Abstract
In the present study, concentration of some selected trace metals (Fe, Mn, Ni, Co, Pb, Zn, Cu, Cr and Cd) are measured in Brahmani, Baitarani river complex along with Dhamara estuary and its near shore. Chemical partitioning has been made to establish association of metals into different geochemical phases. The exchangeable fraction is having high environmental risk among non-lithogeneous phases due to greater potential for mobility into pore water. The metals with highest bio-availability being Cd, Zn and Cr. The metals like Mn, Zn, Cd and Cu represent an appreciable portion in carbonate phase. Fe–Mn oxides act as efficient scavenger for most of the metals playing a prime role in controlling their fate and transport. Among non-lithogeneous phases apart from reducible, Cr showed a significant enrichment in organic phase. Risk assessment code values indicate that all metals except Fe fall under medium-risk zone. In estuarine zone Cd, Zn, Pb and Cr are released to 32.43, 26.10, 21.81 and 20 %, respectively, indicating their significant bio-availability pose high ecological risk. A quantitative approach has been made through the use of different risk indices like enrichment factor, geo-accumulation index and pollution load index. Factor analysis indicates that in riverine zone, Fe–Mn oxides/hydroxides seem to play an important role in scavenging metals, in estuarine zone, organic precipitation and adsorption to the fine silt and clay particles while in coastal zone, co-precipitation with Fe could be the mechanism for the same. Canonical discriminant function indicates that it is highly successful in discriminating the groups as predicted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, it has been established that sediments operate as an active aquatic compartment which performs a fundamental role in redistribution of metals to aquatic biota. In this context, estuarine environment has a significant contribution as these are extremely important biogeochemical zones with the capability of altering the flux of materials between the land via rivers to coastal zones and ultimately to the oceans. Besides the natural processes such as weathering of source rock and soil in the catchment, considerable amount of metals generated by anthropogenic activities like urbanization, industrialization, mining, etc. enter into estuarine environment through rivers (Alagarsamy 2009). Flocculation, adsorption onto inorganic–organic particulates followed by sedimentation is found to be the prime process responsible for accumulation of metals in estuarine systems (Liu et al. 2009).
Sediment-associated metals have the potential to be eco-toxic due to their mobility and bioavailability which subsequently affect the ecosystem through the processes of bio-accumulation and bio-magnification (Ip et al. 2007). However the capacity of mobilization, bioavailability and toxicity of these metals critically depend on their chemical forms. In the absence of anthropogenic influence, metals primarily remain associated with silicates and basic minerals which limit their mobility. In contrast, metals introduced from anthropogenic activities have greater mobility as they remain associated with other easily mobilizable sediment phases such as carbonates, oxides, hydroxides, sulphides, etc. Consequently there is a considerable interest in understanding the association of these metals with different solid phases. Therefore it is essential to perform chemical partitioning studies so as to conform relative affinity of the metals to remain associated to different geochemical phases in the study area.
Application of risk indices such as enrichment factor (EF), pollution load index (PLI), geo-accumulation index (I geo), Risk assessment code (RAC), etc. also provide significant information regarding the quantification of metal contamination in sediments (Rath et al. 2009; Panda et al. 2010). Multivariate statistical treatment of data obtained from experiments is an important tool for environmental analysis as it allows meaningful data reduction and interpretation of multiconstituent chemical and physical measurements (Singh et al. 2004; Simeonov et al. 2003).
Although heavy metal distribution in water and sediments has been investigated in many of the Indian estuaries (Alagarsamy 2006; Krupadam et al. 2006; Ray et al. 2006; Balachandran et al. 2006; Shajan 2001), no significant work has been carried out in the Dhamara estuary in recent years.
In the present study, concentration of some selected trace metals (Fe, Mn, Ni, Co, Pb, Zn, Cu, Cr and Cd) are measured in Brahmani, Baitarani river complex along with Dhamara estuary and its near shore. Chemical partitioning has been made so as to establish association of metals into different geochemical phases. A quantitative approach is made through use of different risk indices. Multivariate statistics has been applied for interpretation of results.
Material and methods
Study area
Brahmani river is the second largest river in Odisha having drainage basin of 39,035-km2 area with total length of 800 km and peak discharge of 22,640 m3 s−1and Baitarani river extending over an area of 8,570 km2 has a total length of 365 km and peak discharge of 14,150 m3 s−1. Both these rivers combine together to form Dhamara estuary before meeting the Bay of Bengal. Brahmani River receives effluents from most of the major industries of Odisha located in Rourkela, Angul, Talcher and Jajpur industrial areas, washings from mines located in Angul-Talcher belt and Sukinda, sewage from a number of major townships and in addition agricultural runoff. As a result, Brahmani water does not represent a healthy aquatic ecosystem. On the other hand, Baitarani River receives mostly agricultural runoff and domestic effluents in addition to mine washings from iron and manganese mines located at Joda and Barbil. The detailed basin status with respect to the anthropogenic scenario is summarized in Table 1.
The adjoining area of the estuary under study is covered with thick alluvium. The basin lies in an Indian shield that consists of Pre-Cambrian rocks such as granites, gneisses, quartzites, schists of Eastern Ghats, amphibolites, pegmatites, khondalites and charnockites and Gondwana rocks like shale, sandstone and coal (Rath et al. 2009). The catchment area of river Baitarani is dominantly granitic terrain. The Dhamara estuary is of high environmental importance due to mangrove forest ecosystem, Bhitarakanika sanctuary, crocodile breeding centre and the Olive Ridley nesting beach.
Thought process
It is a normal practice to consider the different estuarine samples taking them as single population. But considering the diversity of mechanisms that occur for adsorption or removal of trace metals from water by sediments and vice versa, it has been realized to treat them separately on the influence of salinity. In this study, river water zone, i.e. BR1, BR2, BR3, BR4, BT1, BT2, BT3 and BT4 stations; estuarine water zone, i.e. DE1, DE2, DE3 and DE4 stations; coastal water zone, i.e. DC1, DC2, DC3 and DC4 stations have been separated taking salinity range <10, 10–20 and >20 ppt, respectively, as salinity plays a significant role in the flocculation of trace metals. Geostatistical approaches have been undertaken to characterize trace metal association with the bottom sediment with respect to the influence of different water quality in river, estuarine and coastal zones.
