Abstract
This paper evaluates the impact of gold mining activity on surface water quality in the locality of Batouri (East, Cameroon). Water samples were collected from five separate points. These water samples were characterized from a physicochemical point of view. This characterization consisted to determine the hydrogen potential, the alkalinity, the electrical conductivity, the total dissolved solids, the dissolved oxygen, the chemical oxygen demand, the suspended solids and the turbidity as physical properties. Major ions and heavy metals (copper, zinc, nickel, iron, chromium, selenium, lead, arsenic, mercury and cadmium) were also determined using hydrochemical methods. The waters of the gold mining zone were acidic to slightly basic (5.1–8.1), weakly conductive (28.2–40 µm cm−1), turbid (29–721 NTU) and polluted by suspended solids (210–3410 mg L−1). Chemical oxygen demand values were higher than the World Health Organization standards with high dissolved oxygen levels. The results of heavy metals analysis indicated that nickel, iron, chromium, selenium, lead, arsenic, mercury and cadmium exceeded the limits recommended by the World Health Organization. The heavy metal pollution index, the water quality index, the sodium adsorption rate and the percentage of sodium were calculated for each sample in order to assess the overall water quality. The results of the hydrochemical evaluation showed that these waters were chlorinated calcium and calcium sulfate and not suitable for human consumption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fresh water is an essential element in the life of all living organisms (humans, animals, plants). It is a strategic and fundamental resource for the existence of the human beings (Odoulami 2009). Surface waters (rivers, lakes, swamps) play an essential role in the economy of the countries because they are used for drinking, domestic activities, agriculture (irrigation), hydroelectric industry, fishing, tourism and mining activities. Therefore, they deserve special attention, as they may be the target of serious threats due to human activities. In fact, all human activities calling for the mobilization of natural resources lead to the production of waste (solid and liquid) and gaseous effluents capable of causing the transfer of pollutants (Emmanuel et al. 2004). Mining affects freshwater basins like other anthropogenic activities, with water for ore processing and through mine effluent discharges (Bamba 2012). These sources of pollution can be the cause of a growing deterioration in the quality of water resources when the protection of the receiving environment is not guaranteed (Fifi 2010; Wanélus 2016). Water is therefore considered as the victim of mining (Lyon 2010). Trace Metal (TM) pollution is important in water since they could be toxic at very low concentrations (Jain et al. 2010) in aqueous state.
Some authors assessed the impacts of mining activities on the environment and socioeconomic activities (Zobrist et al. 2009; Dogaru et al. 2009). Others studies have been conducted specifically on the evaluation of the physicohemical quality of water resources. Asare-Donkor et al. (2018) evaluated the hydrochemical characteristics of surface water and ecological risk assessment of sediments from settlements within the Birim River basin in Ghana. Gafur et al. (2018) studied the effect of heavy metal pollution in water of bone river by artisanal small-scale gold mine activities in the eastern of Gorantolo in Indonesia. Umer et al. (2020) evaluated the spatial and temporal variations of physicochemical parameters and metal concentrations in Lake Beseka’s water quality in the Ethiopian portion of the East African Rift Valley.
The mining activity has seen great progress since 2001 in Cameroon. The regions where it is mainly developed are the Adamawa and Eastern regions. Gold is the main mineral resource exploited there. The gold sites are exploited in an artisanal and semi-mechanized manner. The exploitation of gold is centered mainly on the alluvial gold generally localized at the edge of rivers, thus predisposing surface waters to serious environmental pollution (Ngoupayou et al. 2016). The Cameroonian areas where mining activity has been developed, like Batouri are subjected to increased environmental degradation due to artisanal and semi-mechanized exploitation. In this locality, two types of mineralization are known: (1) mineralization in the form of quartz veins associated with pyrites; (2) alluvial and eluvial deposits which are subject to intense exploitation (Suh 2008; Akwinga 2010). Then, there is a high risk of pollution of the environment in general and in surface water particularly. Indeed, the use of water for washing gold and effluent discharges from mining sites could lead to a deterioration in the quality and in particular in the physicochemical quality of the surface waters of this locality. Mining activity, because of its clandestine, informal nature and lack of proper regulation by the Cameroonian state has resulted in carelessness and a considerable lack of respect for environmental standards on the part of operators. To this, must be added the deficiency relating to information on the various risks associated with this sector of activity. In Cameroon, very few studies assess the quality of water resources in gold mining areas (Rakotondrabe et al. 2017a; b; Ayiwouo et al. 2020; Mambou et al. 2020). Indeed, a prerequisite for sustainable development must ensure that watercourses are not contaminated (Save Drinking Water Foundation 2017), hence the interest of this study. To date, very few studies have included an assessment of effect of gold mine operations on water pollution. To address this critical gap in knowledge, this study will constitute a basic tool for managing the impacts associated with mining activity on surface water.
