Abstract
Dissipation kinetics of λ-cyhalothrin in mineral and peat soils of Semongok (mineral soil) and Sibu (peat soil) farms was investigated in a laboratory incubation experiment under different temperature and moisture conditions at normal and double application dosages. The soil was spiked with λ-cyhalothrin at 5 and 25 µg/g soil, respectively. The soil moisture content was adjusted to 20, 40, and 60% of field capacity and then incubated in three climatic chambers at 15, 25, and 35 °C. Samples were collected at 0, 7, 21, 42, 70, and 105 days and analysed by Gas Chromatography-Electron Capture Detector (GC-ECD). Pesticides from the soil were extracted via a facile-modified QuEChERS method. Recovery studies of λ-cyhalothrin in mineral and peat soils were carried out at 0.05, 0.1, 0.5 and 1.0 µg/g fortification levels. The percentage of recovered amount was in the range of 81.4–95.0% and 81.3–86.5% for mineral and peat soils, respectively which falls within the acceptable recovery range of 70.0–120.0%. Factors i.e., soil carbon content, moisture, temperature, and applied dosage that render the degradation of λ-cyhalothrin in mineral and peat soils were evaluated. Findings showed that faster λ-cyhalothrin degradation took place in soil that contained low organic carbon content (< 12%), low soil moisture (≤ 20%) and incubated under higher temperatures (≤ 35 °C). Degradation of λ-cyhalothrin was described by first-order kinetics in both mineral and peat soils at various conditions. Half-lives of λ-cyhalothrin in mineral soil were shorter compared to peat soil. This is due to its lower carbon content and lower soil organic matter availability. This study provides significant information to the agriculture industry and farmers on the important factors such as soil properties, environmental conditions and application dosage that will influence the fate of pesticides in soil.
Graphical abstract

Article Highlights
-
1.
Soil with low organic carbon content and moisture in higher temperatures exhibited faster degradation of λ-cyhalothrin.
-
2.
The half-lives of λ-cyhalothrin in mineral soil are shorter than in peat soil.
-
3.
Lessen the risks to the environment and health from the excessive usage of λ-cyhalothrin in humid tropical and temperate countries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pesticide application is an indispensable component in agricultural management practices especially in vegetable cultivation [1]. The application of pesticides depends on various factors such as types of crops, target pests and diseases, the interval time between pathogenic attack and crop harvest as well as the pre-harvest interval prescribed for pesticide [2]. Major pest attacks such as insects and fungi could be curbed by applying pesticides to crops [3]. Pesticide application led to better management in crop production industries which in return has increased the economic benefits and reduced pest problems that perturb the crop production industries. Excessive usage of pesticides, however, leads to major environmental problems such as soil and groundwater contamination which endanger the overall ecosystem [4, 5]. The degradation rate of pesticides is one of the most critical factors that determine a pesticide's fate in the environment. The chemical components in pesticides are mineralized into basic mineral compounds in the environment [5]. The essential factors which will influence pesticides’ fate and behaviour in soil are pH, acidity and salinity, organic matter and clay particle contents, temperature, moisture and humidity, microbes, sunlight and field conditions [6,7,8,9,10]. The physicochemical properties of a pesticide [11] such as hydrophobicity, solubility, sorption capacity, volatility and polarity also contribute to its degradation rate in soil [12]. Higher pesticide degradation rates in tropical countries were reported to be due to higher amounts of rainfall, higher temperature and humidity all year round [13,14,15]. Warm and humid conditions with adequate annual sunlight may also increase the rate of pesticide degradation in the environment.
The toxicity of pesticides has prompted a thorough examination of its effects on the environment [16]. The introduction of safer pesticides with efficiency towards the proliferation of pests and low impact on the environment is the main global concern in modern agriculture. As a result, certain pesticides, such as dichlorodiphenyltrichloroethane (DDT), were banned due to their hazard to humans, animals, and aquatic environments [16, 17]. Previous studies on pesticides have been conducted on the effect of soil temperature and moisture under field and laboratory conditions for organophosphorus (OP), organochlorine (OC) and pyrethroids (Py) pesticides [18, 19]. Endosulfan (OC), acephate (OP), chlorpyrifos (OP), cypermethrin (Py), deltamethrin (Py), fenvalerate (Py) and cyfluthrin (Py) are among other most common pesticides reported in Malaysia. Py has become one of the widely used pesticides due to its greater knockdown effect on insects and low terrestrial vertebrate toxicity [20]. λ-Cyhalothrin is an insecticide that belongs to the group of Py pesticides. It is cheap, readily available in the market and the most commonly used Py in Malaysia. λ-Cyhalothrin is extensively used for agricultural purposes, especially in Sarawak [4] due to its robust effectiveness against a wide range of insect pests. To date, there is no degradation study has been conducted on λ-cyhalothrin degradation in Malaysian tropical soils despite their wide usage in agriculture. Therefore, it is important to determine the behaviour and fate of λ-cyhalothrin in tropical soils such as mineral and peat soils. λ-Cyhalothrin is a colourless solid or yellowish colour in liquid and its properties are reported in Table 1 [20, 21].
Soil components such as clay particles, amorphous mineral matter and soil organic matter are important parameters to control adsorption. This is because λ-cyhalothrin tends to adsorb into soil and sediment, and also the suspended in the water bodies [22]. A high Kow and mean of water-soil organic carbon partition coefficient (Koc) indicate a high potential of adsorption in water for sediment and soil that promotes soil sorption [12]. High Koc is associated with higher organic matter adsorption, which reduces the likelihood of water body contamination. In water bodies, degradation of λ-cyhalothrin is possible even though it has low solubility. Photo-degradation may occur in aqueous solution in the presence of sunlight. However, the chemical breakdown by sunlight may slow down the degradation rate in the adsorbed phases such as soil and sediments than the other soluble molecules of pesticides in water [12]. λ-Cyhalothrin degradation could result in a decrease in toxicity or an increase in toxicity [23]. λ-Cyhalothrin may degrade rapidly in soil under field conditions in 6–40 days. Nevertheless, the half-life of λ-cyhalothrin varied in the water body due to its hydrophobicity and strong adsorption in soil and sediment [24].