Sampling and analytical methods
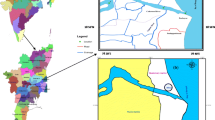
Sixteen stations, four each in rivers Brahmani, Baitarani, Dhamara estuary and coastal sea up to a distance of 3 km have been selected for investigation (Fig. 1). Coastal and riverine samples were collected with the help of fishing trawlers. Surface water samples were collected in acid-cleaned polythene bottles. The pH and salinity of samples were measured in the field.
Surface sediment samples were collected from the stations using a portable Peterson’s grab and samples were homogenized, then air dried. A portion was then taken for textural analysis, i.e. sand, silt and clay percentages. For determination of metal content in bulk sediments, about 1 g dried and powdered samples was taken in a 100-ml Teflon beaker followed by addition of 2 ml of HClO4, 12 ml of HF and 8 ml of HNO3. The samples were tightly closed and kept on hot plate at about 150 °C for 6 h (Li and Thornton 1992). The contents were transferred and finally 10 ml of 10 % HNO3 was used to rinse thoroughly for complete transfer of the contents. Finally, the volume was made up in a 50-ml volumetric flask. Metal concentration of metals Fe, Mn, Co, Ni, Cu, Zn, Cr, Pb and Cd was measured by atomic absorption spectrophotometer (Perkin Elmer, model 1025). All the samples were analyzed in triplicate with blanks similarly treated for metal analysis. The precession and accuracy of the methods were systematically and routinely checked analyzing United States Geological Survey reference samples (GXR), where it has been found that the precession (coefficient of variation of five replicate analyses) were 3 % for Cu and Cr and 4 % for Fe, Mn, Co, Ni, Zn, Pb and Cd.
The metals (Fe, Mn, Co, Ni, Cu, Zn, Cr, Pb and Cd) were sequentially extracted from <63-μm fraction following the method proposed by Tessier et al. 1979 into five phases operationally defined as exchangeable (F1), carbonate (F2), Fe–Mn hydroxide (F3), organic (F4) and residual (F5). The detailed scheme is as follows:
-
1.
Exchangeable fraction (1 M MgCl2, pH 7.0)
-
2.
Carbonate bound fraction (1 M NaOAc adjusted to pH 5.0 with acetic acid)
-
3.
Fe–Mn oxide bound fraction, i.e. reducible phase (0.04 M NH2OH.HCl in 25 % (v/v) HOAc at 96 °C)
-
4.
Organic bound, i.e. oxidized phase (5 ml of 30 % H2O2 and 0.02 M HNO3 3 ml of 30 % H2O2 at 85 °C)
-
5.
Residual fraction (total digestion with a concentrated mixture of HNO3/HClO4/HF).
Metal concentrations in each leachet were analyzed using AAS. The precision and accuracy of the methods were systematically and routinely checked as per the procedure followed for metal analysis in bulk samples.
The estimation of organic carbon was carried out by Walkley and Black method (Trivedy and Goel 1984). All chemicals used in the study were of analytical reagent grade (Merk). Milli-Q water was used throughout. The analytical data quality was ensured through careful standardization, procedural blank measurements, spiked and triplicate samples.
Data treatment and multivariate statistics
Multivariate statistical analysis of the river sediment quality data set was performed through Pearson correlation, factor and discriminant analysis techniques (Simeonov et al. 2003; Singh et al. 2004). Pearson correlation coefficients were calculated to establish relationship between any two random variables. Factor analysis was performed on correlation matrix of rearranged data of some sediment metal concentrations, textural parameters and organic carbon. The variance and factor loadings of the variables with eigen values were computed. Stepwise discriminant analysis using Wilks’ method was carried to investigate differences between the groups on the basis of the variables of the cases, indicating which variables contribute most to the group separation and in addition to test the theory whether cases are classified as predicted. Data were processed using routines taken from Statistical Program for Social Sciences (SPSS-Version17.0) statistical software.
Result and discussion
Metals in bulk sediment
The range, average and standard deviation of the concentrations of metals, organic carbon and textural parameters in the sediments of Brahmani–Baitarani river complex along with Dhamara estuary and coast are summarized in Table 2. In all three different zones of the study area, the first three most abundant metals remain in the order Fe > Mn > Cr. For other metals, the trend is not regular. In the riverine segment, the order of abundance is Zn > Ni > Pb > Co > Cu > Cd; in estuary, i.e. mixed water zone the order is Zn > Co > Ni > Pb > Cu > Cd; and in coastal segment it is Ni > Zn > Co > Pb > Cu > Cd. Except Cd which shows a depleting trend from river to estuary and to coast, all other metals Cu, Co, Ni, Mn, Fe, Zn, Cr and Pb show an increasing trend from river to estuary and then decreasing towards coast. In general Brahmani River sediments are more enriched with metals than Baitarani which is in consistent with some earlier findings of Konhauser et al. 1997.
Heavy metals are subjected to transfer into estuary with hydrodynamic disturbances which leads to low heavy metal content in the sediment of tide-affected river (Weng et al. 2008) as compared to estuarine zone. In estuary, i.e. at the fresh and saline water interface both adsorption and coagulation of the dissolved metals are introduced followed by deposition to the bottom sediments. As a result, the metals accumulate in the bed sediments at the fresh water and saline water interface. With increase of salinity, the amount of dissolved metals in the water column available for coagulation decreases. Therefore accumulation of heavy metals is lower in coastal sediments than in estuary sediments. A depleting trend in concentration is mostly observed in offshore direction. This pattern can be related to the concentration gradient from source to sink. In addition it also demonstrates that dissolved metals may be retained in the coastal area by the circulation regime of the surface water. Relatively higher standard deviation in estuarine water zone as compared to coast indicates existence of significant variability among individual sampling sites. The spatial fluctuation of trace metal content likely represents their input from different sources and also variation in processes those occur in different segments under consideration.
The concentration of Cd, Co, Cr and Pb in the study area are higher than World Surface Rock (WSR) average (Martin and Meybeck 1979) in all the three segments while concentration of Ni remains higher side than WSR in estuarine segment. Also, it has been observed that concentration of metals Zn, Cr and Pb are present above the Indian River average in all segments and Cu, Co, Ni, Mn and Fe are above Indian average in estuarine segment (Subramanian 1987). Metals like Co and Cr also show higher value than World river average (Martin and Meybeck 1979) at all the segments, where as Cd is found to be in higher concentration than World average in riverine and estuarine segment due to anthropogenic activities at upstream. Comparison of average elemental composition of Dhamara estuarine system bed sediments with other Indian estuaries, Indian River average, world river average, Bay of Bengal and surface rock average are given in Table 3.