The main objective of this paper is to evaluate the influence of gold mining on the physicochemical quality of surface water in the locality of Batouri (East Cameroon).
Materials and methods
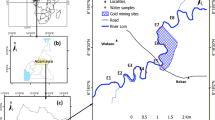
The geographical location of Batouri and the study area are shown in Fig. 1.
Batouri is located in the Kadey department, in the Eastern region of Cameroon. It covers an area of approximately 5.786 km2, latitude 7˚01 N and longitude 14˚53 E. It is limited to the North by the district of Kette; to the East by the Central African Republic; to the southeast by the Kentzou district; to the south by the districts of Mbang and Ndelele and to the west by the Lom and Djerem department. Batouri is subjected as a whole to the influence of a hot and humid equatorial climate with two rainy seasons interspersed with two dry seasons. The hydrographic network consists of many rivers (Belingonga, Mondim, Djengou, Bokoto, Kpwangala, Boungmama, Mama, Bil, Touki, Nol, Dja and Djengue) which all converge on the Kadey River. It is maintained by the Kadey rivers in the North and Doume in the Southwest (Commune de Batouri PNDP 2012) with its main tributaries: Boungmama, Mama, Bil, Touki, Nol, Dja and Djengou. The geological mapping of the Batouri gold district is presented in Fig. 2.
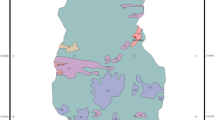
Geology of southeastern Cameroon. a Geological map of Cameroon (modified after Toteu et al. (2001)). The Central African Shear Zone is defined by a system of NE-trending faults comprising Tchollire-Banyo Fault (TBF), Adamawa Fault (AF), Sanaga Fault (SF), and Kribi-Campo Fault (KCF). The inset is the map of the African continent, showing the location of Cameroon relative to the distribution of cratons and mobile belts. NWC, Northwestern Cameroon domain; AYD, Adamawa-Yade Domain; YD, Yaounde Domain. b Regional geological map of southeastern Cameroon showing artisanal gold mining sites and other reported gold indications (modified after Milési et al. (2004)). Toteu et al. (2001) and Toteu et al. (2006) report included geochronological and model ages
Sampling
Five sampling points were chosen. Several criteria guided the choice of sampling points. These include, among other things, criteria of accessibility and representativeness in relation to mining sites, criteria of proximity to mining and/or agricultural sites, the level of demand on these water points both for domestic needs and for mining (ore washing). Sampling was done manually over a three-month period (May–July 2018). Samples were collected between 10 a.m. and 2 p.m. The water samples were taken in 1-L polyethylene bottles and 30 cm below the surface. The geographical coordinates of the selected sampling points were obtained using a GARMIN brand Global Positioning System (GPS) and recorded using a marker in a field note pad. The location of these points and their geographical coordinates are given in Table 1 and the sampling map is presented in Fig. 3.
Water characterization
The water analysis was to determine the physicochemical properties, particularly: pH, alkalinity, electrical conductivity (EC), total dissolved solids (TDS), dissolved oxygen (DO), chemical oxygen demand (COD), suspended solids (SS) and turbidity (Tu). The pH values were determined using a Consort brand pH meter. The alkalinity values were determined by automatic titration with a nitric acid solution (THAM solution) using a Metrohm brand automatic titrator. The EC values were determined by an electrometric method using a conductivity meter brand PCE-PHD 1. The field of application of this method is between 1 and 500,000 µS cm−1. To calculate the total amount of dissolved solids, the method described by Koupaee and Wang (2018) was used. The TDS were obtained by applying the following equation:
where Ke is the conversion factor varying between 0.54 and 0.96 depending on the liquid and atmospheric conditions and σ the conductivity in µS cm−1. As the actual value of the conversion factor was unknown, the value of 0.67 was used in accordance with generality.