Studies on the degradation of various pesticides in tropical soils under laboratory conditions were carried out based on several environmental factors such as temperature, soil moisture and humidity, pH, soil texture and microbial activity [13,14,15]. By controlling these factors, the pesticide degradation rate and pathway in laboratory conditions can be compared to the field conditions in tropical regions as well as in temperate regions [17]. Other pesticides degradation studies in Malaysian tropical soils under laboratory conditions have been reported such as fenvalerate [15], acephate [13], cyfluthrin [11], and chlorpyrifos [14]. In temperate soils, λ-cyhalothrin has a half-life of 12–16 weeks [25]. The half-life of cypermethrin and λ-cyhalothrin in soils under laboratory conditions has been reported as 8–11 days and 6–7 days, respectively [26]. However, there is less evidence available on the fate and behaviour of pesticides used on cultivated organic soils such as peat and mineral soil.
In this study, we investigated the effects of temperature, moisture, application dosage, and soil carbon content on the degradation of λ-cyhalothrin in two types of soil, namely mineral and peat soils, as well as to determine their half-lives under controlled laboratory conditions (temperature and moisture content) and two application dosage. These findings are crucial to measure the potential risks and environmental impact due to increasing λ-cyhalothrin and other Py usage for agriculture at different temperatures and climatic conditions. Thus, Good Agriculture Practices Program (GAP) and remedial actions can be formulated to reduce environmental risks and health hazards from pesticide violations in agriculture sectors.
2 Materials and methodology
2.1 Reagents and chemicals
λ-Cyhalothrin (purity 98.2%) standard was obtained from Ehrenstorfer, Germany. Analytical and residue grades of acetonitrile, dichloromethane, n-hexane, glacial acetic acid, sodium chloride, and sodium sulfate were obtained from J.T. Baker. The silica gel was obtained from Merck. Pesticide stock solution (500 µg/g) was prepared by dissolving appropriate amounts of pesticide standard in n-hexane. An appropriate aliquot of stock solution was diluted with residue grade of n-hexane to make up standard solutions that contained 0.05, 0.1, 0.5, 1.0, 5.0, 10 and 50 µg/g of λ-cyhalothrin. The diluted stock solutions were used for recovery study in the method validation and batch studies.
2.2 Apparatus and instrument
Vortex-Genie model K-550-GE and IKA VORTEX GENIUS 3 were used to shake and homogenise soil extract. The extracted samples were centrifuged using a Thermo Jouan Model B4i multifunction centrifuge to obtain the supernatant. Soil samples were incubated in a Protech Model Cool-300 incubator (± 0.1 °C). An Agilent Model 6890 gas chromatography (GC) equipped with an electron capture detector (GC-ECD) was used for the determination of λ-cyhalothrin. This instrument was configured with a non-polar fused-silica capillary column, Ultra 1, 25 m × 0.32 mm and 0.5 µm, obtained from J&W Scientific, Folsom, California, USA and employing nitrogen gas as carrier gas at 1.2 mL min−1. The column temperature was maintained at 120 °C for 0.5 min, then programmed at 10 °C min−1 to 180 °C followed by another temperature ramp of 6 °C min−1 to 240 °C and subsequently 10 °C min−1 to 280 °C and held constant at 280 °C for 12 min. The injector and detector temperatures were maintained at 260 °C and 300 °C, respectively. The air and hydrogen gas flows were set at 80 mL min−1 and 67 mL min−1, respectively.
2.3 Soil characterization
Mineral and peat soils that are commonly found in Sarawak for vegetable cultivation were used in this study. The soils used in this study were collected from two vegetable farms. One from Semongok, Kuching (mineral soil) and another one from Sg Bidut, Sibu (peat soil) farms (Figure S1). Collected soil samples were air-dried for 5–7 days and sieved (2 mm) to remove stones, plant remnants and root residue. Water was added to the air-dried soils and left for a week to reactivate biological activity. Soil physiochemical characterization was determined by soil analysis procedures [14].
2.4 Method development and validation
In recovery studies, 10 g of mineral and peat soil samples were fortified with 1.5 mL of 0.5, 1.0, 5.0 and 10 µg/g of λ-cyhalothrin working standards to obtain 0.05, 0.1, 0.5 and 1.0 µg/g of λ-cyhalothrin following extraction. The samples were mixed homogeneously and left for 1 h to allow the solvent to evaporate and λ-cyhalothrin to interact with the sample. Each sample was prepared in three replicates. A blank sample was prepared for use as control. Recovery of λ-cyhalothrin was calculated following Eq. 1:
2.5 Degradation studies
Degradation kinetics of λ-cyhalothrin in mineral and peat soils were quantified using laboratory incubation experiments adapted with slight modification [13]. Ten grams of individual soil sample was weighed into a glass bottle. Each sample was prepared in three replicates with a control sample. For samples with initial concentrations of 5 and 25 µg/g, 1.5 mL of 50 µg/g and 250 µg/g of λ-cyhalothrin standard solutions were added to the soil and left for 30 min to allow the n-hexane to evaporate. The λ-cyhalothrin was mixed homogeneously with the rest of the soil. The samples were covered with aluminium foil, pricked on top to allow aeration and stored in a fixed-temperature incubator (± 0.1 °C) according to experimental conditions. The samples were collected at specific time intervals for further extraction and determination. The degradation of λ-cyhalothrin was studied concerning soil moisture (20, 40, and 60% of field capacity), temperature (15, 25, and 35 °C), application dose (5 and 25 g/g), and soil carbon content (low and high). Soil moisture content was adjusted to the desired moisture content of 20, 40, and 60% of the field capacity by adding an amount of water to air-dried soil according to Eq. 2:
Soil carbon content was classified as low (2.3% from Semonggok) and high (36.4% from Sg Bidut, Sibu). The soils were incubated in darkness and retrieved on days 0, 3, 7, 15, 25, 35, 55, 75, and 105 for analysis following the methodology described by earlier investigations on chlorpyrifos [14]. Water content in each bottle was monitored weekly so that the constant moisture content of soils could be maintained. All experiments were carried out in triplicates [13].
2.6 Pesticide extraction and analysis
λ-Cyhalothrin residues in soil were extracted and analyzed using a modified method known as ‘quick, easy, cheap efficient, rugged, and safe’ (QuEChERS) [27]. Acetonitrile containing 1% acetic acid (15 mL) was added into the homogenized soil samples (10 g) in a 50 mL Teflon centrifuge tube and shaken vigorously by hand followed by vortex mixing for 1 min. Anhydrous sodium sulfate (6 g) and sodium chloride (1.5 g) were added to the mixture. The sample was vortexed for 1 min and centrifuged at 3000 g for 1 min. The supernatant was transferred into a test tube and kept for clean-up. For a clean-up, 2 mL extract was transferred into a beaker and left in a fume hood to near dryness. The dry extract was mixed with hexane: dichloromethane (2 mL, 4:1 v/v) and transferred into the Pasteur pipette packed with silica gel (0.2 g). The extract was eluted with hexane: dichloromethane (2 mL, 1:1 v/v) at a flow of 1 mL min−1 and left to dry. After drying, hexane (2 mL) was added to the extract and transferred into a vial before GC-ECD determination.