The concentration of trace metals in sediments cannot be interpreted only with respect to change in grain size. Thus physical transportation is not the only way to control the pattern of distribution of trace metals in estuary and coastal mixing zone. The most important factors controlling the spatial variations of the trace metals in sediments include grain size, the chemical condition (e.g., sorption-adsorption of trace metals, flocculation, etc.) of sedimentary environment and anthropogenic input (Willams et al. 1994).
Enrichment factor
Metal enrichment as a result of contamination can be measured in a number of ways. In heavy metal studies, different authors have compared their data pertaining to a particular environment with that of similar environment in other places of the country/world (Subramanian et al. 1987). In the present study, a method called enrichment factor analysis (Simex and Helz 1981) has been used to study the trace metal concentrations of the study area.
Where, C x is the concentration of metal x.
Fe is chosen as the element for normalization, as anthropogenic sources of iron are small. Again as stated by Forstner and Wittmann 1981 in the case of Fe, particularly the redox-sensitive iron-hydroxide and oxide under oxidative condition constitute significant sink of heavy metals in aquatic system. Many works are also in support of taking Fe as normalizing element (Zhang et al. 2007). A five category ranking system is used to express the degree of anthropogenic influence on concentration of metals in the estuarine sediments (Sutherland 2000). An EF of <2 represents a deficiency to minimum enrichment whereas EF of ∼2–5 represents a moderate enrichment. An EF of ∼5–20 represents a significant enrichment and EF of ∼20–40 and >40 depicts a very high and extremely high enrichment.
The enrichment factors for all metals with respect to Indian River average (Subramanian 1987) except for Cd (calculated with respect to World river average by Martin and Meybeck 1979) for all stations in the study area are presented in box–whisker plots (Fig. 2). EF values ranges from 0.48 to 1.12 for Cu, 0.79–6.97 for Cd, 0.61–2.24 for Co, 0.85–1.97 for Ni, 0.78–1.32 for Mn, 2.05–4.60 for Zn, 1.53–3.97 for Cr and 1.93–4.70 for Pb. A very small percentile of Cu shows minor enrichment indicating their origin predominantly from lithogeneous materials. Half percentile of Mn remains in minor enrichment zone. Full percentile of Co and Ni remain in minor enrichment zone. More than 25 percentile of Cr (mostly estuarine stations) remains in the moderate enrichment zone. Cd shows minor enrichment throughout the study area with a long whisker (EF >6) indicating moderate to severe enrichment in some riverine stations. More than half percentile of Zn lies in the moderately enrichment zone while for Pb it is about 40 %. The analysis shows that the sediments in the study area are polluted with Zn, Pb, Cr and Cd and act as a sink for metals contributed from multitude of anthropogenic sources.
Pollution load index
The PLI for a particular station has been calculated (Tomlinson et al. 1980) by taking the nth root of the n highest contamination factors multiplied together.
Where, ‘n’ is the number of metals (7 in this study).
Concentration of metals in world surface rock average (Martin and Meybeck 1979) has been used as base level for metals. It provides a summative indication of the overall level of heavy metal pollution in a particular sample.
PLI was calculated for the area under consideration by taking seven out of nine metals, considering least toxicity of most abundant metals, i.e. Fe and Mn. Similarly PLI for river (river index) has been calculated taking n th root of n site indices multiplied together. The PLI of Brahmani is slightly higher (1.5) than Baitarani (1.2) due to inputs at upstream. PLI lies within 2.0–3.0 with an average 2.3 in estuary which is quite alarming as far as heavy metal pollution is concerned. At coast it is 0.9–1.2 with an average 1.1.
Geo-accumulation index
Geo-accumulation index (I geo) was first introduced by Muller (1979) to compare the present-day heavy metal concentration with the pre-civilized background values. This index has been used by various workers for their studies (Rath et al. 2005; Krupadam et al. 2006)
Where
- C n :
-
Concentration of element ‘n’,
- B n :
-
Geochemical background values.
The world surface rock average (Martin and Meybeck 1979) has been used as the geochemical background. The factor 1.5 was used to account for possible variation in background data due to lithogenic effect. I geo < 0 (class 0) means pollution free, 0 ≤ I geo < 1 (class 1) refers to pollution free to moderately polluted, 1≤I geo<2 (class 2) refers to moderately polluted, 2≤ I geo < 3(class 3) moderate to strongly polluted, 3 ≤ I geo < 4 (class 4) strongly polluted, 4 ≤ I geo < 5 (class 5) strong to very strongly polluted and I geo ≥ 5 (class 6) refers to very strongly polluted.
Based on world surface rock average, the I geo values are calculated and presented in Fig. 3. The I geo values of Fe, Mn, Cu, Ni and Zn remain in class ‘0’ in all segments including rivers, estuary and coast. In both the rivers and estuary, Cd remains in class ‘4’ which changes to class ‘3’ in coast. For both the rivers Co remains in class ‘1’ while in estuary it remains in class ‘2’ indicating its accumulation due to flocculation/coagulation which again comes to class ‘1’ in coast. In Brahmani River Cr remains in class ‘1’ indicating its inputs from upstream mining activities. Due to accumulation in estuary, I geo for Cr changes to class ‘2’ in estuary. Towards coast it again changes to class ‘1’. In Baitarani river, Pb is in class ‘1’ may be due to the fishing activities and use of boats, mechanical trawlers for transportation of goods, which again changes to class ‘2’ in estuary due to accumulation.
Correlation analysis
Correlation matrix (Table 4) shows that in the riverine zone, heavy metals are not controlled by any size fraction. Here, intensively significant correlation (r > 0.8) among Zn, Cr, Fe and Mn indicates that both oxides/hydroxides of Fe–Mn might be operating as host for scavenging the metals like Zn and Cr. In estuarine zone, significant correlation is found among metals like Cu, Co, Mn, Zn, Cr, Cd with finer particles, i.e. silt + clay. This might be due to the adsorption of these metals on the finer particles in the fresh-saline mixed water zone. In addition, good correlation of metals except Ni, Cr and Pb with organic carbon reveals the formation of organic complexes by flocculation and subsequently influences their distribution due to its high specific surface area. In this zone, OC also shows a good correlation with finer fractions of the sediments. In coastal zone, metals like Cu, Ni, Cr and Pb have significant negative correlation with finer particles, i.e. silt indicating that adsorption into fine particles is not important here. A high correlation (r > 0.9) of Cd and Mn with OC indicates their precipitation as organic complexes.
