Abstract
The quality of the water in a uranium-ore-mining area located in Caldas (Minas Gerais State, Brazil) and in a reservoir (Antas reservoir) that receives the neutralized acid solution leaching from the waste heaps generated by uranium mining was investigated. The samples were collected during four periods (October 2008, January, April and July 2009) from six sampling stations. Physical and chemical analyses were performed on the water samples, and the data obtained were compared with those of the Brazilian Environmental Standards and WHO standard. The water samples obtained from waste rock piles showed high uranium concentrations (5.62 mg L−1), high manganese values (75 mg L−1) and low average pH values (3.4). The evaluation of the water quality at the point considered the limit between the Ore Treatment Unit of the Brazilian Nuclear Industries and the environment (Consulta Creek) indicated contamination by fluoride, manganese, uranium and zinc. The Antas reservoir showed seasonal variations in water quality, with mean concentrations for fluoride (0.50 mg L−1), sulfate (16 mg L−1) and hardness (20 mg L−1) which were low in January, evidencing the effect of rainwater flowing into the system. The concentrations for fluoride, sulfate and manganese were close or above to the limits established by current legislation at the point where the treated mining effluent was discharged and downstream from this point. This study demonstrated that the effluent discharged by the UTM affected the quality of the water in the Antas reservoir, and thus the treatments currently used for effluent need to be reviewed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contamination of the ecosystem resulting from anthropogenic activities is a major ecological concern. In particular, mining activities involve great environmental modifications of the landscape outline, chemistry and biology, and abandoned or inactive mines are of particular concern and require continuous control and monitoring (Oliveira and Ávila 2001). Uranium mines, in particular, produce large amounts of tailings with a potentially negative impact, resulting from both the abandoned radioactive material and high concentrations of highly toxic heavy metals (Lozano et al. 2002; Neves and Matias 2008). Moreover, improper disposal of the mill tailings during the early decades of uranium mining led to substantial contamination of the soils, groundwater and surface water (Abdelouas 2006).
Some studies have reported the impact of uranium mining on water quality. An assessment of the environmental impacts resulting from the uranium production facilities in Cunha Baixa (Portugal) indicated sulfate, manganese and uranium as the main contaminants in the surface water downstream from the mine sites (Neves and Matias 2004). Heavy metal contamination of Lake Dongting by uranium mining activities (China) was described by Qian et al. (2005), and similarly, Muscatello et al. (2008) indicated elevated concentrations of selenium in the water, sediments and biota of lakes downstream from uranium mining and milling operations in Canada. In uranium mining and milling areas in the territory of South Yakutia (Russia), Chevychelov et al. (2010) reported contamination of the surface water by radionuclides and other toxic components at a short distance from the radioactive refuse rock dumps.
According to Sarmiento et al. (2009), predicting the impact of an emission on the environment requires evaluations of both the short-term fate of the pollutants and of their medium and long-term interactions within the ecosystem. Many articles in the literature claim to describe the impacts of uranium mining on the natural environment, and of the placement/disposal and management/mismanagement of the mill tailings. In contrast there are relatively few articles that quantify the level of harm caused by uranium mining and milling residues to the natural aquatic ecosystem, taking into account the seasonal and spatial variation of the water quality of the natural system surrounding the mine.
The present study was carried out in the uranium-ore-mining area located in Caldas (Minas Gerais, Brazil) and in the Antas reservoir that receives the neutralized acid solution leaching from the waste heaps generated by uranium mining. The Antas River hydrographic basins begin at the Antas reservoir head, which was constructed in 1982 for the purpose of providing water for the uranium milling plant of the Ore Treatment Unit of the Brazilian Nuclear Industries (UTM-INB). This mine was commercially explored by UTM-INB from 1982 to 1995, after which the operations were suspended, leaving the INB responsible for remediation of the area. Legal procedures for remediation of the area and facility decommissioning are currently under way (Nóbrega et al. 2008). The main environmental problem of uranium UTM mines is the generation of acid water which contains radionuclides and metals in concentrations above the permissible levels for discharging (Ladeira and Gonçalves 2007). Water from the Antas River is used for crop irrigation and for cattle watering, and the river also supplies the Bortolan dam, near the city of Poços de Caldas (MG, Brazil), and is used as a water supply for the population (Amaral et al. 1988).
The present study was designed to evaluate the water quality of the ecosystems under the influence of uranium mining in Caldas (southern region of Minas Gerais State, Brazil) by way of physicochemical analyses, with particular emphasis on the temporal variability of the water quality. This study is an important contribution to the understanding of the limnological aspects of reservoirs in tropical regions that have experienced the impact of uranium ore mining and milling.
Material and methods
Description of the study area
The study area was located in a tropical climate zone with two distinct seasons. The rainy season is from October to March with an annual average temperature of 20 °C, and the dry season from April to August with an annual average temperature of 14 °C (INB 1999).