2.7 Data analysis
The λ-cyhalothrin degradation data were modelled using a simple first-order model: Ct = C0e−kt where Ct is the λ-cyhalothrin concentration at time t, C0 is the initial concentration of λ-cyhalothrin, and k is the rate constant. Curve fitting was performed using a non-linear least squares regression analysis of λ-cyhalothrin concentration against time. The value of k was obtained by plotting graph ln C (concentration) against time. The half-life value, t1/2 was calculated using the formula, t1/2 = ln 2/k. All data were statistically analyzed with a two-factor analysis of variance (ANOVA) at the significance level of p ≤ 0.05.
3 Results and discussion
3.1 Soil characteristics
The characteristics of the soil have a significant role in understanding the environmental fate of λ-cyhalothrin. Peat and mineral soils were the two types of soils employed in this investigation. The soils were collected from two vegetable farms, one at Semongok (mineral soil) (1.3930° N, 110.3322° E) and another one at Sg. Bidut, Sibu (peat soil) farms (Latitude: 2.30179590595. Longitude: 111.804043114). Soil physiochemical determination is shown in Table 2. The pH of the soils was slightly acidic with a pH of 5.2–5.3, a typical soil pH in humid tropic regions [17]. Soil moisture was 27.0%, and 53.5% from fresh soil of Semongok and Sg. Bidut, respectively. The carbon content determined for mineral and peat soils was 2.3% and 36.4% of carbon, respectively. Peat soil has a higher percentage of carbon content due to the high decomposed organic matter content compared to mineral soil [28]. Semongok mineral soil has a high content of clay (23%). Clay content is also one of the important factors for pesticide dissipation in soil [13].
3.2 Method validation of λ-cyhalothrin in soils
Method validation is important to ensure that the proposed analytical method is accurate and reliable for research work. Additionally, it is a prerequisite for certification, an analytical requirement, and a confirmation of the method being considered about its capability’s consistency, accuracy, and precision. Accuracy and precision were estimated through recovery experiments (n = 3). The accuracy was expressed in the percentage of calculated recovery of the fortified sample. Acceptable mean recoveries are 70–120%, with relative standard deviation (RSD) ≤ 20%, for all compounds within the scope of a method [29, 30]. The limit of detection (LOD) and limit of quantification (LOQ) were estimated based on the signal of the background noise [27]. The level of noise was measured from the chromatograms of the lowest standard at a concentration of 0.05 µg/g. The LOD was calculated as three times the level of noise, and the LOQ was equal to ten times the noise level [13]. The LOD of λ-cyhalothrin was 0.002 µg/g and the LOQ was 0.02 µg/g.
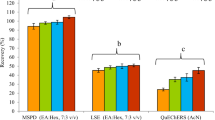
The recovery of λ-cyhalothrin spiked at different concentrations is shown in Table 3. The recoveries of λ-cyhalothrin in Semongok mineral soil at 0.05, 0.1, 0.5 and 1.0 µg/g ranged from 81.4–95.0% with RSD of less than or equal to 11%. While the recoveries of 81.3–86.5% were obtained for λ-cyhalothrin from Sg. Bidut peat soil at 0.05, 0.1, 0.5 and 1.0 µg/g with RSD of less than 11% (Table S1). The results showed that the accuracy of the method in this study was within the acceptable recovery range which complied with the guideline on the pesticide residue analysis that requires an acceptable range of 70–120% and RSD of ≤ 20% [29, 30] (Fig. 1).
A similar finding was also reported by Chai et al. (2014) in a method development study on Py using similar soils, which were mineral and peat soils with recoveries of λ-cyhalothrin ranging from 87.5–111.7% with RSD of less than 3% [27]. Meanwhile, for peat soil (carbon content of 45.3%), the recoveries of λ-cyhalothrin ranged from 89.5 to 101.5% with RSD of less than 4% were obtained. The recoveries of Py pesticides in mineral and peat soils were within the acceptable range and in agreement with the standard pesticide residue analysis guidelines [27]. Similar percentage recoveries of cyfluthrin were also reported within the acceptable range of 83–94% (RSD < 6) at 0.1 and 1.0 µg/g in three different mineral soils with low organic matter (0.55–1.65%) [11].
3.3 Degradation studies of λ-cyhalothrin in soils
3.3.1 Effect of soil carbon content on λ-cyhalothrin degradation
Several studies have been reported on λ-cyhalothrin environmental fate and degradation patterns in soils with varying carbon contents [26, 31]. The influence of soil organic matter content on pesticide fate and behavior in soils has been widely reported. However, information on the behavior and fate of pesticides applied to cultivated organic soil, such as peat soil is scarcely available [32]. Organic carbon and clay are important components in soil that could contribute to the environmental fate of pesticides [32]. Carbon content measured for mineral soil was the lowest with only 2% (Table S2-S5). The peat soil used in this study has a high carbon content which is 36%. The effect of soil carbon on the degradation of λ-cyhalothrin was studied in soils with varying carbon contents (low and high) under laboratory conditions. The degradation of λ-cyhalothrin in mineral and peat soil over time is illustrated in Fig. 2.
λ-Cyhalothrin exhibits an exponential degradation pattern in both soils (Fig. 3). Initial concentration on day 0 of λ-cyhalothrin in mineral (2% carbon) and peat (36% carbon) were 4.18 µg/g and 4.70 µg/g, respectively. On the 75 days, the concentration of λ-cyhalothrin decreased to 1.18 µg/g in mineral soil with 2% of carbon content and 2.41 µg/g in peat soil with 36% of carbon content, respectively (Figure S2).
The mineral soil with low carbon content showed a higher loss of λ-cyhalothrin compared to peat soil that contains higher organic carbon. This is because λ-cyhalothrin is less absorbed to any soil surface of mineral soil which provides more available sites for λ-cyhalothrin to undergo degradation [33]. The rapid loss of λ-cyhalothrin in soil with only 2% carbon content was also attributed to microbial degradation, where λ-cyhalothrin was available more in soil for microbes to utilize [34]. The degradation rate of λ-cyhalothrin in soil was in the order of low > high carbon content. The degradation rate of Py including λ-cyhalothrin residue in organic soil is always lower than in mineral soil due to pesticide adsorption to soil organic matter [32]. The hydrophobic characteristic of λ-cyhalothrin led to its high affinity towards peat soil [6] with high (36%) carbon contents. It was reported that the persistence of pesticides in surface horizons is higher in organic peat soil [32]. Additionally, pesticide residues stay longer in peat soil containing more than 40% carbon compared to mineral soil that contains a lower amount of organic matter [27].
