 ml.
The solution of each digested sample was analyzed with an atomic absorption spectrometer (Buck Sci., Model 200A) for lead (Pb), zinc (Zn), cadmium (Cd), nickel (Ni), cobalt (Co), copper (Cu), and manganese (Mn). For quality control, all sediment samples were analyzed in triplicate, and the mean values were calculated. Laboratory blanks were routinely prepared and analyzed (one blank for every five samples) to monitor the possibility of sample contamination during digestion and subsequent analysis. No detectable contamination was found when aliquots of reagents were processed and analyzed with the samples. The absorption wavelength and detection limits of metals were as follows: 228.8 nm and 0.0006 ppm for Cd, 240.7 nm and 0.007 ppm for Co, 324.7 nm and 0.003 ppm for Cu, 279.5 nm and 0.003 ppm for Mn, 232.0 nm and 0.008 ppm for Ni, 217.0 nm and 0.02 ppm for Pb, and 213.9 nm and 0.002 ppm for Zn. The percentage of blank contribution to the average concentration of the studied metals was evaluated, and it was found to be 0.28 % Cd, 3.7 % Co, 6.2 % Cu, 2.9 % Mn, 1.1 % Ni, 5.4 % Pb, and 4.3 % Zn. No significant contribution was observed from blank values to the measured concentrations in atomic absorption spectrometer analysis.
Statistical analyses
In order to explore the possible associations between heavy metals, Pearson’s correlation coefficient matrix (CA) (Sun et al. 2010; Ghrefat et al. 2011) was carried out on the obtained data. Pearson’s correlation is obtained by standardizing and dividing the distance from the mean by the sample standard deviation. The Pearson’s correlation coefficients for heavy metals in the study area are summarized in Table 3. To obtain more reliable information about the relationships among the variables, the PCA was applied (Bartolomeo et al. 2004; Glasby et al. 2004).
The heavy metal concentrations data were standardized by means of z-scores before HCA and squared Euclidean distances for similarities in the variables were calculated. Then, hierarchical clustering by applying Ward’s method was performed on the standardized data set, and the HCA results for the heavy metals are shown in Fig. 2 as a dendrogram. All the statistical analyses of data were performed using SPSS Version 20. Evaluation of heavy metal pollution in the recent bottom sediments of Mabahiss Bay was carried out using the geoaccumulation index (I geo) of Müller (1979) and the contamination factor (CF) of Hakanson (1980).
Results and discussion
As a matter of fact, metals are present in the marine environments as dissolved ions, complexes, colloids, suspended solids, and solids in sediments (Larocque and Rasmussen 1998).
Metal levels
The results showed that Mn has the highest mean concentration in the recent bottom sediments of the study area, followed by Co, Pb, Ni, Zn, Cu, and Cd (Table 1). Manganese content ranges from 15.5 to 270.6 with an average of 77 ppm (Table 1), with remarkable decreases toward the deeper part of the bay (Table 1, Fig. 1). The average concentration of Cu is 13.4 ppm (Table 1). By comparing the concentration value of Cu with its location (Table 1 and Fig. 1), the Cu concentration increases toward the inner part of the bay. The average concentration of Ni is 34.0 ppm (Table 1). Generally, it increases toward certain spots in the shallow parts of the bay along the shore and around Shaabs as well as toward deeper parts. The average cadmium content is 3.1 ppm (Table 1) where it increases toward the basin center (Table 1 and Fig. 1). Lead content ranges from 10.7 to 254.3, averaging 43.56 ppm in the studied bottom sediments (Table 1). It has more or less general increasing trend toward the deeper parts between Shaabs (Table 1 and Fig. 1) with some spots of increasing concentration near the shore.
Consequently, the results showed that Cu, Ni, Cd, and Pb have similar trends, and their concentrations increase more or less toward the innermost parts of the bay. This suggests that the origin of these metals is from the bay itself rather than from the input of outside terrestrial sediments. Because Mabahiss Bay is used as a harbor for fishermen and cargo boats, the possible sources of the above-mentioned metals are shipment operations inside the harbor, shipyards, domestic sewage, landfill, and dredging. In addition, Cu, Ni, Cd, and Pb are found in the antifouling and anticorrosive paints used to protect ships and coastal structures.
On the other hand, Zn, Co, and Mn showed a common trend of increasing concentration toward the outermost parts of the bay. Consequently, we can deduce that the sources of metal pollution near the shoreline of Mabahiss Bay include dredging, land filling, discharge of municipal wastewaters from tourist centers as well as from fishermen and cargo boats (Frihy et al. 1996).