The DO measurements were made by an electrochemical probe method using a Milwaukee brand oximeter. The COD test was carried out by the closed system reflux method followed by a colorimetric test with potassium dichromate. It is a method applicable for undiluted samples with an ST-COD between 5 and 100 mg L−1 and a chloride concentration not exceeding 1000 mg L−1. The SS values were determined by filtration on a glass fiber filter of the solutions to be studied. The Tu values were determined by the nephelometry method by a Hach brand turbidimeter. This method is based on photometry of cloudy environments and its field of application is between 0.1 and 1000 NTU, but can be extended up to 7500 NTU by modifying the calibration. The physical parameters were analyzed at the laboratory of the University Institute of Technologies (UIT) of the University of Ngaoundere, Cameroon.
Major cations (Na+, Ca2+, Mg2+, K+ and NH4+) and major anions (F−, Cl−, NO3−, PO43−, SO42−) except HCO3− were determined by Dionex ion chromatography. The TMs (copper, zinc, nickel, iron, chromium, selenium, lead, arsenic, mercury and cadmium) were determined by atomic absorption spectrometry (AAS) using SHIMADZU spectrometer. The concentrations of major ions and heavy metals were determined at the laboratories of the Institute of Geological and Mining Research (IRGM) of Yaounde-Nkolbisson and of Hydrogeology and Environment of the University of Yaounde 1, Cameroon.
Evaluation of the overall quality of water and hydrochemical analysis
Five tools were used to assess the overall water quality: the Heavy metal Pollution Index (HPI), the Water Quality Index (WQI) and the Piper, Riverside and Wilcox diagrams. The HPI was used to evaluate overall pollution of water with respect to heavy metals. The WQI was used to assess the overall quality of water for human consumption. The Piper diagram was used to identify the hydrochemical facies and to have an indication of the qualitative aspect of the water. Riverside and Wilcox diagrams were used to assess the suitability of water for irrigation.
The HPI is a parameter used to assess the level of water pollution by heavy metals. This method consists of assigning a weight or an index (Wi) to each metal according to its contribution to the process of water quality degradation (Singh and Rakesh 2016). The Heavy metal Pollution Index (HPI) model proposed is given by Mohan et al. (1996):
where k is the proportionality constants, if the authorized standard value of the parameter i according to the WHO standard (2011). The parameter Wi is the relative weight or index assigned to each metal.
where Vi is the value taken in the field for the parameter i (mg L−1) and Si the limit or standard allowed for said parameter according to WHO standards.
The WQI was obtained by assigning each physicochemical parameter a weight varying from 1 to 5 depending on its importance in assessing the quality of drinking water (Cude 2001). The highest weight (5) is attributed to parameters that have critical effects on the quality of water intended for human consumption compared to the limits recommended by the WHO (Cude 2001). The water quality index was calculated by the following equation:
where qi indicates the grade of quality; Ci the concentration of each chemical parameter and SI the WHO guideline value (2011); SIi is the sub-index of the i parameter; Wi the relative weight of each parameter, wi the weight assigned to each parameter and n is the number of parameters. The categories of WQI are presented in Table 2.
To identify the hydrochemical facies and to have an indication of the qualitative aspect of the water, the graphic representation of the analysis results is an inevitable tool. To achieve this objective, Piper, Wilcox and Riverside diagrams were studied using the Diagrams 6.51 software.
The calculation of the ionic balance (IB) was carried out to check the quality and reliability of the analysis results obtained. It makes it possible to evaluate the relative error on the degree of electric neutrality of ions in solution by evaluating the following ionic balance (Boeglin et al. 2003):
The calculation of the ionic balance was obtained by the following equation:
Results and discussion
The visual impacts of mining on the environment are shown in Fig. 4.
Figure 4 shows the environmental degradation due to the semi-mechanized mining activity. This activity has significant effects on water. Visually, in the study area, its effects were manifested by the diversion of water courses; the turbid state of waters and the creation of numerous holes, which are in most cases not rehabilitated. Given the lack of data related to the quantified assessment of the impact of semi-mechanical operation on water resources, this study, therefore makes it possible to estimate this impact using precise and objective tools to provide methods of adequate remediation. The summary of statistical analysis is presented in Table 3.
