Abstract
The gliding electric discharge in humid air is a source of activated species forming (e.g. •OH, •NO and their derivatives H2O2, ONO2H and NO3H) which are present in a non-thermal plasma at atmospheric pressure. These species are able to degrade organic pollutants in palm oil refinery wastewaters (PORW). The increase in acidity (pH decrease), conductivity and total dissolved solids (TDS) and the decrease in the total organic carbon (TOC) of PORW samples exposed to the discharge are reported. More than 50 % TOC abatement is obtained for 15 min treatment in batch conditions with a laboratory reactor. The organic pollutants of PORW, i.e. mainly fatty acids are degraded according to a pseudo first-order reaction (k* = 0.06 min−1). Post discharge reactions are also observed after having switched off the discharge, which suggests that the pseudo first-order (k ≈ 0.05 min−1) degradation reactions should be attributed to the diffusion of soluble reactive species, e.g. H2O2 and ONOOH in the liquid target.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many developing countries are now concerned with serious water problems, resulting from the increase in their growing population and their developing industry. The disposal of million cubic meters of palm oil refinery wastewaters (PORW) every year raises a major environmental problem which requires urgent solutions especially for the equatorial African countries in general and South Cameroon in particular. PORW have significant pollutant properties which are accounted by high values of total organic carbon (TOC) and turbidity levels, due to the high concentration of a series of organic contaminants that can be divided into two groups. The first group involves oil-soluble components, such as proteins, sterols, tocopherols, hydrocarbons and natural pigments; the second one concerns free fatty acids, peroxides, ketones and aldehydes (Thomas 2002) which may inhibit biological treatment (Ryan and Robards 1998; Cicheli and Solinas 1984; Sayadi et al. 2000). Given the nature of these concentrated chemical compounds present in wastewaters, elimination of these wastes may be performed either by incineration, with the matching production of soot, smokes and air pollution and/or by classic physical–chemical treatments but biological treatments cannot be applied, so that alternative wastewater treatments have to be considered.
During the last decades, the advanced oxidation processes were largely developed for the treatment of wastewaters containing recalcitrant compounds at mild temperature and pressure (Masschelein 1991; Hnatiuc 2002; Ikehata and Gamal El-Din 2005). Various processes have been investigated and among them, the electric discharges. Some of them burn in humid air (i.e. in water sprays) over the liquid target (Brisset et al. 2008); other ones called eletrohydraulic discharges (Locke et al. 2006) use at least one electrode dipped into the liquid. All these techniques lead to the formation of active ·OH radicals by electron collision with water-activated molecules and breaking the covalent bond, since vapour is present either over the liquid or in gas bubbles resulting from Joule's effect:
where H2O* refers to excited molecules (e.g. the X1A1, 3A2, A1B1, A3B1, A3B2 and B1B1 states) (Brisset and Hnatiuc 2012).
A matching reaction
may take place because of the short lifetime of ·OH. Hydrogen peroxide is water soluble and easily dissolves in the aqueous phase.
Each technique presents advantages and drawbacks. For instance electrohydraulic discharges are presented as allowing an improved contact between the plasma and the solute and consequently favouring energy exchange to form active species. The reaction between the plasma species and the solute are then governed by diffusion phenomena in the liquid phase and the presence of one immerged electrode may lead to the formation of matching oxidised or reduced species according to classical electrolysis and induce their neutralisation.
Oppositely, discharges over water, i.e. in a gas, without contact with the liquid target enable us to select the suitable gas to form convenient and useful moieties. Water sprayed air then appears as an efficient solution to form reactive oxygen species (ROS) such as HO• radicals and H2O2 in the same way as electrohydraulic discharges, but also reactive nitrogen species (RNS) such as nitric oxide •NO, which was spectrometrically identified and quantified by Benstaali et al. (2002), and peroxinitrite ONOO− whose occurrence in the condensed phase is suggested by microbial inactivation studies (Naitali et al. 2009). •NO formation takes place in a gliding arc burning in air over an aqueous solution. The starting chemicals are thus N2 and O2 that combine in the arc according to the Birkland process and form •NO provided the quantity of energy transferred from the discharge is suitable. •NO is a precursor for several RNS compounds, e.g. •NO 2 , nitrous acid ONOH and nitric acid HONO2 via a linear intermediate isomer, i.e. peroxynitrous acid ONOOH, as detailed elsewhere (Brisset and Hnatiuc 2012). A noticeable advantage of the gliding discharge in humid air is to form important and controlled quantities of the transient reagents ONOOH/ONOO− by modifying the gas flow and, when possible, the input energy.