Multivariate statistical analysis results
Correlation analysis
Interelement relationships using the Pearson’s correlation coefficients provided interesting information on the sources and pathways of the heavy metals. A significantly positive correlation at P < 0.01 was found between the elemental pairs Cu–Zn (0.46), Cu–Pb (0.73), Zn–Co (0.79), Zn–Ni (0.47), and Co–Ni (0.62) (Table 2). A significantly positive correlation at P < 0.05 was also found between the elemental pairs Zn–Pb (0.35), Zn–Mn (0.32), Co–Mn (0.39), and Ni–Pb (0.36) (Table 2). The high correlated coefficients between metals could be a potential indicator to the same anthropogenic and natural sources.
The long water retention time in the study area, as a result of its semiclosed basin character, may be the cause of the relatively high concentration of heavy metals in the bottom sediment samples. The restricted exchange with the open sea and the limited effect of flushing suggests that bottom sediments receive and retain virtually all of the heavy metals introduced to the bay. Moreover, the increase in the concentration of Zn, Co, Ni, Pb, and Mn may be due to dredging, land filling, decay of organic materials, sewage, and wastewaters discharges, where the bay receives intermittent and variable amounts of municipal wastewater from many outlets of tourist centers as well as from fishermen and cargo boats.
Principal component analysis
In the present study, PCA (varimax with Z normalization) identified three components for all sampled sites, and the percentage of variance explained by each of them were calculated and shown in Table 3. The results showed that the three components explained 77.2 % of the total variance. The principal component 1 (PC1) explained 42.0 % of the total variance and loading heavily on Zn, Co, Ni, and Mn. The principal component 2 (PC2) accounts for 19.5 % of the total variance, which contains a high positive loading of Cu and Pb as well as a negative loadings of Mn. The principal component 3 (PC3) contains a high loading of Cd and a negative loading of Cu, Zn, and Co, accounting for 15.7 % of the total variance. Small (positive or negative) values indicate weak relationships, while large (absolute) loadings indicate stronger relationships.
Hierarchical cluster analysis
In general, the HCA results agreed very well with those of PCA analysis. Two distinct clusters were observed from the dendrograms for the metals in the study area. Cluster 1 includes Mn. Cluster 2 includes Cu, Zn, Cd, Ni, Co, and Pb (two sub-clusters: Co–Pb; Cu, Zn, Cd, and Ni). It is clear from Fig. 2 that there was a remarkable difference from two integrated clusters in terms of Euclidian distances, which possibly implied different sources.
Heavy metal assessment
I geo is used to determine and define metals contamination in sediments, by comparing current concentrations with pre-industrial levels. The formula used for the calculation of I geo is expressed as follows:
where C n is the measured concentration of the examined metal n in the sediment, B n is the geochemical background concentration of the metal n, and the factor 1.5 is used because of possible variations of the background data due to lithological variations. Because we did not have the background values of the metals of interest, we adopt the global average shale data values (Turekian and Wedepohl 1961) in I geo calculation.
Table 4 summarizes classes of I geo (Müller 1981). The highest class (class 6) reflects 100-fold enrichment above the background values. The results of I geo values are depicted in Table 5. I geo values describe recent bottom sediments of Mabahiss Bay as uncontaminated to moderately contaminated for Pb (I geo = 0.2) and the moderately contaminated to strongly contaminated classes with respect to Cd (I geo ≥ 2.0) possibly due to the anthropogenic inputs such as discharge of sewage, oil pollution from ships, use of paints for fishing and tourist boats. However, I geo values indicate that for Zn, Ni, Cu, and Mn, the sampling sites are classified as uncontaminated.
Heavy metals assessment is also carried out using CF (Hakanson 1980). CF is calculated based on the following formula:
where M x is the mean concentration of the target metal in at least five sub-samples, and M b is the concentration of the metal in the selected reference background. Table 6 summarizes CF classes. The values of CF range between <1 and >6. The results of CF (Table 7) demonstrated that there is high contamination by Cd. This result is in agreement with the results of El-Mamony (1995 and Mansour et al. (2007). However, cadmium release from the underlying sediment layer to the water at the low redox potential with the presence of organic-bound metal. Pb and Co are classified as moderate contaminants. CF values indicate that there is no contamination by Zn, Ni, Cu, and Mn. This result is agreement with the results of Mansour et al. (2007) during their study of activates including different aspects of coastal developments along the Red Sea coastal region from Hurghada to Quseir (Mansour et al. 2007).