Physical quality of water
The results of the physical characterization of the water samples collected are presented in Fig. 5.
The pH values of the waters studied were acidic to slightly basic (5.1–8.1) as shown in Table 2. These values were not in agreement with the range of values recommended by the WHO standard (WHO 2011) for E4 sample. E4 was acid. These results reflect those obtained in previous study (pH = 5.42–7.58) (Rakotondrabe et al. 2017a, b). The production of NO3− and SO42− from the biodegradation of cyanide and thiocyanate (Gould et al. 2012) may have contributed to the lower pH value observed in these samples.
The alkalinity ranged from 90.4 to 102.3 mg L−1. The high values of alkalinity were observed during the month of May.
The EC values ranged from 28.42 to 40 μS cm−1. This indicated that these waters were not very conductive. Activities that lead to the release of rock minerals into the water have been noted to influence conductivity of water (Asamoah 2012).
TDS concentrations ranged between 18.9 and 26.8 mg L−1 during the studied duration. TDS values were below the maximum permissible limit of 1000 mg L−1 set by WHO (2011).
The DO values ranged from 3.9 to 7.2 mgO2 L−1. The average value was 5.12. DO of a sample of water measure the quantity of oxygen dissolved in the water. DO of water varies depending on factors, such as temperature, sunlight, atmospheric pressure, salinity and water turbulence (FDEP 2013). DO value is above the WHO for E1 sample, the low oxygen holding capacity of the water as a result the (bio)chemical oxidation of the sulfide ore minerals (Lundgren and Silver 1980).
COD values ranged from 20.2 to 31.8 mgO2 L−1. COD values were below the desirable limit recommended by the Cameroonian standard (NEPIC 1996) at all the sampling points. High concentrations of COD recorded in the water samples could be attributed to the oxygen that is mainly required for the oxidation of both organic and inorganic compounds (Acheampong et al. 2013).
The variations in turbidity were substantially proportional to those of the SS. The turbidity values varied from 29 to 721 NTU. These values exceeded the limits recommended by the WHO (2011) at all the sampling points. The SS values ranged from 0.21 to 3.41 g L−1 exceeded the limit of 0.04 g L−1 set by the Cameroonian standard (NEPIC 1996). These high values (turbidity and suspended solids) were attributed to artisanal and semi-mechanized exploitation, which through the various activities carried out, such as deforestation, digging in the riverbed, or even the exposure of washing discharges, contribute to their increase. These results are in agreement with the results of Asamoah (2012), Rakotondrabe et al. (2017a, b) and Ayiwouo et al. (2020) in gold mining zones.
Major ions
Table 4 shows the concentrations of the major ion concentrations of water samples.
The major ion concentrations in the study area were in the following order: Ca2+ > K+> Na+> SO42−> NH4+ > Mg+ > Cl− > NO3− > HCO3− > PO43− > F−. Most major cations and anions were present at relatively low concentrations. Ca2+ is the dominant cation with concentrations higher than other major cations. SO42− is the dominant anion.
The Ca2+ and Mg2+ contents in water samples varied from 0.74 to 33.19 and 1.56 to 9.53 mg L−1, respectively. The lowest value was recorded in E1 for Ca2+ and in E5 for Mg2+. The highest value was recorded in E3 for Ca2+ and in E1 for Mg2+. Calcium and magnesium are earthy alkaline metals and major components of water hardness. Its content varies essentially according to the nature of the lands that the water flowed over (Nollet 2007). Calcium is one of the parameters responsible for the hardness of water (Li et al. 2014). The geological map (Fig. 2) shows that the study area is made up of alkaline granites. Calcium concentrations could come from the dissolution of this rock.
The K+ content ranged from 8.5 to 18.92 mg L−1. The lowest value was observed in E5 and the highest in E3. In many water quality guidelines, no restrictions were established concerning human health effects.
The Na+ and NH4+ contents varied from 1.47 to 20.45 mg L−1 and 2.42 and 9.77 mg L−1, respectively. The lowest value was recorded in E3 for Na+ and in E1 for NH4+. The highest value was recorded in E5 for Na+ and in E2 for NH4+. Sodium (Na+) could come from the weathering of evaporites and silicates. The high concentrations of NH4+ can contribute to eutrophication.