Factor analysis
R-mode factor analysis with rotation was carried out to clarify the relation among heavy metals, textural parameters and organic carbon in sediments of study area taking in three different sets, i.e. riverine, estuarine and coastal zones. A varimax rotation of principal components or factors was used to clarify the picture for simpler and more meaningful representation of underlying factors as it lowers the contribution to factors of variables with minor significance and increase the more significant one. Factor loadings were calculated using eigen values >1. The factor loadings may be classified as ‘strong’, ‘moderate’ and ‘week’ considering their significant influence in the geochemical processes corresponding to absolute loading values of > 0.70, 0.70–0.50 and 0.50–0.40, respectively (Liu et al. 2003; Panda et al. 2006). The results are reported in Table 5.
In riverine zone, varifactor 1 represents 48.5 % of the total variance and is found to be strong positively loaded with metals like Cr, Cu, Zn, Mn, Co and Fe along with negatively loaded with Cd. Here Fe–Mn oxides/hydroxides seem to play an important role in scavenging metals like Cr, Cu, Zn and Co. Moderate negative loading of clay indicates that adsorption onto finer particles is not important in this zone. This factor may be regarded as ‘reducible factor’. Varifactor 2 accounts for 28.6 % of the total variance. This factor is strong positively loaded with organic carbon, silt along with negatively loaded with sand. The factor is also moderate to weak positively loaded with Clay and Pb. This suggests that organic precipitation and adsorption on finer particles are responsible for enrichment of Pb in the sediments. This factor may be regarded as ‘Precipitation-adsorption factor’. Pb has a special affinity for clay minerals because of similarity in its ionic radii with that of K (Modak et al. 1992). Varifactor 3 accounts for 13.32 % of total variance and is strong positively loaded with Ni and Pb..
In estuarine zone, varifactor 1 accounts for 54.46 % of total variance. The factor is strong positively loaded with Mn, Pb, Cu, Co, Fe, silt, clay and OC along with negatively loaded with sand. Here, organic precipitation and adsorption to the fine silt and clay particles plays an important role. In addition Fe–Mn oxides/hydroxides are also responsible in scavenging the said metals. The distributional characteristics of OC with finer sediments indicate that dominant hydrodynamic processes are the main reason for the concentration of organic matter in the surface sediments (Valdes et al. 2005). This factor may be termed as ‘adsorption factor’. Varfactor 2 accounts for 38.81 % of total variance. It shows strong positive loading of Zn, Cr and negative loading of Cd. The factor is also moderate positively loaded with clay. Cd2+ has a strong affinity for Cl− ions and weak affinity for organic matter compared to the other metals in sea water (Tipping et al. 1998). Thus Cadmium prefers to remain as soluble chloro-complexes than free Cd 2+ ions. Therefore adsorption on finer particles and coordination with organic matter is hindered as a result of poor availability of free Cd 2+ ions. This factor can be considered as a ‘textural factor’ as adsorption into finer particles seems to be the major mechanism.
In coastal zone, Varifactor 1 accounts for 47.21 % of the total variance strong to moderate positively loaded with Cd, Ni, Fe, Pb, Cr and sand while strong negatively loaded with clay. Negative loading of metals with clay (finer particles) indicates that adsorption does not play any role here. Rather co-precipitation with Fe could be the mechanism here. Varifactor 2 accounts for 34.00 % of total variance. It is strong positively loaded with Clay and OC while negatively loaded with Cr. Varifactor 3 accounts for 18.79 % of total variance. It is strong to moderate positively loaded with Co and Mn while strong negatively loaded with Zn.
Discriminant analysis
Stepwise discriminant analysis using Wilks’ method was carried out by entering sampling sites as grouping variables and the metal concentrations, textural parameters, CaCO3, pH and salinity as independent variables. The module was executed by entering the most correlated variable in the beginning and then the second until an additional variable adds no significant change. The summary of canonical discriminate functions is presented in Table 6. Here, first canonical discriminate function accounts for 63.9 % while second function accounts for 36.1 % of the discriminating ability of the discriminating variables. The standardized canonical coefficients indicate that ‘Cd’ and ‘salinity’ largely contribute to the first discriminate score while ‘Zn’ and ‘Fe’ to second discriminating function out of other variables. A very high canonical correlation indicates that there is a high between-group variation of variables and the functions discriminate well. A very small Wilks’ lambda (0.001) for the first discriminating function indicates that it is highly successful in discriminating the groups. The plot of first and second canonical discriminate functions clearly demonstrates the distinctive groupings (Fig 4).
Sequential leaching (metals in different geo-chemical phases)
The average metal content in various geochemical phases of river water, estuarine and coastal zone sediments is presented in Fig. 5 and the relative abundance is presented in Table 7. The importance of individual phases and their association with heavy metals are discussed as follows.
Exchangeable fraction includes those metals held by electrostatic adsorption as well as those specially absorbed on sediment forming materials with large surface area such as clay minerals, amorphous iron and manganese oxides as well as organic substances. The metal content in this phase indicates the environmental condition of the overlying water bodies. The sequence of leaching of metals by IM MgCl2, i.e. in exchangeable phase is Cd (12.46–17.94 %, with average of 15.00 % the total metal content)) > Zn (8.17–15.78 %, average of 12.20 %) > Cr (7.45–13.90 %, average 10.63 %) > Pb (5.70–11.65 % average of 9.50 %) > Cu (4.41–8.41 % average of 6.69 %) >Co (4.22–8.72 % average 6.12 %) > Ni (4.31–7.39 % average 6.03 %) > Mn (1.45–2.62 % average 1.83 %) > Fe (0.60–0.98 % average of 0.73 %). The metals like Cd, Zn, Cr, Pb and Cu contribute appreciably in this phase which may be originating from upstream industrialization and mining activities of both the river catchments particularly in Brahmani along with urban sewage and agricultural washings. Higher availability of exchangeable Cd in the study area may be attributed the higher capacity of principal polarization, resulting in strong adsorption of Cd onto colloids in the sediments (Yang et al. 2009). Estuarine sediments are having high exchangeable Pb (11.65 %).Pb has a special affinity for clay minerals because of its ionic radius which is similar to that of K and therefore remains primarily associated with clay minerals (Modak et al. 1992), which could be the reason to support higher abundance of Pb in estuarine fraction where silt and clay together contribute to 42.96 % of the total sediment. Higher availability of Mn (average 1.83 %) compared to Fe (average 0.74 %) in this phase may be attributed to redox condition in the sediment. Under low redox condition Mn is more available than Fe as the latter is controlled by pH (Schoer et al. 1983). In addition, Mn (II) oxidation is a much slower process than Fe (II). This fraction is having high environmental risk among non-lithogeneous phases due to greater potential for mobility into pore water which threat to pollution with respect to Cd, Zn, Cr, Pb and Cu.