The results of I geo coincide with those of CF. I geo and CF results are in good agreement with those of PCA and HCA. Cd has no essential biological functions and is highly toxic to humans (Selinus et al. 2006; Dong et al. 2007). The major hazards to human health from Cd are its chronic accumulation in kidneys (Alloway 1990) and its absorption and toxicity in women during menstruation related to decrease in iron stores (Berglund et al. 1994), causing human carcinogens (NTP 2002); urinary cadmium excretion in adults is directly correlated to the concentration of cadmium in the renal cortex (Idaho Department of Health and Welfare Division of Health 2000); admixture of Cd, Pb, and Zn can cause hematological effect (Thawley et al. 1977), affecting metallothioneins (Goyer and Clarkson 2001); high exposure to cadmium fumes (which can occur in some occupational settings) can cause acute bronchitis or even chronic disease, such as emphysema or pulmonary fibrosis and lung cancer (Davison et al. 1988). In addition, chronic exposure over several years to low doses of cadmium (cigarette smoking for example) can cause kidney tubular dysfunction and osteoporosis in susceptible populations (elderly women with iron deficiency) (Jarup et al. 1998). Moreover, chronic inhalation of cadmium may cause lung cancer (Goyer and Clarkson 2001).
The study of the relationship between coral reefs and sediment along the Egyptian Red Sea coast showed that there is no relationship between the concentrations of heavy metals in coral reef species and those in sediments indicating that the heavy metals accumulated by coral reef species are not directly derived from the sediments (Madkour 2012), but the bioaccumulation of heavy metals is mainly associated with carbonates where the marine organisms uptake it from surface seawater (Bender and Gagner 1976). On the other hand, by measuring the heavy metals in the hard skeleton of some coral reef species, the highest concentration of Cd is recorded in Acropora humilis at Hurghada Harbour near the anthopogenic sites (Madkour 2012). Consequently, this indicates that the main source of heavy metals is of anthropogenic origin. This variation among coral reef species indicates that bioaccumulation of heavy metals is species specific. This difference in spatial concentration for the different metals suggests that they are influenced by physical and chemical conditions from nature and human activity.
Conclusions and recommendations
Different projects of coastal development such as tourist villages and constructing marinas are observed in the study area. Although all projects has executed an Environmental Impact Assessment study, the study area was subjected to severe coast destruction and shoreline change from landfilling and dredging of the beach and the tidal flat. Such activities increase solid wastes input and change sediment distribution patterns, and may destroy the entire reef systems in the area.
Multivariate analyses, including the correlation matrix analysis, principal component analysis, and cluster analysis used in this study provide an important tool for better understanding the complex dynamics of pollutants. Cluster analysis revealed two distinct clusters for the metals in the study area. Cluster 1 includes Mn. Cluster 2 includes Cu, Zn, Cd, Ni, Co, and Pb. Based on values of geoaccumulation index and contamination factor, the recent bottom sediments of Mabahiss Bay are uncontaminated with respect to Zn, Ni, Cu, and Mn, uncontaminated to moderately contaminated with Pb, and moderately to strongly contaminated with Cd. The elevated values identified for Cd and secondary for Pb are probably resulting from anthropogenic activities in the study area.
The results of the present study demonstrate that metals in the study area are of two sources: artificial and natural. The artificial sources comprise the artificial landfilling resulted from the beach and neighbor areas enhancement by removing the sand hills and sand piles from the areas of investment and also from the nearby sand quarries. The natural source represents by the sediments derived by wadies from the west. Landfilling and dredging in Hurghada coastal areas have serious physical, chemical, and biological impacts on the marine environment. Consequently, from the present study, the following points are recommended:
-
1.
Landfilling, dredging, and other destructive activities along the study area should be stopped and prohibited.
-
2.
Organic contaminants and toxicity should be monitored through detecting the environmental changes throughout the coastal area and within the coral reefs.
-
3.
Planning the suitable use of coastal resources and conservation of coral reefs.
-
4.
Continuous monitoring of the impact of human activities in the area.
-
5.
Continuous measuring of chemical and biologic parameters in the study area to control the toxic effects in marine organisms
References
Alloway, B. J. (1990). Heavy metals in soils. London: Wiley. 371p.
Badr, A. M., & Crossland, C. (1939). Topography of the Red Sea floor. Marine Biology Station Publication, Gharbaqa, Red Sea, 1, 13–20.
Bartolomeo, A. D., Poletti, L., Sanchini, G., Sebastiani, B., & Morozzi, G. (2004). Relationship among parameters of lake polluted sediments evaluated by multivariate statistical analysis. Chemosphere, 55, 1323–1329.
Beltagy, A. I., Ali, Y., & Lotfi, I. (1986). Geochemistry of the Red Sea shelf sediments at Al-Ghardaqa. I- Abundance and distribution of chemical constituents. Bulletin of the Institute of Oceanography and Fisheries, ARE, 12, 57–80.