The F− and HCO3− contents varied from 0.07 to 0.1 mg L−1 and 0.08 to 1.22 mg L−1, respectively. The lowest value was recorded in E1 for F− and in E4 for HCO3−. The highest value was recorded in E2 for F− and in E5 for HCO3−. The fluoride concentrations were below the limit of 1.5 mg L−1 set by WHO (2011) for drinking water. HCO3− derived from the dissolution of the atmospheric carbon (CO2) I water (Garnels and Mackenzie 1971). HCO3− could also come from the weathering of alkaline granite found in the study area.
The NO3− and PO43− contents varied from 0.05 to 1.08 mg L−1 and 0.004 to 1.01 mg L−1, respectively. The lowest value was recorded in E4 for NO3− and in E1 for PO43−. The highest value was recorded in E3 for NO3− and in E2 for PO43−. The presence of NO3− and PO43− can be due to runoff agricultures fields. The high concentrations of these two elements can also contribute to eutrophication of waters.
The Cl− and SO42− contents in water samples varied from 2.23 to 6.22 and 1.37 to 20.12 mg L−1, respectively. These concentrations were below the desirable limits of 200 mg L−1 and 200 mg L−1 recommended by the WHO (2008), respectively. The source of soluble sulfate in the river is sulfur-bearing sedimentary rocks and mainly originates from the oxidation of sulfate ores and the presence of shale (see Fig. 2). Gold moving with iron sulfides (FeS pyrite), small amounts of sulfur are brought into contact with water and oxygen during excavation work for the recovery of ore (gold) which generates sulfates (Aranguren et al. 2008). The results of overall ion balance (IB) are shown in Table 5.
The IB values ranged from 4.02 to 15.06. With the exception of E4, the calculated IB were all less than 10%. The value of the IB is in the range of tolerable values for four (E1, E2, E3, E5) of the five samples analyzed. This rate is an argument in favor of the reliability of the analytical data on major elements. The Piper triangular diagram (Piper 1944) obtained from the water samples analyzed is illustrated in Fig. 6.
Figure 6 shows the calcium dominance (three of the five samples) in the sub-triangle of the cations, while the anions show a sulfated and chlorinated tendency. The percentage of samples falling under alkaline earth (Ca2+ + Mg2+) were between 40 to 80% and the alkalis (Na+ + K+) were 50%. The type of Ca–SO4 water is predominant compared to the samples analyzed. The predominance of this water types is indicative of the dissolution process of minerals, particularly silicate minerals (Rakotondrabe et al. 2017a, b). The Wilcox and Riverside diagrams of water samples are shown in Fig. 7a and b, respectively.
The Wilcox diagram represents the percentage Na and EC at the same time and grades the water samples into five classes, namely class I, class II, class III, class IV, and class V, representing excellent to good, good to permissible, permissible to doubtful, doubtful to unsuitable, and unsuitable water quality, respectively. The sodium level varied from 23.7 to 62.2%. Depending on these percentages of sodium and the electrical conductivity (Wilcox diagram), all samples were suitable (excellent) for irrigation.
The sodium absorption ratio (SAR) was between 0.07 and 1.5. The SAR data represented on the Riverside diagram show that all the samples had a weak alkalizing power. This indicates that the salinity and alkalinity values of nearly all the water samples were very low. These waters were good quality and could be used on any type of soil with a little problem of sodium accumulation.
Heavy metal concentration in water samples
Water contamination by heavy metals was evaluated by comparing their concentrations with the guidelines for water intended for human consumption (WHO 2011). The TMs in the study area decreased in the order Ni > Cr > Pb > Fe > Zn > Se > As > Cd > Hg > Cu. Figure 8 presents the spatial variations of essential heavy metals (oligo-elements).
Some studies have shown that sulfide minerals in the auriferous vein system of the Batouri gold district, southeastern Cameroon, include pyrite, chalcopyrite, sphalerite, galena, covellite and chalcocite (Suh 2008). Four successive ore mineral assemblages were distinguished: (1) Pyrite I; (2) Pyrite II, arsenopyrite, chalcopyrite and pyrrhotite; (3) sphalerite, tetrahedrite, chalcopyrite, galena, pyrite III and gold; and (4) marcasite, greenockite, bornite, covellite, goethite, Mn oxides and gold. Primary gold occurs either as invisible gold within pyrite (all generations of pyrite) and arsenopyrite, or as visible gold along cavities and fractures in pyrite, arsenopyrite and quartz (Akwinga 2010).