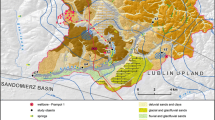
The study involved the evaluation of six sampling stations (Fig. 1). Two of the stations were in the area of the Ore Treatment Unit: one in the region called the waste rock piles ponds (point 1) and the other in the pit mine (point 2). Point 3 was located at the UTM interface with the environment at Consulta Creek. The uranium mine unit evaluated in this study consisted of a pit mine (point 2) covering an area of about 15 km2 (Fernandes et al. 1995). It was estimated that 94.5 × 106 t of rock was removed during the mining operation, but only 2 % of this amount was destined for physical and chemical processing, the rest mainly being stacked in two waste rock piles. The piles of sterile ore constituting the waste rock were deposited at the Consulta Creek (point 3) and Soberbo Creek hogsbacks (Amaral et al. 1988), and the courses of these rivers were deviated to canals. The acid drainage from the waste rock piles (points 1 and 2) was pumped into the treatment unit. The uranium extraction process produces large quantities of liquid and solid wastes that are neutralized to pH 9 using CaCO3 and CaO, and then discharged into a tailing dam for deposition of the solids (Fernandes et al. 1995). In the present case, after treatment, the effluent was released into the Antas reservoir (point 5).
The Antas reservoir is located in the southeast of Minas Gerais State and has a volume of 3.9 × 106 m2, and a drainage area of 51 km2 with an average depth of 4 m (maximum of 8 m) (INB 1999). Samples were taken from the surface of the reservoir at three points (points 4, 5 and 6). The selected sampling sites were located upstream and downstream of the effluent discharge point (point 5). Point 4 (upstream of the mining effluent discharge), located at the head of the reservoir, was about 1.5 m deep. Point 5 was shallower (1.0 m deep) and near the middle of the reservoir, where the treated effluent from the UTM-INB was discharged. Point 6 was downstream of point 5, near the dam, and deeper than the other sites (6.9 m) (Fig. 1).
Sampling was carried out during four periods: October 2008 (before the first rain), January, April and July 2009. The water was collected in 5-L Van Dorn bottles, transferred to previously washed plastic bottles and stored at 4 °C until the moment of analysis. The rainfall and air temperature data were provided by the Meteorological Station of the Poços de Caldas Laboratory, Brazilian Nuclear Energy Commission (Meteorological Station Campbell Scientific, Modelo CR10X, 815 W-1800 N). The rainfall was considered as the amount accumulated per month.
Physical and chemical analyses
The temperature and the values for dissolved oxygen were determined using a WTW OXI-315i oximeter (WTM GmbH, Weilheim, Germany). The values for pH were obtained using a pH meter (model DM-21, Digimed, São Paulo, Brazil), and those for electrical conductivity using a conductivity meter (model DM-31, Digimed, São Paulo, Brazil) with a selective electrode. The reduction–oxidation potential was determined using a Pt electrode (with Ag/AgCl as the reference, model DM-22, Digimed, São Paulo, Brazil). Water samples were taken for the determination of the chlorophyll a concentration according to Lorenzen (1967), and the total suspended solids were analyzed according to Teixeira et al. (1965). The water transparency was measured using a Secchi disk.
Fluoride was estimated potentiometrically with an ion-selective electrode (Nascimento et al. 1988), and sulfate by UV–Vis spectrophotometry as described by ASTM (1980). The hardness of the water (Ca2+ and Mg2+) and the metals were quantified by inductively coupled plasma atomic absorption optical emission spectrometry, ICP-OES, using a Varian sequential equipment, model Liberty RL (Varian, USA). The water samples taken for the metal determinations were preserved by adding nitric acid to pH < 2 (Ultrapure acid, J. T. Baker) and stored at 4 °C. The total metal concentrations (iron, manganese, molybdenum and zinc) were measured using coupled plasma atomic absorption spectrometry (Martin et al. 1994). The uranium and thorium concentrations were measured using spectrophotometry, with arsenazo III as the colorimetric reagent (Fukuma et al. 2001). The total organic nitrogen (Golterman et al. 1978) and total phosphorus (APHA/AWWA/WCPF 1995) contents were also determined.
The results obtained for water transparency (Secchi disk depth), chlorophyll a concentration and total phosphorus were used to evaluate the trophic state of the Antas reservoir. Carlson’s Trophic State Index (TSI) (Carlson 1977) was computed from the Secchi disk depth (TSI (SD)), total phosphorus concentrations (TSI (TP)) and chlorophyll a concentrations (TSI (CHL)).
The chemical data obtained for the water sampled from the UTM (point 3) and from the Antas reservoir were compared with the limits adopted by the Brazilian guidelines (CONAMA Resolution 357/2005) and World Health Organization guidelines for drinking water quality (WHO 2011). That CONAMA resolution regulates the quality of water bodies in Brazil, stipulating the reference limits, and also establishes the conditions and standard practice for effluent disposal. According to the Brazilian legislation, the waters collected from point 3 and from the Antas reservoir were classified in category 2 (water suitable for domestic consumption after conventional treatment, primary contact, recreation, irrigation and the protection of aquatic communities).
Statistical analysis
The results of the physical and chemical analyses were analyzed using ANOVA and Tukey’s test (post hoc test) to detect significant differences between the water samples obtained from the different sites at the different collection times. The above statistical tests were performed using the BioEstat 4.0 program (Ayres et al. 2005). Pearson’s correlation coefficient (r) was used to find correlations amongst the physical and chemical variables for the samples.