This paper aims to test the “glidarc” plasma system as a useful tool for the treatment of recalcitrant substances present in actual wastes, and explore working conditions close to industrial operations. This study was initiated after the successful plasma treatments of tributylphosphate and trilaurylamine in a similar reactor (Moussa and Brisset 2003 and Moussa et al. 2006). Such a feature took place in a gliding arc burning over an aqueous solution with synthetic waste solutions. Thus, we checked their ability to achieve a complete conversion of organic compounds present in wastewaters and we have selected palm oil wastewaters as an illustrative example. The low running and investment costs of the gliding arc technique favour the introduction of the process in countries of developing industrial network.
As a first approximation it is reasonable to consider that PORW contain long-chain carbonaceous compounds, mainly glyceryl palmitate, oleate and β-carotene which are the major components of palm oil. Palmitic acid, or hexadecanoic acid, is a linear saturated acid with the formula CH3·(CH2)14·COOH. Oleic acid is a monounsaturated ω-9 fat acid involving 18 carbon atoms and one double bond at the ninth carbon atom from the carboxylic function (formula: CH3·(CH2)7·CH = CH·(CH2)7·COOH). β-carotene is a polyethylenic hydrocarbide (formula: C40·H56) with 11 conjugate bonds which presents a strong anti-oxidizing power and is responsible for the red-yellowish colour of the waste effluents.
Gliding discharge (“glidarc”)
The gliding arc or “glidarc”, first proposed by Lesueur et al. (1988) and by Czernichowski (1992, 1994), was examined as a convenient source of quenched, non-thermal plasma in several laboratories, mainly for pollution abatement of gases (Czernichowski 1992, 1994). A suitable device consists of a couple of diverging knife shaped electrodes connected with an energy source such as a high-voltage transformer. An arc formed at the narrowest gap is pushed along the electrodes by a gas flow disposed along the axis of the reactor. Previous studies showed that humid air (i.e. water-saturated air) was a suitable source of reactive species. Such a device enables energy transfer to the ambient gas to take place. The arc feet are blown downwards to the tips of the electrodes up to the arc breaks on being short-circuited by a new one. The resulting plasma cloud is composed of the same components as the arc (i.e. a thermal plasma) but its temperature remains close to ambient with an increase less than 12 °C for 1 h exposure, which confers on it the properties of a non-thermal quenched plasma in contact with the liquid target. The discharge leads to the formation of positive and negative ions, photons, electrons and other chemically active species such as molecules and radicals which can react with the solute at the liquid surface or/and in the solution if they are soluble enough.
This discharge was already used for the treatment of gases (Lesueur et al. 1988; Czernichowski 1992, 1994) and more recently for that of liquids (Benstaali et al. 1998; Brisset et al. 2008). Apart from aqueous solutions and spent industrial solvents, such as tributylphosphate and trilaurylamine that are concerned with nuclear industry processes (Moussa and Brisset 2003; Moussa et al. 2006), or chlorine containing solvents (Fanmoe et al. 2003; Krawczyk and Ulejczyk 2003; Indarto et al. 2006), the technique was successfully operated for the pollutant abatement of domestic and industrial wastewaters (Njoyim-Tamungang et al. 2009). It is now developed in the field of bacterial inactivation (Brisset et al. 2008; Naitali et al. 2009) and medicine applications and it appears as a promising technique. Anyhow, we found it necessary to check the chemical properties of the discharge in humid air and to verify the usefulness of the process on targets directly sampled from sewerage brooks that is to work with actual wastes in view of further industrial application instead of synthetic wastes.
Chemical properties of a gliding discharge
The nature of the activated species formed in the discharge depends on that of the feed gas as mentioned (Brisset and Hnatiuc 2012) in the case of discharges in humid air. The activated species derive from N2, O2 and H2O; therefore hydrogen peroxide, ozone or nitrogen oxides NO x are expected although the presence of water vapour is not favourable to the formation of ozone. Emission spectroscopy investigation showed the formation of HO• and NO• radicals in discharges burning in humid air (Benstaali et al. 2002). These species are the main active reagents formed with derivative species such as peroxynitrite or the matching acid (Brisset and Hnatiuc 2012) yielded by their reaction with the parent molecules. These species drift to the liquid surface and react with the solute. Acid effects are ascribed to NO• via nitrous and nitric acids, while the oxidizing effects are essentially related to the occurring HO• radicals to the derived species, i.e. hydrogen peroxide or peroxynitrite. These latter species are water soluble and able to dissolve in the aqueous phase before reacting with the solute, so that peroxynitrous acid ONOOH is an excellent vector to get HO• and NO2 • radicals in the liquid phase where homolysis reaction takes place. The resulting plasma-chemical treatments on aqueous solutions thus induce acid effects (Brisset et al. 2011) which are due to transient HNO2 and ONOOH medium or weak acids and stable HNO3 strong acid, additionally to oxidizing effects related to the strong oxidizing properties of •OH, H2O2 and ONOOH/ONOO−: \( \mathrm{E}{}^{\circ}\left( {\mathrm{ OH}/{{\mathrm{H}}_2}\mathrm{O}} \right)=2.85\ \mathrm{V}/\mathrm{SHE} \); \( \mathrm{E}{}^{\bullet}\left( {{{\mathrm{H}}_2}{{\mathrm{O}}_2}/{{\mathrm{H}}_2}\mathrm{O}} \right)=1.76\ \mathrm{V}/\mathrm{SHE} \); \( \mathrm{E}{}^{\bullet}\left( {\mathrm{O}\mathrm{N}{{\mathrm{O}}_2}\mathrm{H}/\mathrm{N}{{\mathrm{O}}_2}} \right)=2.05\ \mathrm{V}/\mathrm{SHE} \); \( \mathrm{E}{}^{\circ}\left( {\mathrm{O}\mathrm{N}{{\mathrm{O}}_2}^{-}/\mathrm{N}{{\mathrm{O}}_2}} \right)=2.44\ \mathrm{V}/\mathrm{SHE} \).