In Fig. 8, copper ranged from 0.002 to 0.01 mg L−1. These concentrations were below the WHO standard (2011) and Cameroonian standard (NEPIC 1996) at all the sampling points. Copper in optimum quantity in its natural sources may not cause any environmental problem, however, when overabundant quantities of copper are released into the environment, it becomes a problem. The presence of copper in water samples could be due to soil leaching containing the copper and the weathering of minerals, such as chalcopyrite, from the gold vein present in the study area as observed by Suh (2008) and Akwinga (2010).
Zinc concentrations (0.06–0.33 mg L−1) were below the WHO standard (see Table 3) at all the sampling points. The presence of zinc in these water samples could be due to the dissolution of sphalerite mineral. The auriferous vein system of the study area includes this mineral (Suh 2008).
Nickel concentrations (8.33–22.45 mg L−1) exceeded the WHO standard (2011) at all the sampling points. Nickel could come from Ni–Cd batteries used in gold mine sites (Pahimi et al. 2015). In ultrabasic rocks and soils derived from them, Ni and Cr are mainly associated with primary magnesium silicates (olivine, serpentine, chlorite). They are depending on the climate, with secondary phyllosilicates of the smectite and vermiculite type (temperate climate) or with oxides and Fe hydroxides (tropical climate) (Chardot et al. 2007). In addition, nickel is present in the air, in suspended particles, after being released by human activities or natural phenomena, such as volcanic eruptions, forest fires and meteorites from the upper atmosphere (Fay et al. 2005). Nickel is considered to be one of the most potent carcinogens, with initiator genotoxic activity (Costa et al. 1994).
Iron is the most abundant element in the various water samples as well as in the sediments and the geochemical bottom. The iron concentrations varied from 0.05 to 6.78 mg L−1. They were below the WHO standard (2011) for E1 and E2 samples. These samples were before the mining sites. For E3, E4 and E5 samples, iron concentrations exceeded the limit recommended by WHO (2011). Therefore, it has greater bioavailability. The soils of the eastern region of Cameroon belong to the large group of red and yellow ferralitic soils which cover all of the country south of the 8th parallel (Segalen 1967). Red ferralitic soils are red, deep, loose, clayey and porous soils suitable for forest-type vegetation (Ngon Ngon et al. 2009). Their pedogenesis involves complete hydrolysis of rock minerals. The high iron contents in the waters could be first due to the leaching of the lateritic grounds, which confirm the results of Hernandez (2003). Second, this could be due to the hydrolysis of the pyrite or pyrrhotite, which releases iron. The auriferous vein system of the Batouri gold district includes these two minerals (Suh 2008; Akwinga 2010).
Chromium concentrations (6.53–12.59 mg L−1) were above the maximum limit of 0.05 mg L−1 recommended by WHO (2011) and Cameroonian standard (NEPIC 1996). This indicated that the water was polluted with chromium. Chromium is usually found in the trivalent state in the medium ultrabasic (Oze et al. 2004). Trivalent chromium is the most common condition: most soils and rocks contain small amounts of chromic oxide. In view of the high Ni and Cr concentrations, they could come from the same source. Trivalent chromium is an essential trace element for the metabolism of sugar in humans (Chardot et al. 2007). Chromium deficiency can affect the ability of insulin to regulate the level of sugar in the body. High chromium levels could cause many cancers as lung cancer, cancers located in the nasal cavity, larynx or stomach, cancers located in the bones, stomach, prostate, genitals, kidneys, bladder, blood (Pichard et al. 2005).
The Se concentrations ranged from 0.002 to 0.08 mg L−1 and were above the WHO limit (2011) for E2, E3 and E4 samples. Selenium results from the disintegration of rocks, especially clayey shales. According to Fig. 2, which shows the geological context of Batouri, shales are observed. In rocks, selenium could be incorporated into iron sulfides (pyrite, chalcopyrite) under reducing conditions (Gourcy et al. 2011). Selenium could be released by human activities including the combustion of hard coal (Longchamp 2012). It is a micronutrient that in high dose and short term has negative effects on health, including loss of hair, fatigue and a change in the nervous system. Figure 9 shows the spatial variations of non-essential heavy metals.