Results and discussion
Ore treatment unit
For the UTM, low average pH values, high average reduction–oxidation potentials, and the highest values for sulfate and metals were detected in the water samples obtained from the waste rock piles ponds and the pit mine (points 1 and 2, respectively). The average value for the pH at point 3 (5.0) was significantly higher (P < 0.05, Tukey’s test) than those found at points 1 (3.5) and 2 (3.8). With respect to electrical conductivity, higher average values were recorded for the water samples from points 1 (2169 μS cm−1) and 2 (2392 μS cm−1), whereas the lowest average value was recorded for the water samples from point 3 (121 μS cm−1) (P < 0.05, Tukey’s test). The highest reduction–oxidation potential value was recorded for water samples from point 1 (488 mV), whereas the lowest value was recorded for water samples from point 3 (338 mV) (P < 0.05, Tukey’s test) (Table 1).
The average fluoride concentrations at points 1 (82 mg L−1) and 2 (55 mg L−1) were significantly higher (P < 0.05, Tukey’s test) than that at point 3 (2.10 mg L−1). The average concentrations for hardness at points 2 (927 mg L−1) and 1 (389 mg L−1) were significantly higher (P < 0.05, Tukey’s test) than that at point 3 (18 mg L−1). Similarly, the average sulfate concentrations at points 1 and 2 (1275 and 1413 mg L−1, respectively) were significantly higher (P < 0.05, Tukey’s test) than that at point 3 (38 mg L−1) (Table 2). The lowest average concentration for iron was found in water samples from point 3 (0.82 mg L−1) and the highest at point 1 (26 mg L−1) (P < 0.05, Tukey’s test). The average concentrations for manganese (75 mg L−1) and uranium (5.62 mg L−1) in the water samples from point 1 were significantly higher (P < 0.05, Tukey’s test) than those at point 3. With respect to zinc, the lowest average concentration was found in the water samples from point 3 (0.52 mg L−1) and the highest at points 1 and 2 (14.10 and 11.13 mg L−1, respectively) (P < 0.05, Tukey’s test) (Table 2).
In a study on the UTM-INB, Campos et al. (2011) observed the presence of bioleaching microorganisms (Acidithiobacillus ferrooxidans and A. thiooxidans) in the water from points 1 and 2. These findings could indicate a bio-lixiviation action of the A. ferrooxidans and A. thiooxidans on the uranium ore and a possible release of radionuclides into the environment. The action of bioleaching microorganisms on uranium ore causes the release of metals and radionuclides into the water column, with severely adverse effects on the adjacent aquatic ecosystem (Maier et al. 2000). The fundamental condition for bacterial leaching from uranium ores is the presence of metallic sulfides such as pyrite (FeS2) in association with the ore, which is the case in the ore and waste rock from the uranium-mining area of Caldas (Minas Gerais State, Brazil). These findings indicate that acid mine drainage occurs in this region, which should therefore be considered as a critical site in the event of decommissioning.
Uranium may cause both chemical and radiological toxicity for terrestrial and aquatic organisms (Sheppard et al. 2005). As one of the geochemically most mobile heavy metals, U moves rapidly from a multitude of mining-related point and non-point sources into the aquatic environment, polluting both stream waters and groundwaters contained in aquifers (Winde 2010). According to Fernandes et al. (2008), contamination of the groundwater at UTM was to be expected, due to infiltration from contaminated discharges, and the hydrology and general geo-hydrology of the UTM drainage basin show characteristics suggesting a connection between the surface water and groundwater (Campos et al. 2011).
According to the limits stipulated by Brazilian environmental regulations, the present study indicated metal contamination in the water from Consulta Creek. Water samples from point 3 had average concentrations of fluoride (2.10 mg L−1), manganese (3.40 mg L−1), uranium (0.13 mg L−1) and zinc (0.52 mg L−1) all above the limits established by the CONAMA Resolution 357 (CONAMA 2005) and those recommended by the WHO (2011) (Table 2). The bioavailability and toxicity of metals in water has been recognized to depend on chemical variables such as the pH and hardness (Penttinen et al. 1998; Zeman et al. 2008). In the natural environment metals are generally an order of magnitude more available in soft than in hard waters, due to competition between the metal and Ca2+ and Mg2+ ions for the uptake sites in organisms (Pyle et al. 2002; Kozlova et al. 2009) in hard waters. The effect of pH on metal toxicity is twofold. The H+ concentration may exert its effect either directly by affecting metal uptake, or indirectly by affecting the chemical speciation and bioavailability of the metals (Peterson et al. 1984). At lower pH levels, more free metal ions may be present in the water column and be taken up by living organisms, hence inducing toxicity (Kim et al. 2001; Clifford and McGeer 2009; Greig et al. 2010). Since point 3 was classified as soft (average value for hardness of 18 mg L−1) and acidic (average pH value close to 5), higher bioavailability of metals in that environment could be expected.