The sequence of leaching of metals by 1 M NaOAc (pH 5.0) , i.e. carbonate phase, is Mn (11.11–17.61 %, average 13.18 %) > Zn (10.17–14.16 %, average 11.66 %) > Cd (7.58–14.83 %, average 11.63 %) > Cu (7.46–15.94 %, average 11.55 %) > Co (7.33–11.21 %, average 9.48 %) > Ni (7.59–10.39 %, average 8.44 %) > Pb (5.06–10.15 %, average 6.98 %) > Cr (3.29–6.81 %, average 5.27 %) > Fe (1.64–4.62 %, average 2.83 %). The metals like Mn, Zn, Cd and Cu represent an appreciable portion of the total metal content which is most likely due to similarity in their ionic radii to that of calcium (Zhang et al. 1998). In addition, these metals have special affinity for carbonate, which facilitate their co-precipitation with carbonate minerals, i.e. these metal ions get adsorbed into the surface of carbonate minerals followed by incorporation into the crystal lattice forming Cdx Ca1−x CO3 solid solution (Billon et al. 2002). Most Mn bound to carbonate mineral is well dispersible on calcite surface (Billon et al. 2002). The higher availability of Mn in carbonate phase is in consistent with the results of Wang et al. 2010 for Jinzhou bay. In this fraction, contribution of Fe is relatively low.
The sequence of leaching with 0.04 M NH2OH·HCl and 25 % HOAc, i.e. reducible phase, is Cr (13.03–19.01 %, average 17.03 %) > Ni (13.91–20.00 %, average 16.79 %) > Pb (16.01–17.44 %, average 16.74 %) > Zn (14.04–19.85 %, average 15.89 %) > Cu(10.66–17.57 %, average 14.55 %) > Co (10.06–16.63 %, average 13.39 %) > Mn (9.56–12.00 %, average 10.91 %) > Cd (6.97–10.94 %, average 8.64 %) > Fe (8.74–10.62 %, average 9.64 %). Fe–Mn oxides act as efficient scavenger for most of the metals thereby playing a prime role in controlling their fate and transport in the study area. The association of metals in this phase is due to adsorption into colloids of Fe–Mn and coagulation of colloidal materials under high salinity conditions (Jenne 1968). The higher availability of Mn than Fe in this phase is attributed to the flocculation of colloids of Mn. Cd has a strong affinity for chloride and thereby prefers to remain as different chlorocomplexes than free Cd2+ ions in estuary and coast. The result being adsorption on Fe–Mn oxides and coordination with organic matter are appreciably hindered due to the decreased availability of free Cd2+ ions despite of the fact these materials have a strong affinity for the same (Turner et al. 2008).
Metals in organic phase remain bound to active sites like carboxyl, phenolic –OH, etc. of organic molecules and/or precipitated as sulphides. However the analytical procedure adopted here does not allow distinguishing between organic and sulphide-bound metals. The contribution of metals in this organic/sulphide fraction is in the order Cr (8.41–17.80 %, average 12.48 %) > Mn (9.21–12.74 %, average 10.97 %) > Cd (6.73–15.38 %, average 10.45 %) >Pb (7.75–12.61 %, average 9.88 %) > Zn (6.63–11.04 %, average 8.73 %) > Cu (5.70–10.47 %, average 8.37 %) > Ni (7.34–10.21 %, average 8.34 %) > Co (4.39–9.65 %, average 6.93 %) > Fe (0.25–13.33 %, average 6.21 %). Among non-lithogeneous phases apart from Fe–Mn phase Cr showed a significant enrichment in this phase which is in well agreement with the findings of river Rio de Janeiro (De Souza et al. 1986).
The metal concentration in residual phase, i.e. inert fraction is higher than any of preceding extraction, which represents normally more than 50 % of total concentration except Zn and Cr in Brahmani River and estuary, Cd in estuary and coast and Pb in estuarine sediments in the present study. A major portion of Fe (average 79.16 %), Mn (average 64.83 %) and Co (average 64.43 %) is found to be associated with this phase represents their detrital origin, while Zn (51.62 %), Cd (average 54.28 %), Cr (54.59 %) and Pb (56.95 %) are having relatively less contribution in this phase. The contribution of metals towards this phase is Fe (77.46–80.74 %, average 79.16 %) > Mn (62.50–67.60 %, average 64.83 %) > Co (60.99–70.29 %, average 64.43 %) > Ni (57.13–66.51 %, average 60.40 %) > Cu (51.78–68.16 %, average 58.84 %) > Pb (48.51–61.39 %, average 56.95 %) > Cr (48.37–63.88 %, average 54.59 %) >Cd (49.04–62.29 %, average 54.28 %) > Zn (44.15–60.85 %, average 51.62 %) .
The dominant fraction among all non-lithogeneous fractions for Cu, Co, Ni, Cr and Pb is reducible phase. For Cd, exchangeable phase is the major pool. For Mn, carbonate phase is the major pool with exception of river Baitarani where organic phase dominates. For Zn and Fe, reducible phase is the major pool. However for Zn, exchangeable phase dominates in river Brahmani while for Fe, organic phase dominates in estuarine segment. The predominance of reducible fraction is a consequence of the presence of detrital Fe–Mn oxides with metals adsorbed into the mineral surfaces (Sullivan and Taylor 2003). In estuary, the second dominating fraction being exchangeable for Zn, Cr while organic phase for Pb and carbonate for Cu, Co, Cd and Ni. The enrichment of Pb in organic fraction for estuary may be attributed to its affinity for organic substances and clay minerals. The affinity towards organic substances could be attributed to high stability of lead-organic complexes while affinity towards clay minerals might be due to its ionic radii (Irving and Williams 1953). In coast, the second dominating fraction being exchangeable for Cu and Cr, organic for Co, Cd, Ni, Pb and Fe while carbonate for Zn only.