Note that the nitrogen-containing derivatives are also able to react with organic solutes and form nitro and nitroso products. Such a feature is probably the key of the bacterial inactivation properties of the plasma treatments (Bonnefont-Rousselot et al. 2005; Briviba et al. 1999).
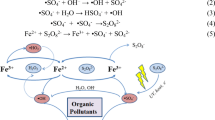
Specific reactivity of peroxynitrite
Many molecules are oxidised and/or nitrated by peroxynitrite including protein tyrosine residues, thiols and unsaturated fatty acids (Sekher Pannala et al. 1999; Rubbo et al. 2009; Botti et al. 2004; O’Donnell et al. 1999; Radi et al. 1991; Radi et al. 2000). The oxidizing reactivity of peroxynitrite is highly pH dependent and both peroxynitrite anion (ONOO−) and its matching acid (ONOOH) can participate in direct one- and two-electron oxidation reactions. ONOOH oxidises organic molecules directly or through H+ or CO2-catalysed homolysis yielding nitrogen dioxide (•NO2), hydroxyl (•OH) or carbonate anion radical (•CO3 −) (Radi et al. 1991). This proton catalysed decomposition into •OH and •NO2 radicals may also be realized in hydrophobic media, resulting in the initiation of lipid oxidation (Fig. 1). •NO and superoxide (O2 • −) combine to form peroxynitrite which rapidly reacts with CO2 to yield •NO2 and CO3 • −, the last one causing inhibition of lipid oxidation. On the other hand, ONOOH homolyses to •NO2 and •OH and these species abstract H to unsaturated fatty acids, initiating lipid oxidation. Lipid oxidation is very sensitive to inhibition by •NO, which mainly depends on •NO termination reactions with COO•. More important, •NO2 can react with lipid-derived radicals and lead to nitro-fatty acids (Rubbo et al. 2009). The reaction of peroxynitrite-derived radicals with lipids leads not only to oxidation (Botti et al. 2004; O’Donnell et al. 1999; Radi et al. 1991) but also to formation of nitrated products (Fig. 2) (O’Donnell et al. 1999; Rubbo et al. 1994). Nitrogen dioxide coming from ONOOH homolysis can mediate oxidation and nitration of unsaturated fatty acids under anaerobic or aerobic conditions: •NO2 reacts with unsaturated fatty acids through a radical pathway yielding nitrovinyl, nitroallyl or nitrohydroxy derivatives. Isomerisation of lipid derivatives is favoured by increased oxygen partial pressure. Alternatively, ONOOH homolysis can induce the formation of nitro-epoxy products (Balazy 1994).
Overview of peroxynitrite reactivity with lipids adapted from Rubbo et al. (2009)
Mechanisms of nitro-fatty acid formation by peroxynitrite adapted from Rubbo et al. (2009)
Plasma–liquid interaction
We focused on palm oil refinery wastewaters as a demonstrative application example. The recent literature shows that degradation of organic wastewater persistent pollutants was performed by using advanced oxidation processes, i.e. •OH, H2O2, O3 and UV light or any suitable combination (Masschelein 1991; Hnatiuc 2002; Ikehata and Gamal El-Din 2005).
Aqueous solutions or dispersions can be exposed to such plasma, so that chemical reactions may occur at the liquid/gas interface. The liquid target is thus subjected to the chemical properties of the impinging activated species present in the discharge after they react with water vapour. Many parameters such as the composition of the solution, the nature and flow rate of the working gas, the electrode material and shape, as well as the power supply voltage play important roles in the degradation of an organic pollutant and on the variation of pH, conductivity and electrical potential of the solution. The species present in the plasma phase are governed by the composition of the feed gas, the nature of the target solute and by the discharge parameters. For example, a discharge burning in a mixture of N2 and O2 yields nitrogen oxides such as NO which are able to react at the liquid surface with suitable organic molecules. NO is thus the source of transient nitrous and peroxynitrous acids and stable nitric acid which form in a complex set of reactions (Brisset and Hnatiuc 2012). Apart from pH lowering, oxidation phenomena also result from the impinging species.