Comparisons with the results of ecotoxicological studies suggest that point 3 contains average concentrations of manganese (3.40 mg L−1), uranium (0.13 mg L−1) and zinc (0.52 mg L−1) sufficient to cause toxicity to aquatic organisms. The inhibitory concentration values of Mn for the alga Scenedesmus quadricauda (IC50 = 1.94 mg L−1, WHO 2004) and the cladoceran Ceriodaphnia dubia (IC25 = 3.10 mg L−1, Lasier et al. 2000) were exceeded at that point. The U concentration at point 3 was close to the value showing a toxic effect on the alga Chlorella sp. (IC50 = 0.08 mg L−1, Franklin et al. 2000) and sub-lethal effects on the cladoceran Daphnia magna (50 % reduction in reproduction at 0.09 mg L−1, Zeman et al. 2008). Similarly, the Zn concentration exceeded the toxicity levels for Selenastrum capricornutum (IC50 = 0.05 mg L−1, Turbak et al. 1986), the cladoceran D. magna (LC50 = 0.07 mg L−1, Mount and Norberg 1984) and the fish Oncorhynchus mykiss (LC50 = 0.52 mg L−1, Spear 1981). Moreover, aquatic biota (e.g. phytoplankton, mollusks and some fish) can accumulate significant amounts of Mn and Zn at lower trophic levels (Eisler 1993; USEPA 2004). Thus, the chemical evaluation made suggests that water from point 3 may represent a hazard to the aquatic biota and to humans exploring freshwater resources in the area.
Antas reservoir
The rainfall and air temperature data showed the characteristic local seasonal pattern (Fig. 2), with two periods during the present study: hot rainy season (October to March) and cold dry season (April to September). The average water temperature during the rainy period (25 °C in January 2009) was higher than that registered in the dry period (17 °C in July 2009) (P < 0.05, Tukey’s test). The average values were near 21 °C at all the sampling points (Table 1). The average suspended solids contents were significantly higher (P < 0.05, Tukey’s test) in January 2009 (9.16 mg L−1) than those observed in July (2.60 mg L−1). The average values for suspended solids at the sampling points remained around 5 mg L−1 (Table 1).
The main force functions controlling the biogeochemical processes in reservoirs are the rain, wind, outflow rates, retention time and pulses. In reservoirs, pulses can be considered as any type of sudden change caused by natural or artificial anthropogenic factors, with an effect on the physical, chemical and biological variables (Tundisi et al. 1999). In the Antas reservoir, for example, the contribution of suspended matter coming from the banks of the reservoir and the occurrence of re-suspension of material present in the sediment to the water column was much greater in the rainy season (January 2009) than in the dry season (July 2010), and a positive correlation was found between the rainfall and the suspended matter (r = 0.90) in the reservoir. This seasonality of the matter generated by rainfall produces a pulse of inorganic or organic matter within the reservoir (Wetzel 1993).
Seasonally, there were small spatial fluctuations in the values for pH and dissolved oxygen, but they were not significant (P > 0.05, Tukey’s test) (Table 1). Spatially, the average values for dissolved oxygen and pH were around 7 mg L−1 and 6.5, respectively. Water electrical conductivity showed the highest average value in October 2008 (608 μS cm−1) and the lowest in April 2009 (87 μS cm−1) (P < 0.05, Tukey’s test). The average values registered for electrical conductivity were between 177 μS cm−1 (point 4) and 396 μS cm−1 (point 5). The highest average value for the reduction–oxidation potential was recorded for water samples in January 2009 (282 mV), whereas the lowest value was recorded for water samples in July (123 mV) (P < 0.05, Tukey’s test). The average values for the reduction–oxidation potential ranged from 204 mV (point 5) to 251 mV (point 4) (Table 1).
The lowest values for water transparency (Secchi disk depth) were registered at points 4 (1.30 m) and 5 (0.60 m), and the highest Secchi disk depth at point 6 (3.00 m) (Table 1). The results for chlorophyll a indicated average values from 0.47 μg L−1 (point 4) to 2.67 μg L−1 (point 5). Seasonally, the values for chlorophyll a varied between 0.52 μg L−1 (January/09) and 3.56 μg L−1 (July/09) (Table 1). The average values for total organic nitrogen ranged from 746 μg L−1 (October 2008) to 1003 μg L−1 (January 2009), and the average concentrations for total organic nitrogen varied from 805 to 960 μg L−1 (points 4 and 6, respectively) (Fig. 3). With respect to the total phosphorus concentrations, the highest average value was found in the samples collected in January (13.2 μg L−1), whilst the lowest value was found in October 2008 (2.6 μg L−1) (P < 0.05, Tukey’s test). The average values for total phosphorus varied from 6.3 μg L−1 at point 5 to 8.7 μg L−1 at point 6 (Fig. 3).
The determination of the trophic state index is an important aspect of water quality, and the monitoring of eutrophication is part of the assessment of aquatic systems. The average values for the trophic state index (TSI) of the Antas reservoir were below 40 in October/08, April/09 and July/09, and between 40 and 50 in January/09. The reservoir was classified as oligotrophic, with the exception of January, when it was mesotrophic. The results reported here suggest that the Antas reservoir is not eutrophic.
The average fluoride concentration was significantly higher (P < 0.05, Tukey’s test) in October (2.30 mg L−1) than in January (0.50 mg L−1). The average fluoride concentration varied from 0.80 mg L−1 at point 4 to 1.54 mg L−1 at point 5. With respect to hardness, the lowest average value was found in January/09 (20 mg L−1) and the highest in October/08 (230 mg L−1) (P < 0.05, Tukey’s test). The average values recorded for hardness were between 71 mg L−1 (point 4) and 122 mg L−1 (point 5). With respect to sulfate, the highest level (269 mg L−1) occurred in October and the lowest (16 mg L−1) in January, and the statistical analysis showed that these two values differed significantly (P < 0.05, Tukey’s test). The average values recorded for sulfate varied between 59 mg L−1 (point 4) and 113 mg L−1 (point 5) (Table 2).