Ecological risk assessment
Metals remain bound to different geochemical phases with different strengths. The strength of this association provides a clear indication of sediment reactivity as well as the processes involved in the release of these metals under changing environment which ultimately helps in assessing the risk connected with their presence in an aquatic environment (Jain 2004). The relative abundance of metals in non-lithogeneous (sum of first four phases excluding the residual phase) and lithogeneous (residual phase) phases are given in Table 7.
The contribution of non-lithogeneous (NL) phases is comparatively less than that of lithogeneous phases, with the highest NL contribution of 55.85 % for Zn followed by Cr (51.63 %) in Brahmani River and for Pb (51.44 %) in estuarine zone. The NL contributions of Mn, Co, Fe, Ni, Cu, Pb, Cr, Cd and Zn are 32.14–37.50, 29.71–39.70, 19.26–22.54, 33.49–42.87, 31.84–48.22, 38.84–51.44, 36.12–51.63, 37.71–53.10 and 39.15–55.85 %, respectively. This indicates that the enrichment of Cu, Pb, Cr, Cd and Zn in the study area is from anthropogenic sources. A higher percentage of trace metals except Cd binding to NL fraction is found in estuarine zone than coastal zone which suggest that the trace metals might simply be affected by sorption–adsorption processes (Tessier et al. 1979). This also points towards the fact that a larger fraction of the sediment in the estuary is freshly deposited and is formed by the adsorption of trace metals. The saturation of mineral cations at fresh and salt water interface lead to interaction of metal ions and mineral cations causing precipitation. In addition, upstream of estuarine zone receives large input of metals through industrial discharge, extensive mining activities and also agricultural runoff from the catchment of Brahmani and Baitarani rivers. Flocculation as well as aggregation of particles into larger sizes via biological mediation (Chester 2000) might have also contributed. With increase of salinity, the amount of dissolved trace metals in the water column available for the process of coagulation decreases. Thus the concentration of trace metals in the NL fraction of the sediment is decreased in the coastal area.
The mobility of metals in different non-lithogeneous fractions is different in different environmental conditions. Researchers have different opinion regarding the importance of these mobile phases. Morrison (1989) suggested that complexation with humic substances reduces metal bioavailability and in turn their potential toxicity. Again Tessier’s procedure which involves the treatment by HNO3 and H2O2 for organic-bound metals includes metal fraction bound to organic matter as well as sulphides. The sulphide-bound metals are not easily bio-available (Soto-Jimenez and Paez-Osuna 2001). However, these sulphide-bound metals becomes bioavailable in oxidizing environment where oxidation releases H+ and SO4 2− ions into sediments which operate as powerful leaching agents causing secondary release of heavy metals (Pagnanelli et al. 2004). As per Fernandes et al. 1994 Fe–Mn hydroxide phase is very effective in binding metals but only in anoxic conditions these compounds are reduced to lower oxidation states and get released into the pore water. The discussion indicates that the release of Fe–Mn and organic-bound metals is mostly decided by the redox condition and pH. However exchangeable and carbonate bound metals easily equilibrate with water column and thereby make themselves more readily bio-available (Singh et al. 2005). The criteria of risk assessment code (RAC) as given below indicates that the sediment that can release <1 % of the total metal in exchangeable and carbonate fractions is considered safe, i.e. in no risk category while that release >50 % of the total metal in the same fraction is considered to be under very high risk category. Low risk is there when the release is 1–10 %, medium risk is there when the release is 11–30 % and high risk is there when the release is 31–50 % (Perin et al. 1985).
The average RAC values for Rivers Brahmani and Baitarani along with the Dhamara estuary and Coast are plotted and shown in Fig. 6. In the study area all metals except Fe (2.36–5.60 %) fall under medium risk zone. For Brahmani River, Cu and Zn are released to the extent of 21.86 and 29.95 % in exchangeable + carbonate phase, respectively while in estuarine zone Cd, Zn, Pb and Cr are released to 32.43 % (high risk), 26.10, 21.81 and 20 %, respectively, indicating their significant bio-availability which may pose significant ecological risk. In coastal region, Cd is released to the extent of 27.36 % in exchangeable + carbonate phase.
Conclusion
The present study indicates that the concentration of heavy metals like Cd, Co, Cr and Pb in the study area are higher than World Surface Rock (WSR) average due to anthropogenic activities at up-stream catchment. Also, it has been observed that concentration of metals Zn, Cr and Pb are present above the Indian River average in all segments. Enrichment factor of different metals indicates that more than half percentile of Zn, 25 percentile of Cr (mostly estuarine stations) remains in the moderate enrichment zone while for Pb it is about 40 %. Cd shows moderate to severe enrichment in some riverine stations. The PLI of Brahmani is slightly higher than Baitarani. Average PLI of estuary is 2.3 which is quite alarming as far as heavy metal pollution is concerned. In both the rivers and estuary, I geo values of Cd remains in class ‘4’ which changes to class ‘3’ in coast. Due to accumulation in estuary, I geo for Cr, Co and Pb remain in class ‘2’ in estuary due to accumulation.
Factor analysis indicates that in riverine zone, Fe–Mn oxides/hydroxides seem to play an important role in scavenging metals like Cr, Cu, Zn and Co. In estuarine zone, organic precipitation and adsorption to the fine silt and clay particles pose the important role. In coastal zone, adsorption does not play any role rather co-precipitation with Fe could be the mechanism.
The sediments of study area are having high exchangeable Cd. Estuarine sediments are having high exchangeable Pb (11.65 %). The metals like Mn, Zn, Cd and Cu represent an appreciable portion of the total metal content in carbonate phase. Fe–Mn oxides act as efficient scavenger for most of the metals thereby playing a prime role in controlling their fate and transport in the study area. Among non-lithogeneous phases apart from Fe–Mn phase Cr showed a significant enrichment in organic phase. The metal concentration in residual phase, i.e. inert fraction is higher than any of preceding extraction, which represents normally more than 50 % of total concentration except Zn and Cr in Brahmani River and estuary, Cd in estuary and coast and Pb in estuarine sediments in the present study. RAC values indicate that in the study area all metals except Fe (2.36–5.60 %) fall under medium risk zone.