The degradation of λ-cyhalothrin followed the first-order kinetics. The regression coefficient (r2) value obtained was higher than 0.95 and the degradation rate coefficient (k) ranged from 0.090 to 0.186 day−1 (Table 4) for the two soils. Degradation of λ-cyhalothrin was observed to be faster in soil with low carbon content compared to the high carbon content. The half-lives significantly increased from low to high soil carbon content and varied from 3.7 to 7.7 days (Table 4). The rates of λ-cyhalothrin degradation were in order of low > high carbon content. That means peat soils with high carbon content are likely to contain more λ-cyhalothrin residue compared to mineral soil with low carbon content. A similar finding was also reported by Chai et al. (2014) where peat soil generally contains more pesticide residues as compared to mineral soil.
λ-Cyhalothrin persisted longer in soil with higher carbon content. Soil with the least carbon content showed a 1.8–2.1 times faster degradation rate of λ-cyhalothrin. As the percentage of carbon content in soil increased, the degradation rate observed for λ-cyhalothrin decreased. This indicated that the soil carbon content could adsorb λ-cyhalothrin readily, thus inhibit the degradation of λ-cyhalothrin in soil [27].
3.3.2 Effect of temperature on λ-cyhalothrin degradation in mineral and peat soils
The effect of temperature on the degradation of λ-cyhalothrin in mineral and peat soils has also been investigated (Table S6-S12 & Figure S3, S4). λ-Cyhalothrin degraded exponentially with time in mineral and peat soils (Fig. 3A, B). For mineral soil, the concentration of λ-cyhalothrin was reduced consistently until day 105 with a comparable degradation rate of 3.57 µg/g followed by 3.42 µg/g and 3.69 µg/g at 15 °C, 25 °C and 35 °C, respectively in mineral soil. λ-Cyhalothrin has degraded in peat soil by 1.97 µg/g, and 3.34 µg/g at 15 °C, 25 °C and 35 °C, respectively.
The initial concentration of λ-cyhalothrin was reduced rapidly in mineral soil due to a higher number of active soil microorganisms that facilitate the breakdown of λ-cyhalothrin [35]. Evaluation of peat soil showed that the initial residue loss of λ-cyhalothrin was comparable to mineral soil at 25 °C and 35 °C. Figure 3B showed rapid degradation of λ-cyhalothrin in peat soil at 25 and 35 °C. However, slower degradation was observed at 15 °C. Slower degradation degradation was observed at 15 °C. Generally, lower temperature leads to a slower degradation rate [36]. The high persistency of λ-cyhalothrin in peat soil at 15 °C may be due to the pesticide aging in the redistribution of chemicals whether from weaker to stronger adsorption sites or slow sequestration between λ-cyhalothrin and soil organic matter such as organic carbon [37].
At 25 °C, λ-cyhalothrin showed a lower amount of reduction compared to 15 °C and 35 °C in the mineral soil. This phenomenon could be due to the increasing amount of non-extractable residue of λ-cyhalothrin in the soil as the temperature increased to 25 °C [36]. As the temperature increased further to 35 °C, the degradation became more rapid. There is a possibility that volatilization of λ-cyhalothrin occurred from the soil surface as a result of vapour pressure (> 10–6 mm Hg at 25 °C) increment with the increasing temperature [1, 7, 15].
In this study, the degradation of λ-cyhalothrin was faster in mineral soil compared to peat soil. The percentage of λ-cyhalothrin losses from mineral soil throughout 105 days was 76.8% (15 °C), 82.0% (25 °C), and 86.4 (35 °C). From this figure, it can be inferred that λ-cyhalothrin degradation in mineral soil was very pronounced at 35 °C. A similar observation was reported for the degradation of other pesticides such as acephate in the same mineral soil used in this study [13]. In contrast, the total loss of λ-cyhalothrin in peat soil until 105 days was less than in mineral soil. The amount of λ-cyhalothrin loss was 42.9%, 75.1% and 72.3% at 15 °C, 25 °C and 35 °C, respectively. The increasing temperature (25–35 °C) also contributed to the fast reduction of λ-cyhalothrin in peat soil (> 70%) [15]. From this study, it was observed that overall degradation rates were faster at higher temperatures (25–35 °C) for both soils. However, a slower rate was observed in peat soil probably due to the high carbon content which contributed to the strong adsorption and thus retention of pesticides causing the residue to persist longer in the soil [27].
3.3.3 Half-lives of λ-cyhalothrin degradation in mineral and peat soils at different temperature
The reported half-life and dissipation time of most pesticides in the field were generally shorter in the tropics with higher surrounding temperatures compared to temperate regions with lower surrounding temperatures [8, 17]. This affirmed the findings from the current study where higher temperature resulted in faster degradation of λ-cyhalothrin in both mineral and peat soil. The degradation of λ-cyhalothrin in mineral soil was fitted into the first-order kinetics, with a regression coefficient (r2) of more than 0.93. The degradation rate coefficient (k) of λ-cyhalothrin ranged from 0.164 to 0.223 day−1. The half-lives obtained in mineral soil at 15 °C, 25 °C and 35 °C decreased from 4.2 to 2.8 days, respectively (Table 5). The differences in the half-lives were only 1.3–1.4 times higher at 15 °C compared to 25 °C and 35 °C.
Peat soil exhibits a similar degradation pattern to mineral soil and the degradation also fits the first-order kinetics, where the regression coefficient (r2) values were more than 0.84. The degradation rate coefficient (k) varied from 0.065 to 0.140 day−1. Half-lives of λ-cyhalothrin in peat soil at 15 °C, 25 °C and 35 °C were 10.7, 5.0, and 5.8 days, respectively (Table 5). Peat soil had the longest half-life of λ-cyhalothrin at lower temperatures (15 °C). A similar study reported for other pesticides, florasulam in soil also showed slower degradation at lower temperatures (10–20 °C) [36]. It can be concluded that degradation of λ-cyhalothrin in mineral and peat soils was faster at 25 and 35 °C compared to 15 °C. Overall half-lives of λ-cyhalothrin obtained for the soils were in the order of 15 °C > 25 °C > 35 °C. A similar finding was reported for the degradation of acephate with the highest reduction of concentration in tropical soils, which generally has higher temperature [13]. Increasing temperature accelerated the degradation rate of λ-cyhalothrin which resulted in its shorter half-life. Another similar study was also reported on the degradation of fenvalerate insecticide (Py) under laboratory conditions at 30–35 °C. It showed a significant reduction of half-lives with increasing temperature of 5 °C in peat, sandy clay and sandy clay loam soils [15]. The half-life of fenvalerate was also reported to be longer in peat soil compared to sandy clay and sandy clay loam soils. A shorter half-life of fenvalerate insecticide was obtained for the three soils as the temperature increased to 35 °C [15].