For the iron concentration, the lowest average value was found in the samples collected in July (0.23 mg L−1), while the highest was found in January 2009 (0.94 mg L−1) (P < 0.05, Tukey’s test). The average iron concentration varied from 0.45 mg L−1 at point 4 to 0.54 mg L−1 at point 5. The average value for manganese determined in October (1.02 mg L−1) was significantly higher (P < 0.05) than that quantified in July (0.41 mg L−1). With respect to manganese, average concentrations were detected ranging from 0.66 mg L−1 (point 5) to 0.83 mg L−1 (point 6) (Table 2).
The quality of the water in the reservoir showed seasonal variations, the average values for fluoride, hardness and sulfate being higher in October than in January. The Antas reservoir is subject to seasonal variation in relation to the level of pollution by metals and other compounds. The low rainfall and high water residence time characteristic of the dry season (April to September) may have contributed to the accumulation of ions in the water from the Antas reservoir, resulting in high ion concentrations in October (before the first rain). Moreover, the highest mean values for electrical conductivity were detected in the water samples in October. An association between these data was confirmed by positive correlations between the electrical conductivity values and the sulfate concentrations (r = 0.85) and hardness (r = 0.80). On the other hand, the rainfall acted as a diluting factor in the system (Naselli-Flores 1999), resulting in the lowest ion values being recorded during the rainy season (January). Similar to the present results, Tutu et al. (2008) found low concentrations of ions in rivers close to a South African mining area during rainy periods.
Uranium mining and milling operations can release trace elements such as U, As, Mn and Mo into the receiving aquatic ecosystem (Abdelouas 2006; Muscatello et al. 2008). The fluoride levels exceeded the limits defined by the CONAMA Resolution 357 (CONAMA 2005) and that recommended by the WHO (2011) in the samples from points 5 (October 2008 and July 2009) and 6 (October 2008). The sulfate concentrations were also found to be above the CONAMA and WHO limits at point 5 in October (387 mg L−1) (Table 2). All the manganese concentrations determined in the water from the reservoir were above the CONAMA limit (CONAMA 2005) and WHO drinking water standard (WHO 2011) (Table 2). The total manganese concentrations in natural freshwaters seldom reach 1.0 mg L−1 and are usually less than 0.2 mg L−1. Although manganese is an essential nutrient in low doses, chronic exposure to high doses may be harmful to both humans and animals (USEPA 2004). When the manganese concentrations observed in the water from the Antas reservoir were compared with those in the literature for aquatic bodies located in uranium mining areas, some important differences emerged. For example, Trontelj and Ponikvar-Zorko (1998) determined a Mn concentration below 0.01 mg L−1 in a stream receiving waste water from a uranium mine in Slovenia, whereas Brandenberger et al. (2004) found a Mn concentration of 0.4 μg L−1 in water from Lake Corpus Christi (Texas), located in a uranium mining area.
In contrast to the values observed in the studies cited above, the occurrence of high Mn levels (0.35–1.04 mg L−1) in the Antas reservoir is of environmental concern, since they could be sufficient to induce acute toxicity in the cladoceran D. magna (EC50 = 0.80 mg L−1 Mn, Reimer 1999) and chlorophyll inhibition in the green algae S. quadricauda (EC50 = 1.9 mg L−1, Fargašová et al. 1999), and contribute to chronic toxicity in the fish O. mykiss (low egg hatching percentage at 1 mg L−1 Mn, WHO 2004). In the Antas reservoir, the zooplankton community includes the cladoceran C. cornuta and D. ambigua (Ferrari 2010), and the phytoplankton community is composed of the green algae Chlamydomonas sp. and Scenedesmus sp. (Roque et al. 2009). In order to derive reliable toxicity data for the Antas reservoir, ecotoxicological studies of the water using local species as the test organisms should be performed.
Uranium is commonly found in very small amounts in the environment. There are regions with higher concentrations of this metal in the surface and groundwaters due to geological conditions, but mining activities can lead to the U enrichment of natural waters (Baborowski and Bozau 2006). Although there is a risk of radiological toxicity from orally ingested natural uranium, the principal health effects are related to its chemical toxicity (Weir 2003). The present study detected uranium concentrations close to the maximum limit permitted by Brazilian legislation (Resolution 357) at point 5 (0.02 mg L−1 in July). These results were lower than the values obtained by Semaan et al. (2001), who observed a U concentration range between 0.02 and 0.20 mg L−1 in an aquatic environment considered as contaminated, near a uranium mine in Australia.