References
Alagarsamy, R. (2006). Distribution and seasonal variation of trace metals in surface sediments of the Mandovi Estuary, West Coast of India. Estuarine, Coastal and Shelf Science, 67(1–2), 333–339.
Algarsamy, R. (2009). Geochemical variability of copper and iron in Oman Margin sediments. Microchemical Journal, 91, 111–117.
Balachandran, K. K., Laluraj, C. M., Martin, G. D., Srinivas, K., & Venugopal, P. (2006). Environmental analysis of heavy metal deposition in a flow restricted tropical estuary and its adjacent shelf. Environmental Forensics, 7, 345–351.
Biksham, G., & Subramanian, V. (1988). Elemental composition of Godavari sediments (Central and Southern Indian Subcontinent). Chemical Geology, 70, 275–286.
Billon, G., Ouddane, B., Recourt, P., & Boughriet, A. (2002). Depth variability and some geochemical characteristics of Fe, Mn, Ca, Mg, Sr, S, P, Cd and Zn in anoxic sediments from Authie Bay (Nortern France). Estuarine, Coastal and Shelf Science, 55, 167–181.
Chester, R. (2000). Marine geochemistry. Malden: Blackwell Science.
De Souza, M. M. C., Pestana, M. H. D., & Lacerda, L. D. (1986). Geochamical partitioning of heavy metals in sediments of three estuaries along the coast of Rio de Jeniro (Brazil). The Science of the Total Environ, 58, 63–72.
Fernandes, H. M., Bidone, E. D., Veiga, L. H. S., & Patchineelam, S. R. (1994). Heavy metal pollution assessment in the coastal lagoons of Jacarepagua, Rio de Janeiro, Brazil. Environmental Pollution, 85, 259–264.
Forstner, U., & Wittmann, G. T. W. (1981). Metal pollution in the aquatic environment (2nd ed.). Berlin: Springer.
Ip, C. C. M., Li, X. D., Zhang, G., Wai, O. W. H., & Li, Y. S. (2007). Trace metal distribution in sediments of the Pearl River Estuary and the surrounding coastal area, South China. Environmental Pollution, 14, 311–323.
Irving, H. M. N. H. & Williams, R. J. P. (1953). The stability of transition-metal complexes, Journal of the Chemical Society 3192–3210.
Jain, C. K. (2004). Metal fractionation study on bed sediments of river Yamuna, India. Water Research, 38, 569–578.
Jain, S. K., Agarwal, P. K., & Singh, V. P. (2007). Hydrology and water resources of India. Springer, Heidelberg. ISBN: 13978-1-4020-5180-7 (e-Book), pp. 622–625.
Jenne, E. A. (1968). Controls of Mn, Fe, Co, Ni, Cu, and Zn concentration in soils and water. The significant role of hydrous Mn and Fe-oxide. American Chemical Society, Advances in Chemistry Series, 73, 337–387.
Konhauser, K. O., Powel, M. A., Fyfe, W. S., Longstaffe, F. G., & Tripathy, S. (1997). Trace element geochemistry of river sediment, Orissa state, India. Journal of Hydrology, 193, 258–268.
Krupadam, R. J., Smita, P., & Wate, S. R. (2006). Geochemical fractionation of heavy metals in sediments of the Tapi estuary. Geochemical Journal, 40, 513–522.
Li, X. D., & Thornton, I. (1992). Multi-element contamination in soil and plant in the old mining area. Applied Geochemistry, 52, 51–56.
Liu, C. W., Lin, K. H., & Kuo, Y. M. (2003). Application of factor analysis in the assessment of ground water quality in a blackfoot disease area in Taiwan. Science of the Total Environment, 313, 77–89.
Liu, C., Xu, J., Liu, C., Zhang, P., & Dai, M. (2009). Heavy metals in the surface sediments in Lanzhou Reach of Yellow River Chaina. Bulletin of Environmental Contamination and Toxicology, 82, 26–30.
Martin, J. M., & Meybeck, M. (1979). Elemental mass-balance of material carried by major world rivers. Marine Chemistry, 7, 173–206.
Modak, D. P., Singh, K. P., Chandra, H., & Ray, P. K. (1992). Mobile and bound forms of trace metals in sediments of the lower Ganges. Water Research, 26(11), 1541–1548.
Morrison, G. M. P. (1989). Trace element speciation: analytical methods and problems. In: G. E. Batley (Ed.), Trace Element Speciation: Analytical Methods and Problems (p. 343). Boca Raton, Florida: CRC Press.
Muller, G. U. (1979). Schwermetalle in den sediments des Rheins: Veranderungen seit 1971. Umschau in Wissenschaft und Technik, 79, 778–783.
OPCB (Orissa Pollution Control Board). (2007). Water quality of Major Rivers of Orissa. pp-9–19
Pagnanelli, F., Moscardini, E., Giuliano, V., & Toro, L. (2004). Sequential extraction of heavy metals in river sediments of an abandoned pyrite mining area: pollution detection and affinity series. Environmental Pollution, 132, 189–201.
Panda, U. C., Rath, P., Sundaray, S. K., Majumdar, S., & Sahu, K. C. (2006). Study of geochemical association of some trace metals in the sediments of Chilika lagoon—a multivariate statistical approach. Environmental Monitoring and Assessment, 123, 125–150.
Panda, U. C., Rath, P., Bramha, S. N., & Sahu, K. C. (2010). Application of factor analysis in geochemical speciation of heavy metals in the sediments of a lake system—Chilika (India): a case study. Journal of Coastal Research, 26(5), 860–868.
Perin, G., Craboledda, L., Lucchese, M., Cirillo, R., Dotta, L. & Zanetta, M. L. (1985). Heavy metal speciation in the sediments of northern Adriatic Sea. A new approach for environmental toxicity determination. In: T. D. Lakkas (ed.) Heavy metals in the environment. Edinburg: CEP Consultants.
Ramesh, R., Subramanian, V., & Van Grieken, R. (1990). Heavy metal distribution in sediments of Krishna river basin, India. Environmental Geology Water Science, 15, 207–216.