The short lifetime of the •OH radicals formed in the discharge favours the formation of dimmers.
which react with the solutes at the liquid surface more slowly than •OH. Additionally, gas phase reactions may lead to the formation of reactive water soluble peroxynitrous acid ONOOH at the immediate neighbourhood of the liquid phase (Brisset and Hnatiuc 2012). H2O2 and ONOOH are thus able to dissolve in water and react with solutes.
Materials and methods
The plasma reactor
The glidarc reactor used in batch conditions is sketched in Fig. 3. It consists in a cylindrical vessel 23 cm long and 10 cm inner diameter. Compressed air passes through a distilled water-filled bubbling flask, becoming water-saturated before being injected in the reactor through a nozzle along the axis of two diverging electrodes at the flow rate (800 L h−1) which was optimised in the range 500–900 L h−1 explored with other studies. The two aluminium electrodes were connected to an Aupem Sefli HV transformer (10 kV; 50Hz in open conditions; maximum intensity delivered 100 mA). When the circuit is connected, an electric arc forms between the electrodes at the minimum gap (close to 3 mm). The arc is pushed away by the feeding gas flow, sweeps along the increasing electrode gap and breaks in a large plasma plume. A new arc then appears and develops according to the same procedure. The arc is actually a thermal plasma; however, its length increases and the volume of the ionised channel also increases as the arc “glides” along the electrodes. The gas temperature then reduces so that the medium becomes a non-thermal plasma subsequent to the arc breaking. The resulting plasma that reacts at the liquid surface is a quenched plasma which has no noticeable thermal effect on solutions for short exposures. The plasma plume “licks” the liquid surface 30–35 mm far from the tips and allows chemical reactions to take place at the interface. The contact surface was increased by means of magnetic stirring of the target solution.
The reported experimental results are obtained with a reactor operated in batch conditions. Temperature measurements performed on the occasion of microbial inactivation studies (Brisset et al. 2008) showed that the temperature increase was limited to 10 °C for t* = 10 min exposure time and tends to a plateau for longer t*: (e.g. 10< t*, min <60).
Collecting the liquid samples
The liquid samples were collected directly out of the oil workshop near Kribi, South Cameroon, every 1 h from 7 am to 2 pm (during periods of intense production). At the end of the refining operations of palm oil, a heavily loaded effluent from the mill is released untreated into the environment with all the dangers that it implies. The samples were then stored in cold storage room at a temperature of 3 ± 1 °C before analyses or treatments. The organoleptic parameters of the samples, i.e. appearance, feeling to touch and smell, were immediately checked before storage and measurements of the physico-chemical parameters, which are temperature, pH, total dissolved solids (TDS), colour and conductivity. The samples were stored in the cold before the measurements of parameters related to pollution, especially TOC, were performed. One part of the samples was devoted to the plasma treatments and analysed accordingly for pH, TDS, temperature and conductivity. All these parameters were measured using a portable Hanna electronic multimeter. This unit evaluates the TDS (mg L−1), conductivity (μS cm−1) and temperature in degrees Celsius (°C). Total organic carbon (mgC L−1) was determined using a Shimadzu total organic carbon analyser model 5050A, which is the most reliable and rapid method to determine the overall concentration of organic compounds in the treated solution. The working parameters were optimised and the gas flow rate fixed at 800 L h−1.
The PORW solutions S1 diluted 1/10 (V = 500 mL, TOC = 1,278 mg L−1) was circulated through the reactor and exposed to the wet air plasma. To clarify the notation, we will call So and S1 respectively crude and distilled water-diluted samples. Due to the high turbidity level and TOC concentration, S0 was filtrated and diluted to obtain S1 = S0/10 before being exposed to the discharge.
Results and discussion
Effluent characterisation
At the end of the palm oil refining process, a yellowish-brownish heavily polluted effluent from the refinery is rejected in the environment without any treatment with all the dangers that this implies. The composition of samples from these sampling points is recorded in Table 1 below.
Analysis of the results presented in Table 1 shows that the wastewater discharges from the oil mill are acidic with a pH value slightly below the limit value of Cameroonian environmental standards and inspection procedures for industrial facilities and trade (2001). We also note that these waters are troubled, with a high concentration of total dissolved solids and conductivity. The parameter values of organic pollution indicators are above standards.
Since the aim of our treatment lies in reaching a pollutant abatement as large as possible, but not getting directly drinkable (and colourless) water, it was not found necessary to achieve spectrophotometry absorbance measurements at this stage.