This investigation demonstrated the detrimental effect of the release of treated uranium mine radioactive effluents into the Antas reservoir. The liquid effluents from the UTM (points 1 and 2), when characterized chemically before treatment for discharging into the environment, present elevated zinc, uranium, manganese, fluoride and sulfate contents, amongst other elements. In the reservoir, the values for the chemical variables (fluoride, sulfate and manganese) were close to or above the limits established by current legislation at the point of effluent discharge (point 5) and downstream from this point (point 6), indicating harmful environmental conditions at that part of the reservoir. In order to reduce pollution in the Antas reservoir from the treated mining effluent, the use of efficient techniques such as sorption to remove chemical contaminants from the mining effluent is one of the important measures that should be adopted.
Conclusion
The monitoring of the Consulta waters (point considered the limit between the UTM and the environment) showed the need to control the contents of uranium and other elements at acceptable levels. The evaluation of the water quality in the Antas reservoir showed seasonal variations and indicated contamination by fluoride, sulfate and manganese due to effluent discharge by the uranium mining company. Such results support the need for reviewing the treatment actions currently used for effluents from UTM. The authors suggest that an ecotoxicological characterization of the aquatic environment under the influence of the UTM uranium mine should be carried out, in order to better assess the toxic effects and consequences of the chemicals with respect to the aquatic biota.
References
Abdelouas, A. (2006). Uranium mill tailings: geochemistry, mineralogy, and environmental impact. Elements, 2(6), 335–341.
Amaral, E. C. S., Godoy, J. M., Rochedo, E. R. R., Vasconcellos, L. M. H., & Pires do Rio, M. A. (1988). The environmental impact of the uranium industry: is the waste rock a significant contributor? Radiation Protection Dosimetry, 22(3), 165–171.
American Public Health Association; American Water Work Association; Water Control Federation (APHA/AWWA/WCPF). (1995). Standard methods for examination of water and wastewater (20th ed.). Washington: American Public Health Association.
American Society for Testing and Materials (ASTM). (1980). Annual book of ASTM standards. Philadelphia: American Society for Testing and Materials.
Ayres, M., Ayres, M., Jr., Ayres, D. L., & Santos, A. S. (2005). BioEstat 2.0: aplicações estatística na área das Ciências Biológicas e Médicas. Mamirauá: Editora Gráfica Ltda.
Baborowski, M., & Bozau, E. (2006). Impact of former mining activities on the uranium distribution in the River Saale (Germany). Applied Geochemistry, 21(6), 1073–1082.
Brandenberger, J., Louchouarn, P., Herbert, B., & Tissot, P. (2004). Geochemical and hydrodynamic controls on arsenic and trace metal cycling in a seasonally stratified US sub-tropical reservoir. Applied Geochemistry, 19(10), 1601–1623.
Campos, M. B., Azevedo, H., Nascimento, M. R. L., Roque, C. V., & Rodgher, S. (2011). Environmental assessment of water from uranium mine (Caldas, Minas Gerais state, Brazil) in decommissioning operation. Environmental Earth Sciences, 62(4), 857–863.
Carlson, R. E. (1977). A trophic state index for lakes. Limnology and Oceanography, 22(2), 361–369.
Chevychelov, A. P., D’yachkovskii, A. P., Sobakin, P. I., & Kuznetsova, L. I. (2010). Surface water radioactive pollution in South Yakutia anthropogenic landscapes. Contemporary Problems of Ecology, 3(4), 381–385.
Clifford, M., & McGeer, J. C. (2009). Development of a biotic ligand model for the acute toxicity of zinc to Daphnia pulex in soft waters. Aquatic Toxicology, 91(1), 26–32.
CONAMA Resolução nº 357, de 17 de março de 2005 (2005) “Resolução do CONAMA para a classificação dos corpos de água para o seu enquadramento, bem como estabelecimento das condições e padrões de lançamento de efluentes,” seção I, pp. 58–63. Brasília.
Eisler, R. (1993). Zinc hazards to fish, wildlife, and invertebrates: a synoptic review. Laurel: U.S. Contaminant Hazard Reviews Department of the Interior Fish and Wildlife Service Patuxent Wildlife Research Center.
Fargašová, A., Bumbálová, A., & Havránek, E. (1999). Ecotoxicological effects and uptake of metals (Cu+, Cu2+, Mn2+, Mo6+, Ni2+, V5+) in freshwater alga Scenedesmus quadricauda. Chemosphere, 38(5), 1165–1173.
Fernandes, H. M., Franklin, M. R., & Gomieiro, L. A. (2008). Critical analysis of the waste management performance of two uranium production units in Brazil-part I: Poços de Caldas production centre. Journal of Environmental Management, 87(1), 59–72.
Fernandes, H. M., Veiga, L. H. S., Franklin, M. R., Prado, V. C. S., & Taddei, J. F. (1995). Environmental impact assessment of uranium mining and milling facilities: a study case at the Poços de Caldas uranium mining and milling site, Brazil. Journal of Geochemical Exploration, 52(1–2), 161–173.
Ferrari, C. R. (2010). Avaliação de efeitos de efluentes radioativos de mineração de urânio sobre características físicas, químicas e diversidade da comunidade zooplanctônica na Unidade de Tratamento de Minérios, na Represa das Antas e Represa Bortolan, Poços de Caldas (MG). Dissertação. Universidade de São Paulo
Franklin, N. M., Stauber, J. L., Markich, S. J., & Lim, R. P. (2000). pH-dependent toxicity of copper and uranium to a tropical freshwater alga (Chlorella sp.). Aquatic Toxicology, 48(2-3), 275–289.