Rath, P., Panda, U. C., Bhatta, D., & Sahoo, B. N. (2005). Environmental quantification of heavy metals in the sediments of Brahmani and Nandira rivers Orissa, India. Journal of Geological Society of India, 65, 487–492.
Rath, P., Panda, U. C., Bhatta, D., & Sahu, K. C. (2009). Use of sequential leaching, mineralogy, morphology and multivariate statistical technique for quantifying metal pollution in highly polluted aquatic sediments—a case study: Brahmani Nandira rivers, India. Journal of Hazardous Materials, 163, 632–644.
Ray, A. K., Tripathi, S. C., Patra, S., & Sharma, V. V. (2006). Assessment of Godavary estuarine mangrove ecosystem through trace metal studies. Environmental International, 32, 219–223.
Sarin, M. M., Borole, D. V., & Krishnaswamy, S. (1979). Geochemistry of sediments from the Bay of Bengal. Proceedings of the Indian Academy of Science, 88, 131–154.
Schoer, J. U., Hong, Y. T., & Forstner, U. (1983). Variation of chemical forms Fe, Mn and Zn in suspended sediments from Elbe and Weser rivers during estuarine mixing. Environmental Technology Letters, 4(6), 277–282.
Shajan, K. P. (2001). Geochemistry of bottom sediments from a river–estuary-shelf mixing zone on the southwest coast of India. Bulletin of the Geological Survey of Japan, 52, 371–382.
Shaofeng, W., Yongfeng, J., Shuying, W., Xin, W., He, W., Zhixi, Z., & Bingzhu, L. (2010). Fractionation of heavy metals in shallow marine sediments from Jinzhou Bay, Chaina. Journal of Environmental Sciences, 22(1), 23–31.
Simeonov, V., Stratis, J. A., Samara, C., Zachariadis, G., Voutsa, D., Anthemidis, A., Sofoniou, M., & Kouimtzis, T. H. (2003). Assessment of surface water quality in northern Greece. Water Research, 37, 4119–4124.
Simex, S. A., & Helz, G. R. (1981). Regional geochemistry of trace elements in Chesapeake Bay sediments. Environmental Geology, 3, 315.
Singh, K. P., Malik, A., Mohan, D., & Sinha, S. (2004). Multivariate statistical techniques for the evaluation of spatial and temporal variation in water quality of Gomati river (India)—a case study. Water Research, 38, 3980–3992.
Singh, P. K., Mohan, D., Singh, V. K., & Malik, A. (2005). Studies on distribution and fraction of heavy metals in Gomti river sediments—a tributary of the Ganges, India. Journal of Hydrology, 312, 14–27.
Soto-Jimenez, M., & Peaz-Osuna. (2001). Cd, Cu, Pb and Zn in lagoonal sediments from Mazatlan Harbour (SE Gulf of California): bioavailability and geochemical fractioning. Bulletin of Environmental Contamination and Toxicology, 66, 350–556.
Subramanian, V. (1987). Environmental geochemistry of Indian river basins, a review. Journal of Geological Society of India, 29, 205–220.
Subramanian, V., Van Grieken, R., & Vant Dack, L. (1987). Heavy metal distribution in the sediments of Ganges and Brahmaputra rivers. Environmental Geology and Water Sciences, 9, 93–103.
Subramanian, V., Jha, P. K., & Van Grieken, R. (1988). Heavy metals in the Ganges estuary. Marine Pollution Bulletin, 19, 290–293.
Sullivan, P., & Taylor, K. G. (2003). Sediment and porewater geochemistry in a metal contaminated estuary, Dulas Bay, Anglesey. Environmantal Geochemistry and Health, 25(1), 115–122.
Tessier, A., Campbell, P. G. C., & Bison, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51(7), 844–851.
Tipping, E., Lofts, S., & Lowlar, A. J. (1998). Modelling the chemical speciation of trace metals in the surface waters of the Humber system. Science of the Total Environment, 210–211, 63–77.
Tomlinson, D. C., Wilson, J. G., Harris, C. R., & Jeffrey, D. W. (1980). Problems in assessment of heavy metal levels in estuaries and the formation of a pollution index. Helgol Meeresunters, 33, 566–575.
Trivedy, R. K., & Goel, P. K. (1984). Chemical and Biological methods for water pollution studies. Karad: Env. Publ.
Turner, A., Le Roux, S. M., & Millward, G. E. (2008). Adsorption of cadmium to iron and manganese oxides during estuarine mixing. Marine Chemistry, 108, 77–84.
Valdes, J., Vargas, G., Sifeddine, A., Ortlieb, L., & Guinez, M. (2005). Distribution and enrichment evaluation of heavy metals in Mejillones Bay (23° S), Northen Chile, geochemical and statistical approach. Marine Pollution Bulletin, 50(12), 1558–1568.
Weng, H. X., Zhu, Y. M., Qin, Y. C., Chen, J. U., & Chen, X. H. (2008). Accumulation discrepancy heavy metal and organic pollutants in three near-shore depositional environments, south eastern China. Journal of Asian Earth Sciences, 31, 522–532.
Willams, T. P., Bubb, J. M., & Lester, J. N. (1994). Metal accumulation within salt-marsh environments: a review. Marine Pollution Bulletin, 28, 277–290.
Yang, Z., Wang, Y., Shen, Z., Niu, J., & Tang, Z. (2009). Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the Yangtze River catchment of Wuhan, China. Journal of Hazardous Materials, 166, 1186–1194.
Zhang, J., Huang, W. W., & Martin, J. M. (1998). Trace metal distribution in Huanghe (Yellow river). Estuarine, Coastal and Shelf Science, 26, 499–516.
Zhang, L. P., Ye, X., Feng, H., Jing, Y. H., Tong, O. Y., YU, X. T., Liang, R. Y., Gao, C. T., & CXhen, W. Q. (2007). Heavy metal contamination in western Xiamen bay sediments and its vicinity, China. Marine Pollution Bulletin, 54, 974–982.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asa, S.C., Rath, P., Panda, U.C. et al. Application of sequential leaching, risk indices and multivariate statistics to evaluate heavy metal contamination of estuarine sediments: Dhamara Estuary, East Coast of India. Environ Monit Assess 185, 6719–6737 (2013). https://doi.org/10.1007/s10661-013-3060-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3060-3