Plasmachemical treatment of the effluents
pH variations
Exposing S1 solutions to the discharge for various times t* (3, 5, 15 and 30 min) shows that the pH of the sample gradually decreases as the exposure time (t*) increases (Fig. 4). This evolution confirms that new chemicals species with high-acidic properties form in the discharge and are incorporated into the target solution. This feature is attributed to the formation of acid species (e.g. HNO3), as considered elsewhere (Brisset et al. 2011; Brisset and Hnatiuc 2012).
Such a feature was already observed several years ago (Brisset et al. 2011; Moussa et al. 2005; Brisset et al. 1990) but contrary to the results reported for the plasma treatments of alkaline solutions, the pH decrease relevant to PORW is rather slow and limited to about one pH unit within 30 min treatment. This limited variation is attributed to the slightly acidic nature of our samples, and mainly to the high concentration of the solutes which favours the buffering effect of the medium, for example by neutralizing a large part of the carboxyl groups of the waste molecules:
and leading to the formation of the R'COOH/R'COO− buffer.
Conductivity measurements
Conductivity measurements (at 25 °C) show the increasing concentration of ions in a liquid medium. We observe that the conductivity value of the starting sample is largely superior to the authorized values for wastes and sensibly increases with the exposure time (Fig. 5). This feature agrees with the pH abatement, for a couple of reasons, since the increase in the proton concentration induces that of the conductivity of the solution, due to its high equivalent ionic conductance compared with that of other cations.
Assuming that the waste effluent is composed of organic species (i.e. weak acids RCO2H, bases R1R2NH and RCO2 −) and other neutral species R'H such as sugars apart inorganic salts (typical example NaCl and less soluble CaCO3), incorporating nitric acid in the solution by exposing the target to the discharge increases the number and/or the ionic conductance of the conductive species. Thus, H+ and NO3 − which result from the standard evolution of NO in plasma conditions and the disproportionation of nitrous acid into NO3 − and NO make the solution more conductive; R1R2NH2 + is formed by neutralizing the relevant bases; CaCO3 is more soluble and dissociated in acidic medium. Peroxynitrous acid ONOOH which is present in solution as another intermediate does not affect the conductivity because of its weakness (pK a = 6.8).
TDS measurements
The plasma-chemical evolution of TDS, i.e. a regular increase with the exposure time t*, confirms the oxidizing action of the discharge on the solutes: molecular bonds are broken and chemical functions are oxidised so that sparingly soluble compounds are transformed into lighter, more polar or ionized compounds (Fig. 6). Table 2 gathers the relevant results.
TOC abatement induced by plasma treatment
The mineralisation of organic pollutants in treated target was followed by instantaneous measurement of TOC and confirmed by the increase in TDS. The observed TOC abatement of the plasma treated sample accounts for the breaking of carbon–carbon bonds and the formation of light products. These organic compounds may be volatile or directly transformed into carbon oxides. They are thus no more present in the solution. The variations of TOC with the direct exposure time are reported in Table 2 and Fig. 7 illustrates the relevant abatement as TOC% vs t* (min):
The ratio R is calculated from the TOC value at time t* (i.e. TOC t*) and the starting TOC value for t* = 0 (i.e. TOC t*=0).
Figure 7 shows that the major part of the plasmachemical degradation of the organic solutes or dispersed matter occurs by exposing the sample to the plasma for only 15 min: over 50 % of initial TOC (1278 mg L−1) is thus reduced (628 mg L−1). Such a laboratory result was obtained by operating the reactor in batch conditions, which is essential for evidencing the success of the treatment. However, improved degradation yields could be obtained by using a reactor fitted with a circulating device, according to our experience. Relevant under progress work will be more tightly adapted to industrial application. It can also be guessed that the proposed reaction model (Fig. 2) could hold on for plasmachemical treatments in the circulating mode, since the model involves the key species formed in the discharge.
Kinetic considerations
The kinetic evolution of TOC with the exposure time, i.e. the parameter which globally accounts for the organic charge of PORW suggests first-order degradation kinetics. Such a kinetic law:
or as the integral form:
which involves the TOC values at time t* and at infinite time. The experimental result with TOC∞ = 300 is:
A first-order kinetic was already observed for the treatment of dilute solutions: it was assigned to a diffusion process that takes place in the solution and concerns the soluble plasma species and/or betters their derivatives. It is then surprising to observe a first-order kinetic in the present case because the treated solutions are concentrated. This feature may be tentatively explained by considering that the hydrophobic fatty tails of the compounds gather in a barrier layer at the gas/liquid surface interface. Thus the active species have to diffuse through the layer and reach the polar head of the waste compounds.