Bender, M. L., & Gagner, C. (1976). Dissolved copper, nickel and cadmium in the Sargasso sea. Journal of Marine Research, 34, 327–339.
Berglund, M., Askesson, A., Nermell, B., & Vahter, M. (1994). Intestinal absorption of dietary cadmium in women depends on body stores and fiber intake. Environmental Health Perspectives, 102, 1058–1065.
Bettinetti, R., Giarei, C., & Provini, A. (2003). Chemical analysis and sediment toxicity bioassays to assess the contamination of the River Lambro (Northern Italy). Archives of Environmental Contamination and Toxicology, 45, 72–78.
Calmano, W., Ahlf, W., Forstner, U. (1990). Exchange of heavy metals between sediments components and water. In J.A.C. Brockaert, S. Gvear, F. Adams (Eds.), Metal speciation in the environment, NATO ASI series G 23503, 645p.
CRI (1994). Report on studies and recommendations for alleviating the negative impacts of the infringements on the coastal zone shoreline located between km 15 to km 23.7 Hurghada/Safaga road on the Red Sea. Technical Report, 1, 15p.
Crossland, C., 1939. Some coral formations. In: Report on the preliminary expedition for the exploration of the Red Sea in the RRS Mabahith. Marine Biology Station Publication, Ghardaqa, Red Sea 1, 21–35
Davison, A. G., Taylor, A., & Darbyshire, J. (1988). Cadmium fume inhalation and emphysema. Lancet, 26, 663–667.
Diagomanolin, D., Farhang, M., Ghazi-Khansari, M., & Jafarzadeh, N. (2004). Heavy metals (Ni, Cr, Cu) in the Karoon waterway river. Iran Toxicological Letter, 151, 63–68.
Dong, J., Mao, W. H., Zhang, G. P., Wu, F. B., & Cai, Y. (2007). Root excretion and plant tolerance to cadmium toxicity—A review. Plant Soil Environment, 53(5), 193–200.
El Sayed, M. K. (1984). Recfal sedimenls of Al-Ghardaqa, northern Red Sea. Egypt. Marine Geology, 56, 259–271.
El Waked, S.K., El Sayed, M.Kh., Husseini, S.A. (1984). Relative abundance of carbonate minerals in common reef-building corals at Al-Ghardaqa, Red Sea Egypt. Proceeding Symposium Coral Reef Environment, Red Sea (Jeddah), pp. 276–292.
El-Mamony, M.H. (1995). Evaluation of terrestrial contribution to the Red Sea sediments. Ph.D. thesis, Faculty of Science, Alex. Univ., Egypt, 146p.
Forstner, U., & Wittmann, G. T. W. (1983). Metal pollution in the aquatic environment. New York: Springer. 486p.
Frihy, O. E., Fanos, A. M., Khafagy, A. A., & Abu Aesha, K. A. (1996). Human impacts on the coastal zone of Hurghada, northern Red Sea, Egypt. Geo-Marine Letters, 16, 324–329.
Ghrefat, H., Abu-Rukah, Y., & Rosen, M. (2011). Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Kafrain Dam, Jordan. Environmental Monitoring and Assessment, 178, 95–109.
Glasby, G. P., Szefer, P., Geldon, J., & Warzocha, J. (2004). Heavy-metal pollution of sediments from Szczecin Lagoon and the Gdansk Basin, Poland. The Science of Total Environment, 330, 249–269.
Goyer, R. A., & Clarkson, T. M. (2001). Toxic effects of metals. Chapter 23. In C. D. Klaassen (Ed.), Casarett and Doull’s toxicology (pp. 811–868). New York: McGraw-Hill.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control. A sedimentological approach. Water Research, 14, 975–1001.
Hodgson, E. S. (1983). Coral reef community structure at Al-Ghardaqa Red Sea. Bulletin of the Institute of Oceanography and Fisheries, 9, 116–123.
Hollert, H., Keiter, S., Konig, N., Rudolf, M., Ulrich, M., & Braunbeck, T. (2003). A new sediment contact assay to assess particle-bound pollutants using zebrafish (Danio rerio) embryos. Journal of Soils Sediments, 3, 197–207.
Hoz, L. R., Edwards, A. C., & Hernandez, M. L. (2000). Heavy metals in sediments of a large turbid tropical lake affected by anthropogenic discharge. Environmental Geology, 39/3(4), 378–383.
Idaho Department of Health and Welfare, Division of Health. (2000). Coeur d’Alene River Basin environmental health assessment (p. 67). Atlanta: Agency for Toxic Substances and Disease Registry.