Fukuma, H. T., De Nadai Fernandes, E. A., Nascimento, M. R. L., & Quinelato, A. L. (2001). Separation and spectrophotometric determination of thorium contained in uranium concentrates. Journal of Radioanalytical and Nuclear Chemistry, 248(3), 549–553.
Golterman, H. L., Clymo, R. S., & Ohnstad, M. A. M. (1978). Methods for physical and chemical analysis of freshwaters. Oxford: Blackwell.
Greig, H., Niyogi, D. K., Hogsden, K. L., Jellyman, P. G., & Harding, J. S. (2010). Heavy metals: confounding factors in the response of New Zealand freshwater fish assemblages to natural and anthropogenic acidity. Science of the Total Environment, 408(16), 3240–3250.
INB (1999). Indústrias Nucleares do Brasil Relatório Ambiental—Complexo Industrial do Planalto de Poços de Caldas—CIPC para solicitar Licença de Operação junto ao IBAMA. Diretoria de Recursos Minerais, Poços de Caldas: DRM da INB
Kim, S. D., Gu, M. B., Allen, H. E., & Cha, D. K. (2001). Physiochemical factors affecting the sensitivity of Ceriodaphnia dubia to copper. Environmental Monitoring and Assessment, 70(1–2), 105–116.
Kozlova, T., Wood, C. M., & McGeer, J. C. (2009). The effect of water chemistry on the acute toxicity of nickel to the cladoceran Daphnia pulex and the development of a biotic ligand model. Aquatic Toxicology, 91(3), 221–228.
Ladeira, A. C. Q., & Gonçalves, C. R. (2007). Influence of anionic species on uranium separation from acid mine water using strong base resins. Journal of Hazardous Materials, 148(3), 499–504.
Lasier, P. J., Winger, P. V., & Bogenrieder, K. J. (2000). Toxicity of Manganese to Ceriodaphnia dubia and Hyalella azteca. Archives Environmental Contamination and Toxicology, 38(3), 298–304.
Lorenzen, C. J. (1967). Determination of chlorophyll and phaeopigments: spectrophotometric equations. Limnology and Oceanography, 12(2), 343–346.
Lozano, J. C., Rodriguez, B. P., & Tomé, F. V. (2002). Distribution of long-lived radionuclides of the 238U in the sediments of a small river in a uranium mineralized region of Spain. Journal Environmental Radioactivity, 63(2), 153–171.
Maier, R. M., Pepper, I. L., & Gerba, C. P. (2000). Environmental microbiology. San Diego: Academic.
Martin, T. D., Creed, J. T., & Brockhoff, C. A. (1994). EPA method 200.2 sample preparation procedure for spectrochemical determination of total recoverable elements, EPA/600/R-94/111. Cincinnati: EPA.
Mount, D. I., & Norberg, T. J. (1984). A seven life-cycle cladoceran toxicity test. Environmental Toxicology and Chemistry, 3(3), 425–434.
Muscatello, J. R., Belknap, A. M., & Janz, D. M. (2008). Accumulation of selenium in aquatic systems downstream of a uranium mining operation in northern Saskatchewan, Canada. Environmental Pollution, 156(2), 387–393.
Nascimento, M. R. L., Fukuma, H. T., & Hortellani, M. A. (1988). Projeto Itataia—Controle de processo na produção de ácidos fosfórico e urânio. (Manual de Métodos e Análises Químicas). Poços de Caldas: INB.
Naselli-Flores, L. (1999). Limnological aspects of Sicilian Reservoir: A comparative ecosystemic approach. In M. Straskraba & J. G. Tundisi (Eds.), Theoretical reservoir ecology and its applications (pp. 293–312). São Carlos: Backhuys.
Neves, O., & Matias, M. J. (2004). Focos de poluição na área mineira da Cunha Baixa (Viseu, Portugal). Cadernos do Laboratorio Xeolóxico de Laxe, 29, 187–202.
Neves, O., & Matias, M. J. (2008). Assessment of groundwater quality and contamination problems ascribed to an abandoned uranium mine (Cunha Baixa region, Central Portugal). Environmental Geology, 53(8), 1799–1810.
Nóbrega, F. A., Andrade, H. M. L., & Leite, A. L. (2008). Análise de múltiplas variáveis no fechamento de mina—Estudo de caso da pilha de estéril BF-4, Mina Osamu Utsumi, INB Caldas, Minas Gerais. Revista Escola de Minas, 61(2), 197–200.
Oliveira, J. M. S., & Ávila, P. F. (2001). Geoquímica na área envolvente da Mina da Cunha Baixa (Mangualde, no centro de Portugal). Estudos, Notas e Trabalhos, Tomo. Mangualde: Instituto Geológico e Mineiro.
Penttinen, S., Kostamo, A., & Kukkonen, J. V. K. (1998). Combined effects of dissolved organic material and water hardness on toxicity of cadmium to Daphnia magna. Environmental Toxicology and Chemistry, 17(12), 2498–2503.
Peterson, H. G., Healey, F. P., & Wagemann, R. (1984). Metal toxicity to algae: a highly pH dependent phenomenon. Canadian Journal of Fisheries and Aquatic Sciences, 41(6), 974–979.