In this study, the half-life and degradation trend for λ-cyhalothrin in soils obtained at 15 °C was comparable to those pesticide studies reported for temperate climates [38]. The overall pesticide degradation is significantly faster in tropical soils than in temperate soils due to different climatic conditions and soil physicochemical properties [15]. Moreover, higher temperatures under tropical climates could be more favorable conditions for the enhancement of pesticide degradation in soil [17].
3.3.4 Effect of moisture level on λ-cyhalothrin degradation in mineral and peat soils
The effects of soil properties and temperature on the degradation of λ-cyhalothrin in mineral and peat soils were further investigated by introducing different moisture levels. In this study, soil moisture level was adjusted to 20%, 40% and 60% of field capacity to simulate the conditions as in field condition. The degradation patterns of λ-cyhalothrin in mineral and peat soils were observed at three different soil moisture levels.
Figure 4A, B showed that λ-cyhalothrin degraded exponentially over time in mineral and peat soils. The initial residue of λ-cyhalothrin at day 0 in mineral soil was 4.18 µg/g, 4.48 µg/g and 4.57 µg/g at 20, 40 and 60% soil moisture, respectively. For peat soil, the initial residue was 4.45 µg/g, 4.71 µg/g and 4.76 µg/g at 20%, 40% and 60% soil moisture, respectively. The initial residue of λ-cyhalothrin in mineral soil decreased by 3.42 µg/g followed by 3.04 µg/g and 3.15 µg/g at 20, 40 and 60% soil moisture, respectively (Table S13-S19). In contrast, λ-cyhalothrin residue decreased by 3.34 µg/g in the peat soil at soil moisture of 20% followed by 1.37 µg/g and 1.41 µg/g at soil moisture of 40 and 60%, respectively (Figure S5 and S6). Mineral and peat soils showed comparable degradation trends at 20% moisture level. However, as the moisture level increased from 40 to 60%, the degradation pattern observed was a little bit different for peat soil. At 20% soil moisture the degradation of λ-cyhalothrin was rapid in both mineral and peat soils. The amount of λ-cyhalothrin residue declined steadily at 40% and 60% moisture in mineral soil while in peat soil residue, the concentration declined at a very slow rate. The slower rate of λ-cyhalothrin degradation was observed in peat soil as the moisture increased. This phenomenon could be due to the flooded soil condition with soil moisture as high as 60% [13]. The flooded soil may cause the inactivation of soil microbial activities that could inhibit the breakdown reaction of λ-cyhalothrin in soil [14]. Soil moisture is an important factor in the soil environment affecting soil microbes’ activities in enhancing λ-cyhalothrin degradation in soils [8, 10].
The soil moisture and humidity in the peat soil are higher than in the mineral soil. Higher moisture content in peat soil may reduce the degradation rate of λ-cyhalothrin. This is due to the λ-cyhalothrin's non-polar nature and hydrophobic properties, which make them susceptible to sequestration, a delayed sorption mechanism [37]. The mechanism may reduce bio-availability of λ-cyhalothrin thus inhibiting continuous degradation in soils [39, 40]. The degradation rate is directly correlated to soil properties and λ-cyhalothrin interaction with soil [31]. λ-Cyhalothrin has low water solubility (0.005 mg/L) (Table 1) and degrades faster in 20% moisture of soil mainly due to its hydrophobicity [41]. This hydrophobic property could uphold strong adsorption to soil particles and cause more formation of bound residue of λ-cyhalothrin in the soil.
λ-Cyhalothrin degrades rapidly in mineral soil. Overall λ-cyhalothrin residue loss in mineral soil at 20%, 40% and 60% of soil moisture were 82%, 67.9% and 68.9%, respectively. Similarly, at 20% soil moisture level, λ-cyhalothrin residue in peat soil decreased by 75.1%. However, at 40% and 60% soil moisture levels, only a minute amount of residue loss was observed. This accounted for only 29.1% and 32.2% of residue loss at 40% and 60% soil moisture level % respectively. From these observations, it can be inferred that in both minerals and peat soil, λ-cyhalothrin degradation occurs more rapidly at 20% soil moisture level compared to 40% and 60%. This showed that the degradation of λ-cyhalothrin in soils was significantly affected by soil moisture levels. Differences in soil moisture also seem to have more impact on the degradation of pesticides than soil temperature, especially for the degradation of λ-cyhalothrin in peat soil [10]. A similar finding was reported on the effect of soil moisture of 30%, 60% and 90% on the herbicide, pendimethalin which showed higher persistency with increasing moisture levels in soils [38].
3.3.5 Half-lives of λ-cyhalothrin degradation in mineral and peat soils at different moisture
The degradation of λ-cyhalothrin in soil with moisture of 20%, 40% and 60% followed the first-order kinetics. Mineral soil showed a good regression coefficient (r2) value of more than 0.95 and the degradation rate coefficients (k) varied from 0.130–0.207 day−1. Meanwhile, the regression coefficient (r2) values of peat soil were higher than 0.81. The degradation rate coefficients (k) of λ-cyhalothrin were ranged from 0.035–0.139 day−1 (Table 6).
Half-lives of λ-cyhalothrin obtained in mineral soil at 20%, 40% and 60% moisture contents were slightly increased i.e., 3.4, 5.3 and 4.7 days, respectively. This indicated that they were not greatly affected by different levels of soil moisture with a slight increase of 1.4–1.6 times. Conversely, the increase of half-lives obtained in peat soil were 5.0, 16.1, and 19.7 days at 20%, 40% and 60% moisture contents, respectively (Table 6). The fastest λ-cyhalothrin degradation was in the soil with the least moisture, while the slowest degradation was in the soil with higher moisture. As a result, the degradation of λ-cyhalothrin in mineral soil was comparable as it only increased by 1.6–1.4 times when the soil moisture increased. In contrast, the half-lives of λ-cyhalothrin increased 3.2–3.9 times higher in peat soil with increasing soil moisture. The overall rate of removal observed for λ-cyhalothrin residue in soils was in the order of 20% > 40% > 60%. Accumulation of λ-cyhalothrin residue in peat soil could cause longer persistence of λ-cyhalothrin due to strong adsorption by organic carbon [27, 42].