Temporal post-discharge reactions
A new kinetic mechanism called “temporal post-discharge reactions” (TPDR) has been recently identified (Doubla and Brisset 2006; Brisset et al. 2008). It consists in the development of the degradation reactions of the solute in the solution after the discharge is switched off which is of major importance from the financial point of view, since the resulting running energy cost of the process is reduced. This behaviour was first identified and confirmed in case of synthetic wastes, i.e. aqueous solutions of a single solute, such as dyes (Moussa et al. 2007; Doubla et al. 2008; Laminsi et al. 2012), and more recently in case of bacterial inactivation (Kamgang Youbi 2007; Moussa et al. 2010). We acquired some industrial experience on TPDR occurring after the plasma treatment of brewery, palm oil refinery or slaughterhouse effluents from workshops of developing counties, which suggests the general character of the feature. For example Gnokam Zumgang et al. (2010) considered the TPDR degradation of slaughterhouse effluents which could be observed several days after the discharge was switched off.
Figure 8 illustrates the TOC abatement of samples exposed to the discharge for known t* values after post discharge times t PD of 0, 60 and 120 min: it thus confirms the occurrence of TPDR for the degradation of palm oil wastes. The economical advantage of the feature is evident: we may for instance notice that a plasma treatment of 15 min abandoned for 60 min in TPDR conditions induces a TOC abatement close to that resulting from a 30-min direct exposure.
Evolution of PORW solutions in post-discharge conditions for various exposure times and various post-discharge times t pd/t pd = 0 (black squares); t pd = 60 (medium grey diamonds), and t pd = 120 min (light grey triangles and dotted line). Relevant pseudo first-order kinetic constants, 0.028, 0.050, and 0.057 min−1
A rapid examination of the relevant kinetics shows that the TOC abatements follow a pseudo first-order law after the first five minutes, for exposure times of 60 and 120 min. This feature suggests that diffusion processes govern the degradation reactions and may be attributed to the only soluble species presenting a lifetime long enough, i.e. H2O2 and ONOOH. The matching degradation kinetic constants in TPDR conditions are higher than for immediate measurements (t tpdr = 0; k = 0.03 min−1) and increase with the exposure time t*. For example the pseudo first-order constants are close to 0.05 and 0.06 min−1 for post-discharge times 60 and 120 min, respectively.
The occurrence of these post-discharge phenomena are obviously essential for industrial applications. The limited values of the kinetic constant relevant to the TOC abatement confirm that the degradation process is complicated and involves several steps governed by the transfer into the solution of H2O2 and ONOOH. Improving the process requires the increase in the mentioned number of transferred species which can result either from increasing the surface exchange or from using a more powerful electric source. For example, a reactor fitted with a pump for liquid circulation is the first step to a continuous process which leads to faster degradation for a given displayed energy.
Conclusions
A non-thermal plasma technique of the gliding arc type was used at the laboratory scale (i.e. with a plasma source of limited input power, close to 1 kW) for abating the organic charge of a palm oil mill waste from an industrial plant in Cameroon. The decrease in the total organic carbon abatement by 50 % resulted from an exposure time shorter than 15 min when the reactor was operated in batch conditions in order to verify the feasibility of the process. Since oxidizing degradation reactions of linear hydrocarbon chains are usually more difficult to realise than for aromatic compounds, the key feature remains the feasibility of the treatment and the role of the activated species formed in the discharge, i.e. •OH and •NO radicals and their derivatives H2O2, HNO3 and ONOOH. Additionally, this work confirms the destruction of recalcitrant molecules such as tributylphosphate or trilaurylamine which are widely used in nuclear processes (Moussa and Brisset 2003; Moussa et al. 2006) or other surfactants species.
However, the abatement rate remains too low for installing the laboratory device in the plant without improvements. A work in progress deals with the technical development of the device, on the basis of our experience. The work is developed by following several ways to improve the yield of the treatment while remaining in a reasonable running cost range: i.e. (1) using more powerful plasma sources and (2) fitting the reactor with a recycling device, before proposing the device to the relevant industry. Incidentally, the sanitary state of the whole human population around the plant and the collecting river should be largely improved because of the lethal effect of the discharge components on micro organisms (Brisset et al. 2011; Naitali et al. 2009).
References
Balazy, M. (1994). Peroxynitrite and arachidonic acid. Identification of arachidonate epoxides. Polish Journal of Pharmacology, 46, 593–600.
Benstaali, B., Moussa, D., Addou, A., & Brisset, J.-L. (1998). Plasma treatment of aqueous solutes: some chemical properties of a gliding arc in humid air. European Physical Journal Applied Physics, 4, 171–179.
Benstaali, B., Boubert, P., Cheron, B., Addou, A., & Brisset, J.-L. (2002). Density and rotational temperatures measurements of the ·NO and ·OH radicals produced by a gliding arc inhumid air and their interaction with aqueous solutions. Plasma Chemistry and Plasma Processing, 22, 553–571.
Bonnefont-Rousselot D., Beaudeux J. L., & Delattre J. (2005). Radicaux Libres et Stress Oxydant. Lavoisier Eds. Paris France, ch.6, pp.147–167.