Jarup, L., Berglund, M., Elander, C., Nordberg, G., & Vahter, M. (1998). Health effects of cadmium exposure—A review of the literature and a risk estimate. Scandinavian Journal of Work, Environment & Health, 24(Suppl 1), 1–52.
Larocque, A. C. L., & Rasmussen, P. E. (1998). An overview of trace metals in the environment, from mobilization to remediation. Environmental Geology, 33, 85–90.
MacFarlane, G. R., & Burchett, M. D. (2000). Cellular distribution of Cu, Pb and Zn in the gray mangrove Avicenna marina (Forsk) Vierh. Aquatic Botany, 68, 45–59.
Madkour, H.A. (2012). Impacts of human activities and natural inputs on heavy metal contents of many coral reef environments along the Egyptian Red Sea coast. Arabian Journal of Geoscience, 6, 1739–1752.
Mansour, A. M. (1999). Changes of sediment nature by environmental impacts of Sharm Abu Makhadeg area, Red Sea. Egypt. Sedimentology of Egypt, 2, 1–12.
Mansour, A. M., Nawar, A. H., & Mohamed, A. W. (2000). Geochemistry of coastal marine sediments and their contaminant metals, Red Sea, Egypt: A legacy for the future and a tracer to modern sediment dynamics. Sedimentology of Egypt, 8, 231–242.
Mansour, A. M., Mohamed, A. W., El-Dien, G. K., & Sayed, S. M. (2007). Sedimentological and environmental impacts of development projects along the coast of Hurghada, Red Sea, Egypt. Egyptian Geology of Aquatic Research, 33(1), 59–84.
Moussa, A. A., El Askary, M. A., Nasr, S. M., & El Mamoney, M. H. (1991). Unconsolidated reefal sediments in the Jubal Strait region. Red Sea, Egypt. Marine Geology, 97, 379–390.
Müller, G. (1979). Schwermetalle in den Sedimenten des Rheins—Veränderungen seit 1971. Umschau, 24, 778–783.
Müller, G. (1981). Die Schwermetallbelstung der sedimente des Neckars und seiner Nebenflusse: eine Bestandsaufnahme. Chemiker-Zeitung, 105, 156–164.
Nriagu, J. O. (1989). A global assessment of the natural sources of atmosphere trace metals. Nature, 338, 47–49.
NTP (2002). 10th Report on carcinogens. Washington: US Department of Health and Human Services, Public Health Service.
Oregioni, B., Aston, S.R. (1984). The determination of selected trace metals in marine sediments by (flameless/flam-atomic absorption spectrophotometry). IAEA Monaco Laboratory Internal Report, 75p.
Radwan, G.E. (2003). Impact of human activities on coral reef sedimentary environment along Hurghada Coast-Red Sea. M.Sc. thesis, Geology Department, Cairo University, 132p.
Selinus, O., Finkelman, R.B., Centeno, J.A. (2006). Human health and ecosystems. In I. S. Zektser (Ed.), Geology and ecosystems. Berlin: Springer, 390p.
Shukri, N. M. (1953). Bottom deposits of the Red Sea. Nature, 155, 306.
Stauber, J. L., & Florence, T. M. (1987). Mechanism of toxicity of ionic copper and copper complexes to algae. Marine Biology, 94, 511–519.
Sun, Y., Zhou, Q., Xie, X., & Liu, R. (2010). Spatial, sources and risk assessment of heavy metal contamination of urban soils in typical regions of Shenyang, China. Journal of Hazardous Materials, 174, 455–462.
Thawley, D. G., Pratt, S. E., & Selby, L. A. (1977). Antagonistic effect of zinc on increased urinary delta-aminolevulinic acid excretion in lead intoxicated rats. Environmental Research, 14, 463–475.
Turekian, K. K., & Wedepohl, K. H. (1961). Distribution of the elements in some major units of the earth’s crust. Geological Society of America, 72, 175–192.
Veena, B., Radhakrishnan, C. K., & Chacko, J. (1997). Heavy metal induced biochemical effects in an estuarine teleost India. Journal of Marine Science, 26, 74–78.
Wen, X., & Allen, H. E. (1999). Mobilization of metals from Le An River sediments. Science of the Total Environment, 227, 101–108.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Attia, O.E.A., Ghrefat, H. Assessing heavy metal pollution in the recent bottom sediments of Mabahiss Bay, North Hurghada, Red Sea, Egypt. Environ Monit Assess 185, 9925–9934 (2013). https://doi.org/10.1007/s10661-013-3302-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3302-4