Moisture can be considered as a hydrolytic agent in soil [7]. The nature and properties of λ-cyhalothrin are highly hydrophobic and insoluble in water which is closely correlated to the effect of soil moisture [40]. The pesticide itself reacts in the soil depending on the soil's physicochemical properties, soil characteristics and environmental parameters. λ-Cyhalothrin has higher octanol–water partitioning, Kow value of 7.0 (Table 1) compared to other synthetic Pys such as cypermethrin (6.6) and permethrin (6.1). The higher the Kow value of a pesticide, the more hydrophobic is its nature [32]. Thus, the hydrophobic pesticide easily binds to soil organic carbon because it is the only soil constituent with a hydrophobic character.
3.3.6 Effect of application dosage on λ-cyhalothrin degradation in mineral and peat soils
Although temperature and moisture in soils may have some impact on the degradation of λ-cyhalothrin, it is also important to examine the amount of λ-cyhalothrin applied to the soil. This is owing to the possibility that significant contamination on the site resulted from repeated applications at a greater rate and soil accumulation of pesticide residue [1, 8]. The impact of large pesticide application dosages could endanger soil ecosystems and pose a high toxicity risk to nearby organisms [8]. Furthermore, there is a paucity of information regarding the repeated use of different pesticides in typical tropical soils.
In this work, the degradation of λ-cyhalothrin in different types of soils was assessed at 5 g/g and 25 g/g. Figure 5A, B showed that λ-cyhalothrin degraded exponentially with time in mineral and peat soils. The degradation of λ-cyhalothrin showed a steady decline in mineral soil with proximity of the amount reduced by 3.42 µg/g and 3.61 µg/g at 5 and 25 µg/g, respectively (Table S20-S26). The initial concentration of λ-cyhalothrin was also observed in the peat soil and decreased by 3.34 µg/g and 2.34 µg/g at 5 and 25 µg/g, respectively (Figure S7 and S8).
At lower concentrations of λ-cyhalothrin (5 µg/g), mineral soil showed a faster-decreasing amount of residue for the entire degradation curve than peat soil. However, a five-fold increase in dosage (25 g/g) of λ-cyhalothrin had no impact on the rate of degradation in mineral soil [8]. When the soil is exposed to different levels of pesticide concentration, there will be an increasing growth of soil bacteria that are sensitive to high concentrations [34]. A lower concentration of λ-cyhalothrin applied on peat soil showed a faster degradation rate. The effect was more pronounced at low application dosage (5 µg/g). At high application dosage (25 µg/g), soil microorganisms utilized λ-cyhalothrin at the same rate, although much slower, due to the high concentration level of λ-cyhalothrin in soil. Higher λ-cyhalothrin concentration levels needed a longer time to degrade in peat soil. These are correlated to the efficiency and adaptability of soil microbes to degrade λ-cyhalothrin after being exposed to the different application dosages with the ability to adapt the reaction with the chemicals [39]. A similar finding was also reported on fipronil where a higher concentration applied on fresh soil could affect the soil microorganism’s activity resulting in slower degradation [39].
The application dosage showed no significant difference in the λ-cyhalothrin degradation in mineral and peat soils. Although higher spiking levels of λ-cyhalothrin may be highly toxic to soil microorganisms in the soils, the sorption of λ-cyhalothrin to clays or organic matter content may not appear to influence the degradation [14]. Concentration levels have little effect on the degradation rates of acephate and monocrotophos in tropical soils [13]. Reddy et al. (2013) reported that pyraclostrobin showed a proximity of the half-lives obtained for two different fortification levels which implied that fortification level did not influence the rate of dissipation of pyraclostrobin in soil [43]. λ-Cyhalothrin showed better degradation capability and availability in mineral soil than peat soil. This is attributable to the fact that the residue level was still high despite a 75.1% and 56.4% drop, respectively, at day 105. There was no significant difference between the soils and different application dosages. It can be summarized that the degradation of λ-cyhalothrin in mineral soil and peat soil were comparable for both application dosages.
3.3.7 Half-lives of λ-cyhalothrin degradation in mineral and peat soils at different rates of application
There were only slight differences observed for λ-cyhalothrin's half-lives in both soils at 5 µg/g and 25 µg/g application rates as previously mentioned. Degradation of λ-cyhalothrin fitted the first-order kinetics (Table S22) as well with regression coefficient (r2) > 0.87 while the rate coefficient (k) values varied in the range of 0.123–0.207 day−1 (Table 7).
The half-lives of λ-cyhalothrin obtained for mineral soil at 5 µg/g and 25 µg/g were 3.4 and 3.6 days, respectively. On the other hand, the half-lives of λ-cyhalothrin in peat soil obtained for both concentrations were slightly longer but comparable (Table 7). A higher amount of pesticide application on organic soils contributes to a slight delay in degradation rate due to the different soil properties in peat [32]. Therefore, no significant difference in λ-cyhalothrin half-life was observed in mineral and peat soils. The proximity of the half-lives observed for various pesticide application dosages showed that the larger pesticide treatment dosage (5 times) did not significantly impact the degradation rate of λ-cyhalothrin in soils. The possibility of soil matrix existing in the soils could also influence microbial degradation. Soil matrix promotes high sensitivity to chemical substances such as λ-cyhalothrin and rapid mineralization by soil microbes [39]. Similar studies also reported on the degradation of chlorpyrifos with only a slight increase of half-lives (1–2 times) [14]. While no significant differences of acephate's half-lives in humid tropical soils applied at similar application rates [13]. However, the half-life of λ-cyhalothrin under laboratory conditions would likely have a significant difference compared to field studies. Photodegradation is limited under laboratory conditions. Photodegradation of pesticides on soil surfaces in field studies is a key factor for faster dissipation and degradation due to the formation of by-products to reduce the toxic effects of pesticides. In field studies, the shorter half-life reported for λ-cyhalothrin degradation in tropical soils without crops was 5 days [35, 44]. For the other Py, the half-life of cypermethrin in soil was 5.6–7.6 days.