Pyle, G. G., Swanson, S. M., & Lehmkuhl, D. M. (2002). The influence of water hardness, pH, and suspended solids on nickel toxicity to larval fathead minnows (Pimephales promelas). Water, Air, and Soil Pollution, 133(1–4), 215–226.
Qian, Y., Zheng, M. H., Gao, L., Zhang, B., Liu, W., Jiao, W., et al. (2005). Heavy metal contamination and its environmental risk assessment in surface sediments from Lake Dongting, People’s Republic of China. Bulletin Environmental Contamination and Toxicology, 75(1), 204–210.
Reimer, P. S. (1999). Environmental effects of manganese and proposed freshwater guidelines to protect aquatic life in British Columbia. MSc thesis, University of British Columbia.
Roque, C. V., Dellamano de Oliveira, M. J., Nascimento, M. R. L., Ronqui, L. B., Campos, M. B., Ferrari, C. R., Rodgher, S. & Azevedo, H. (2009). Effects of uranium effluents (Caldas, Southeastern Brazil) on the aquatic biota: preliminary study on the phytoplankton community. International Nuclear Atlantic Conference. INAC 2009. Rio de Janeiro (RJ). Associação Brasileira de Energia Nuclear.
Sarmiento, A. G., Olías, M., Nieto, J. M., Cánovas, J. D., & Delgado, J. (2009). Natural attenuation processes in two water reservoirs receiving acid mine drainage. Science of the Total Environment, 407, 2051–2062.
Semaan, M. D., Holdway, A., & van Dam, R. A. (2001). Comparative sensitivity of three populations of the cladoceran Moinodaphnia macleayi to acute and chronic Uranium exposure. Environmental Toxicology, 16(5), 365–376.
Sheppard, S. C., Sheppard, M. I., Gallerand, M. O., & Sanipelli, B. (2005). Derivation of ecotoxicity thresholds for uranium. Journal Environmental Radioactivity, 79(1), 55–83.
Spear, P. A. (1981). Zinc in the aquatic environment: chemistry, distribution, and toxicology. Ottawa: National Research Council of Canada. Publication NRCC 17589.
Teixeira, C., Tundisi, J. G., & Kutner, M. B. (1965). Plankton studies in a mangrove II. The standing stock and some ecological factors. Boletim Instituto Oceanográfico, 14, 13–42.
Trontelj, A., & Ponikvar-Zorko, P. (1998). Influence of a uranium mine on the macrozoobenthic communities of the streams in the nearest environs, Slovenia. Water Science Technology, 37(8), 235–241.
Tundisi, J. G., Matsumura-Tundisi, T., & Rocha, O. (1999). Theoretical basis for reservoir management. In M. Straskraba & J. G. Tundisi (Eds.), Theoretical reservoir ecology and its applications (pp. 505–528). São Carlos: Backhuys.
Turbak, S. C., Olson, S. B., & McFeters, G. A. (1986). Comparison of algal assays systems for detecting waterborne herbicides and metals. Water Resource, 20(1), 91–96.
Tutu, H., McCarthy, T. S., & Cukrowska, E. (2008). The chemical characteristics of acid mine drainage with particular reference to sources, distribution and remediation: the Witwatersrand Basin, South Africa as a case study. Applied Geochemistry, 23(12), 3666–3684.
U.S. Environmental Protection Agency (2004). Drinking water health advisory for Manganese. Office of Water 4304T. Health and Ecological Criteria Div CAS Registry EPA-822-R-04-003 Number 7440-50.8. Washington, DC: EPA.
Zeman, F. A., Gilbin, R., Alonzo, F., Lecomte-Pradines, C., Garnier-Laplace, J., & Aliaume, C. (2008). Effects of waterborne uranium on survival, growth, reproduction and physiological processes of the freshwater cladoceran Daphnia magna. Aquatic Toxicology, 86(3), 370–378.
Weir, E. (2003). Uranium in drinking water, naturally. Canadian Medical Association Journal or its Licensors, 170(6), 951–952.
Wetzel, R. G. (1993). Limnologia. Fundação Calouste Gulbenkian: Trad. Maria José Boavida. Lisboa.
Winde, F. (2010). Uranium pollution of the Wonderfonteinspruit, 1997–2008 Part 1: Uranium toxicity, regional background and mining-related sources of uranium pollution. Water SA, 36(3), 239–256.
World Health Organization (WHO). (2004). Manganese and its compounds: environmental aspects concise international chemical assessment document 63. Geneva: World Health Organization.
World Health Organization (WHO). (2011). Guidelines for drinking-water quality (4th ed.). Geneva: World Health Organization.
Acknowledgments
The authors are grateful to Dr. Ana T. Lombardi for her comments on this work. This study was supported by the Brazilian National Research Council (CNPq proc. no. 381412/2008-3) and the State of Minas Gerais Research Aid Foundation (FAPEMIG proc. no APQ-7807-5.04/07).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodgher, S., de Azevedo, H., Ferrari, C.R. et al. Evaluation of surface water quality in aquatic bodies under the influence of uranium mining (MG, Brazil). Environ Monit Assess 185, 2395–2406 (2013). https://doi.org/10.1007/s10661-012-2719-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2719-5