Botti, H., Batthyany, C., Trostchansky, A., Radi, R., Freeman, B. A., & Rubbo, H. (2004). Peroxynitrite-mediated α-tocopherol oxidation in low-density lipoprotein: a mechanistic approach. Free Radical Biology & Medicine, 36, 152–162.
Brisset, J.-L., & Hnatiuc, E. (2012). Peroxynitrite: a re-examination of the chemical properties of non-thermal discharges in humid air over aqueous solutions. Plasma Chemistry and Plasma Processing, 32, 655–674. doi:10.1007/s11090-012-9384x.
Brisset, J.-L., Lelièvre, J., Doubla, A., & Amouroux, J. (1990). Interaction with aqueous solutions of the air corona products. Rev Phys Appl, 25, 535–543.
Brisset, J.-L., Moussa, D., Doubla, A., Hnatiuc, E., Hnatiuc, B., Kamgang Youbi, G., Herry, J.-M., Naitali, M., & Bellon-Fontaine, M.-N. (2008). Chemical reactivity of discharges and temporal post-discharges in plasma treatment of aqueous media. Example of gliding discharge treated solutions. A review. Industrial and Engineering Chemistry Research, 47, 5761–5781.
Brisset, J.-L., Benstaali, B., Moussa, D., Fanmoe, J., & Njoyim-Tamungang, E. (2011). Acidity control of plasma-chemical oxidation: applications to dye removal, urban waste abatement and microbial inactivation. Plasma Sources Science and Technology (Special issue), 20, 034021.
Briviba K., Klotz L.-O., & Sies H. (1999) Defenses against peroxynitrite. In: L. Packer (Ed). Nitric Oxide, Part C. New York :Academic Press. Chap 32, pp 301–311.
Cicheli, A., & Solinas, M. (1984). Phenolic compounds of olives and olive oil. Riv Merceol, 23, 55–56.
Czernichowski, A. (1992). Gliding discharge reactor for H2S valorisation or destruction. In: B. Penetrante & S, Schultheis (eds). "Non-thermal Plasma Techniques for Pollution Control". Nato ASI Series G34 B: 371–387.
Czernichowski, A. (1994). Gliding arc: applications to engineering and environment control. Pure and Applied Chemistry, 66, 1301–1310.
Doubla, A. & Brisset, J.-L. (2006). Post-discharge kinetics associated with a plasmachemical nucleophilic substitution and application to the analysis of plasma activated CO. Journal of Applied Electrochemistry, 36, 77–85.
Doubla, A., Bouba Bello, L., Fotso, M., & Brisset, J.-L. (2008). Plasma chemical decolourisation of bromothymol blue by gliding electric discharge at atmospheric pressure. Dyes Pig, 77, 118–124.
Fanmoe, J., Kamgan, J. O., Moussa, D., & Brisset, J. L. (2003). Application de l’arc glissant d’air humide au traitement des solvents industriels: cas du 1, 1,1-trichloroéthane. Phys Chem News, 14, 1–4.
Gnokam Zumgang, F., Doubla A., & Brisset, J.-L. (2010). Temporal post-discharge reactions in plasma-chemical degradation of slaughterhouse effluents. Chemical Engineering Communications, 98, 483–493.
Hnatiuc, E. (2002). “Procédés electriques de mesure et de traitement des polluants” (in French), Tec & Doc, Paris.
Ikehata, K., & Gamal El-Din, M. (2005). Aqueous pesticide degradation by ozonation and ozone-based advanced oxidation processes. A review. Ozone sci Technol, 27, 173–202.
Indarto, A., Choi, J.-W., Lee, H., Song, H. K., & Coowanitwong, N. (2006). Discharge characteristics of a gliding arc plasma in chlorinated methans diluted in atmospheric air. Plasma device Oper, 14, 15–26.
Kamgang Youbi, G., Briandet, R., Herry, J.-M., Brisset, J.-L., & Naitali, M. (2007). Destruction of planktonic, adherent and biofilm cells of Staphylococcus epidermidis using gliding discharge in humid air. Journal of Applied Microbiology, 103, 621–628.
Krawczyk, K., & Ulejczyk, B. (2003). Decomposition of chloromethanes in gliding discharges. Plasma Chemistry and Plasma Processing, 23, 265–281.
Laminsi S., Acayanka E., Teke Ndifion P., Djore Tiya A., & Brisset J.-L. (2012). Direct and post discharge chemical reactions of Fe(II) complexes in non-thermal plasma Desalination and Water Treatment, 37, 38–45.
Lesueur H, Czernichowski A, & Chapelle J (1988). Dispositif de génération de plasma basse température par formation de décharges électriques glissantes. Brevet Français, No 2639172.