4 Conclusion
Factors such as soil carbon content, soil moisture level, surrounding temperature and application dosage that render the degradation of λ-cyhalothrin in mineral and peat soils were evaluated. From this study, soils having a lower percentage of organic carbon content (< 12%), low moisture level (≤ 20%) and kept under higher temperatures (≤ 35 °C) led to rapid degradation of λ-cyhalothrin. Degradation of λ-cyhalothrin was described by first-order kinetics in both mineral and peat soils for all factors tested. Half-lives of λ-cyhalothrin in mineral soil were shorter compared to peat soil. Soil with higher organic carbon content such as peat soil led to longer half-lives of λ-cyhalothrin. This was inferred that strong adsorption of λ-cyhalothrin onto soil organic matter hindered λ-cyhalothrin microbial degradation by limiting its bioavailability to soil microbes. λ-Cyhalothrin degradation in soils was accelerated at higher temperatures as the increasing temperature promotes higher microbial activity in the soils as well as other degradation processes. Higher soil moisture content decreased the degradation rate of λ-cyhalothrin in mineral and peat soils. The findings obtained from this laboratory incubation study are significant in providing knowledge and information to the farmers and the pesticide user on factors that may impact the degradation rate of a pesticide, especially λ-cyhalothrin in mineral and peat soils under humid tropical and temperate countries. Thus, the issue of λ-cyhalothrin contamination in the environment can be avoided. These findings can also be used as input for GAP programs to lessen the risks to the environment and health caused by the excessive usage of λ-cyhalothrin. Although the degradation of pesticides studies have been done worldwide by many researchers, much work is still needed to fully understand the degradation pattern of specific pesticides and surrounding factors and conditions that could inhibit their degradation following their application to soils. The application dosage of pesticides needs to be carefully considered, considering the specific pesticide type and soil characteristics, to minimize risks to the environment and human health.
Data availability
All experimental data generated in this study are available on request to the corresponding author if required.
References
Chai L-K, Mohd-Tahir N, Bruun Hansen HC. Dissipation of acephate, chlorpyrifos, cypermethrin and their metabolites in a humid-tropical vegetable production system: dissipation of insecticides in a tropical vegetable system. Pest Manag Sci. 2009;65:189–96. https://doi.org/10.1002/ps.1667.
Chai L-K, Zaidel N-D, Hansen HCB. A rapid multi-residue method for the determination of pesticide residues in choi sum, yardlong beans and aubergines. Food Chem. 2012;131:611–6. https://doi.org/10.1016/j.foodchem.2011.09.037.
Zhang Z-Y, Liu X-J, Yu X-Y, et al. Pesticide residues in the spring cabbage (Brassica oleracea L. var. capitata) grown in open field. Food Control. 2007;18:723–30. https://doi.org/10.1016/j.foodcont.2006.04.001.
Mattah MM, Mattah PAD, Futagbi G. Pesticide application among farmers in the catchment of Ashaiman irrigation scheme of Ghana: health implications. J Environ Public Health. 2015;2015:1–7. https://doi.org/10.1155/2015/547272.
Liyanage JA, Watawala RC, Mallawatantri AP, et al. Degradation of 14C ring labeled pesticides in selected soils of Sri Lanka. J Radioanal Nucl Chem. 2007;272:477–81. https://doi.org/10.1007/s10967-007-0607-1.
Shahgholi H, Ahangar AG. Factors controlling degradation of pesticides in the soil environment: a review. Agric Sci Dev. 2014;3:273–8.
Frank MP, Graebing P, Chib JS. Effect of soil moisture and sample depth on pesticide photolysis. J Agric Food Chem. 2002;50:2607–14.
Ciglasch H, Busche J, Amelung W, et al. Insecticide dissipation after repeated field application to a northern Thailand Ultisol. J Agric Food Chem. 2006;54:8551–9. https://doi.org/10.1021/jf061521u.
Mukherjee I. Influence of organic amendments on the degradation of endosulfan. Bull Environ Contam Toxicol. 2012;89:334–9. https://doi.org/10.1007/s00128-012-0676-x.
Ngigi A, Dörfler U, Scherb H, et al. Effect of fluctuating soil humidity on in situ bioavailability and degradation of atrazine. Chemosphere. 2011;84:369–75. https://doi.org/10.1016/j.chemosphere.2011.03.068.
Choo LY, Ismail BS, Salmijah S, Halimah M. Persistence of cyfluthrin in three Malaysian agricultural soils under laboratory conditions. J Environ Biol. 2013;34:957–61.
He L-M, Troiano J, Wang A, Goh K. Environmental chemistry, ecotoxicity, and fate of lambda-cyhalothrin. In: Whitacre DM, editor. Reviews of environmental contamination and toxicology. New York: Springer; 2008. p. 71–91.
Chai L-K, Wong M-H, Mohd-Tahir N, Hansen HCB. Degradation and mineralization kinetics of acephate in humid tropic soils of Malaysia. Chemosphere. 2010;79:434–40. https://doi.org/10.1016/j.chemosphere.2010.01.046.
Chai L-K, Wong M-H, Bruun Hansen HC. Degradation of chlorpyrifos in humid tropical soils. J Environ Manage. 2013;125:28–32. https://doi.org/10.1016/j.jenvman.2013.04.005.
Ismail BS, Maznah Z. Persistence of fenvalerate in three malaysian agricultural soils. Bull Environ Contam Toxicol. 2005;75:789–96. https://doi.org/10.1007/s00128-005-0820-y.
Porto ALM, Melgar GZ, Kasemodel MC, Nitschke M. Biodegradation of pesticides. In: Pesticides in the modern world - pesticides use and management. Stoytcheva M.(Ed.)//Tech. 2011;1:407–38.
Racke KD, Coats JR, American Chemical Society. Enhanced biodegradation of pesticides in the environment. Washington, DC: American Chemical Society; 1990.
Ngaini Z, Henry MC, Chai L-K, Farooq S. Chlorothalonil dissipation as influenced by growing season and cultivation systems on green mustard and soil. Chemistry Africa. 2022;5:1127–38. https://doi.org/10.1007/s42250-022-00384-7.
Henry MC, Ngaini Z, Chai L-K, Farooq S. The impact of growing season and cultivation systems on the dissipation of profenofos and λ-cyhalothrin on Brassica juncea: a comparative study. SN Appl Sci. 2023;5:283. https://doi.org/10.1007/s42452-023-05517-2.
Schleier JJ III, Peterson RKD. CHAPTER 3. Pyrethrins and pyrethroid Insecticides. In: Lopez O, Fernandez-Bolanos J, editors. Green chemistry series. Cambridge: Royal Society of Chemistry; 2011. p. 94–131.
Xie J, Wang P, Liu J, et al. Photodegradation of lambda-cyhalothrin and cypermethrin in aqueous solution as affected by humic acid and/or copper: Intermediates and degradation pathways. Environ Toxicol Chem. 2011;30:2440–8. https://doi.org/10.1002/etc.655.
Ali MA, Baugh PJ. Sorption–desorption studies of six pyrethroids and mirex on soils using GC/MS-NICI. Int J Environ Anal Chem. 2003;83:923–33. https://doi.org/10.1080/03067310310001608759.
Villaverde J, Kah M, Brown CD. Adsorption and degradation of four acidic herbicides in soils from southern Spain. Pest Manag Sci. 2008;64:703–10. https://doi.org/10.1002/ps.1545.
Jurisic AD, Petrovic AP, Rajkovic DV, Nicin SD. The application of lambda-cyhalothrin in tick control. Exp Appl Acarol. 2010;52:101–9. https://doi.org/10.1007/s10493-010-9346-z.
Garcia M, Römbke J, de Brito MT, Scheffczyk A. Effects of three pesticides on the avoidance behavior of earthworms in laboratory tests performed under temperate and tropical conditions. Environ Pollut. 2008;153:450–6. https://doi.org/10.1016/j.envpol.2007.08.007.
Liu T, Sun C, Ta N, et al. Effect of copper on the degradation of pesticides cypermethrin and cyhalothrin. J Environ Sci. 2007;19:1235–8. https://doi.org/10.1016/S1001-0742(07)60201-0.
Chai L-K, Elie F, Jinang C. Determination of 24 pesticides residues in mineral and peat soils by modified QuEChERS method and gas chromatography. Int J Environ Anal Chem. 2014;94:519–30. https://doi.org/10.1080/03067319.2013.871713.
Adon R, Bakar I, Wijeyesekera DC, Zainorabidin A. Overview of the sustainable uses of peat soil in Malaysia with some relevant geotechnical assessments. Int J Integr Eng. 2012;4:4.
SANCO (2011) Guidance document on analytical quality control and validation procedures for pesticide residues analysis in food and feed. European commission health & consumer protection directorate-general
Pihlström T, Fernández-Alba AR, Gamón M, et al. Analytical quality control and method validation procedures for pesticide residues analysis in food and feed. Sante. 2017;11813:21–2.
Rodríguez-Liébana JA, Mingorance MD, Peña A. Sorption of hydrophobic pesticides on a Mediterranean soil affected by wastewater, dissolved organic matter and salts. J Environ Manage. 2011;92:650–4. https://doi.org/10.1016/j.jenvman.2010.10.009.
Fortin J. Fate of pesticides in organic soils. In: Parent LE, Ilnicki P, editors. Organic soils and peat materials for sustainable agriculture. Boca Raton: CRC Press; 2003. p. 151–73.
Lu JLDP. Multipesticide residue assessment of agricultural soil and water in major farming areas in Benguet, Philippines. Arch Environ Contam Toxicol. 2010;59:175–81. https://doi.org/10.1007/s00244-010-9478-5.
Cycoń M, Piotrowska-Seget Z, Kaczyńska A, Kozdrój J. Microbiological characteristics of a sandy loam soil exposed to tebuconazole and λ-cyhalothrin under laboratory conditions. Ecotoxicology. 2006;15:639–46. https://doi.org/10.1007/s10646-006-0099-8.
Laabs V, Amelung W, Pinto AA, et al. Pesticides in surface water, sediment, and rainfall of the Northeastern Pantanal Basin, Brazil. J Environ Qual. 2002;31:1636–48. https://doi.org/10.2134/jeq2002.1636.
Krieger MS, Pillar F, Ostrander JA. Effect of temperature and moisture on the degradation and sorption of florasulam and 5-hydroxyflorasulam in soil. J Agric Food Chem. 2000;48:4757–66. https://doi.org/10.1021/jf000009k.
Gevao B, Semple KT, Jones KC. Bound pesticide residues in soils: a review. Environ Pollut. 2000;108:3–14. https://doi.org/10.1016/S0269-7491(99)00197-9.
Swarcewicz MK, Gregorczyk A. The effects of pesticide mixtures on degradation of pendimethalin in soils. Environ Monit Assess. 2012;184:3077–84. https://doi.org/10.1007/s10661-011-2172-x.
Masutti CSM, Mermut AR. Degradation of fipronil under laboratory conditions in a tropical soil from sirinhaém pernambuco, Brazil. J Environ Sci Health B. 2007;42:33–43. https://doi.org/10.1080/03601230601017981.
Oudou HC, Alonso RM, Bruun Hansen HC. Voltammetric behaviour of the synthetic pyrethroid lambda-cyhalothrin and its determination in soil and well water. Anal Chim Acta. 2004;523:69–74. https://doi.org/10.1016/j.aca.2004.03.069.
Mekebri A, Crane DB, Blondina GJ, et al. Extraction and analysis methods for the determination of pyrethroid insecticides in surface water, sediments and biological tissues at environmentally relevant concentrations. Bull Environ Contam Toxicol. 2008;80:455–60. https://doi.org/10.1007/s00128-008-9382-0.
Anwar T, Ahmad I, Tahir S. Determination of pesticide residues in soil of Nawabshah District, Sindh, Pakistan. Pakistan J Zool. 2012;44:87–93.
Reddy SN, Gupta S, Gajbhiye VT. Effect of moisture, organic matter, microbial population and fortification level on dissipation of pyraclostrobin in Soils. Bull Environ Contam Toxicol. 2013;91:356–61. https://doi.org/10.1007/s00128-013-1045-0.
Laabs V, Amelung W, Pinto A, et al. Leaching and degradation of corn and soybean pesticides in an Oxisol of the Brazilian Cerrados. Chemosphere. 2000;41:1441–9. https://doi.org/10.1016/S0045-6535(99)00546-9.
Acknowledgements
The authors would like to thank Universiti Malaysia Sarawak and the Ministry of Education for the research fund. The authors also wish to acknowledge Philip Gudom, Winnie Rosime and Tay Guan Tong from the Pesticides Residue Laboratory for technical assistance.
Funding
This work was supported by the Ministry of Higher Education Malaysia, fundamental research grant scheme, FRGS/1/2019/STG01/UNIMAS/01/1.
Author information
Authors and Affiliations
Contributions
Experimental work was performed by C.R., and M.C.H. The Manuscript was written and edited by Z.N., S.F., M.C.H and A.C. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This article does not contain any of the authors’ research on human participants or animals.
Consent for publication
The authors agree to publish this article.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Generative AI in scientific writing
The application dosage of pesticides needs to be carefully assessed by considering the specific pesticide type and soil characteristics to minimize risks to the environment and human health.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ngaini, Z., Rudy, C., Farooq, S. et al. Differential degradation dynamics of λ-cyhalothrin in mineral and peat soils: a comparative study under laboratory condition. Discov Appl Sci 6, 78 (2024). https://doi.org/10.1007/s42452-024-05712-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05712-9