Locke, B. R., Sato, M., Sunka, P., Koffmann, M. R., & Chang, J. S. (2006). Electrohydraulic discharges and non-thermal plasma for water treatment. Industrial and Engineering Chemistry Research, 45, 882–905.
Masschelein W. S. (1991). “Ozone et Ozonation des Eaux” (in French), Tec & Doc, Paris.
Moussa, D., & Brisset, J.-L. (2003). Disposal of spent tributylphosphate by gliding arc plasma. Journal of Hazardous Materials, B102, 189–200.
Moussa, D., Abdelmalek, F., Benstaali, B., Addou, A., Hnatiuc, E., & Brisset, J.-L. (2005). Acidity control of the oxidation reactions induced by non-thermal plasma treatments of aqueous effluents in pollutant abatement processes. The European Physical Journal—Applied Physics, 29, 189–199.
Moussa, D., Brisset, J.-L., Hnatiuc, E., & Decobert, G. (2006). Plasmachemical destruction of trilaurylamine from nuclear laboratories of reprocessing. Industrial and Engineering Chemistry Research, 45, 23–29.
Moussa, D., Doubla, A., Kamgang Youbi, G., & Brisset, J.-L. (2007). Post-discharge long life reactive intermediates involved in the plasma-chemical degradation of an azo dye. IEEE Transactions on Plasma Science, 35, 444–453.
Moussa, D., Naitali, M., Herry, J.-M., Hnatiuc, B., & Brisset, J.-L. (2010). Reactions induced by electrical discharges in pollutant abatement and bacterial inactivation. IEEE Conf. Optim, 2010, 1329–1335.
Naitali M., Hnatiuc B., Herry J.-M., Hnatiuc E., Bellon-Fontaine M.-N., & Brisset J.-L. (2009) Decontamination of chemical and microbial targets using gliding electrical discharges. In: G. Brelles Marino (ed). Biological and environmental applications of gas discharges plasmas”. Nova Science Publisher, Hauppauge, N.Y.; chap 6.
Njoyim-Tamungang, E., Ghogomu, P., Nzali, S., Laminsi, S., Doubla, A., & Brisset, J.-L. (2009). Coupling gliding discharge treatment and catalysis by oyster shell powder for pollution abatement of surface waters. Industrial and Engineering Chemistry Research, 48, 9773–9780.
O’Donnell, V. B., Eiserich, J. P., Bloodsworth, A., Chumley, P. H., Kirk, M., & Barnes, S. (1999). Nitration of unsaturated fatty acids by nitric oxide-derived reactive species. Methods in Enzymology, 301, 454–470.
Radi, R., Beckman, J. S., Bush, K. M., & Freeman, B. A. (1991). Cytochrome c-catalyzed oxidation of organic molecules by hydrogen peroxide. Archives of Biochemistry and Biophysics, 288, 481–487.
Radi, R., Denicola, A., Alvarez, B., Ferrer-Sueta, G., & Rubbo, H. (2000). Nitric oxide biology and pathobiology. In L. J. Ignarro (Ed.), Nitric Oxide: Biology and Pathobiology (pp. 57–82). San Diego: San Diego Academic Press.
Rubbo, H., Radi, R., Trujillo, M., Telleri, R., Kalyanaraman, B., Barnes, S., Kirk, M., & Freeman, B. A. (1994). Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. Journal of Biological Chemistry, 269, 26066–26075.
Rubbo, H., Trostchansky, A., & O’Donnell, V. B. (2009). Peroxynitrite-mediated lipid oxidation and nitration: mechanisms and consequences. Archives of Biochemistry and Biophysics, 484, 167–172.
Ryan, D., & Robards, K. (1998). Phenolic compounds in olives. Analyst, 123, 31–44.
Sayadi, S., Allouche, N., & Jaoua Aloui, F. (2000). Detrimental effects of high molecular-mass polyphenols on olive mill wastewaters biotreatments. Process Biochemistry, 35, 725–735.
Sekher Pannala, A., Singh, S., & Rice-Evans, C. (1999). Interaction of carotenoids ans tocopherols with peroxynitrite. In: L. Packer (ed.). Methods in Enzymology Vol. 301: Nitric Oxide, Part C. New York: Academic Press. Chap 34. pp 319–332.
Thomas A. (2002). Fats and fatty oils. Ullmann's Encyclopedia of Industrial Chemistry, Release, 6th Edition. www.foretcommunale-cameroun.org/. 2001 Normes environnementales et procédures d’inspection des installations industrielles et commerciales au Cameroun.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mountapmbeme-Kouotou, P., Laminsi, S., Acayanka, E. et al. Degradation of palm oil refinery wastewaters by non-thermal gliding arc discharge at atmospheric pressure. Environ Monit Assess 185, 5789–5800 (2013). https://doi.org/10.1007/s10661-012-2984-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2984-3