In Fig. 9, lead concentrations varied from 1.59 to 3.58 mg L−1. These concentrations were above the WHO standard (2011) at all the sampling points. The presence of lead in mining sites could be due to the excavation activities of miners resulting in weathering and leaching metal from waste rock dumps or can be from exhaust pipes of heavy-duty trucks, vehicles and mining machinery (Aranguren et al. 2008). The presence of lead in water samples could also be due to the weathering of minerals containing lead in particular lead sulfides, such as galena (PbS). Lead concentrations were higher than lead levels in the two gold zones of Bonikro and Betare-Oya (Bamba 2012; Rakotondrabe et al. 2017a, b).
Arsenic concentrations ranged from 0 to 0.02 mg L−1 and were above the maximum limit of 0.01 mg L−1 recommended by the WHO (2011) for E1, E2, E3 and E4 samples. These concentrations were below the Cameroonian standard (NEPIC 1996). The soils contain arsenic primary minerals derived directly from the weathering of the bedrock of the subsoil where the most frequent species of primary mineralization are arsenopyrite (FeAsS), realgar (AsS), orpiment (As2S3) (Aranguren 2008). As arsenic is often associated with deposits of Au, Bi, Cd, Co, Fe, Hg, Mo, Ni, Pb, Pt, Sb, Se, Sn, U, W and Zn (Smedley and Kinniburgh 2002), it has been used as an indicator for mineral prospecting, especially gold (Dictor et al. 2004).The auriferous vein in the study area includes arsenopyrite (Suh 2008; Akwinga 2010). Then, the presence of arsenic in water samples could be due to chemical alteration of this mineral. Naturally, arsenic occurs in rocks and gold ore, such as arsenopyrite (Asamoah 2012). If undisturbed, they may remain in the rocks and beneath the earth’s surface for many years. Human activities, however, often result in the release of them into the environment.
Mercury concentrations ranged from 0 to 0.02 mg L−1 exceeded the standard set by the WHO (0.01 mg L−1) at all the sampling points excepted E1 sample. The mercury is used for the gold amalgamation. In this process, the mercury vapors are deposited in the surrounding rivers. This indicated that the presence of mercury in the water was due to mining activity. The use of mercury is prohibited by the mining code of Loi n° Cameroon (2016). As quicksilver, mercury is found in nature in various forms as released into the environment through natural processes, such as volcanic eruptions, the weathering of rocks, and forest fires, and a number of anthropogenic activities (UNEP 2002). Mercury does not break down in the environment, but instead is cycled between the atmosphere, land, and water, and can travel large distances from the original source (Tay et al. 2008). Mercury and its compounds affect kidneys and can disturb immune processes. The toxic effects of mercury were mainly associated with the central nervous system; inorganic mercury also produces cardiotoxicity (Su and Chen, 1979; Brawer et al. 1998).
Cadmium concentrations ranged from 0.001 to 0.02 mg L−1 exceeded the maximum permissible limit of 0.003 mg L−1 recommended by the WHO (2011) for E4 and E5 samples. These concentrations were higher than that observed in Djouzami gold mining site (Ayiwouo et al. 2020). Sphalerite is the main source of cadmium. Dissolution of sphalerite present in gold vein at Batouri could be the cause of water pollution. Cadmium is one of the most toxic elements with reported carcinogenic effects in humans (Goering et al. 1995). Rieuwerts (2015) noted that ingestion of high level of cadmium can result in toxicity of the kidney and skeletal system and may result in cardiovascular diseases.
Assessment of overall water quality
The results of the HPI and WQI calculation of the water samples are given in Fig. 10a and b.
In Fig. 10a, the HPI ranged from 4.12 to 14.01. The values of the samples downstream were all higher than the value of E1. The waters downstream of sampling point E1 were contaminated by heavy metals. The E4 sample had the highest overall contamination level.
In Fig. 10b, below the green horizontal line, the WQI indicated good quality water. Above the red horizontal line, the WQI indicated water unfit for consumption. Spatial variations in the WQI indicated a significant deterioration in water quality from upstream to downstream. The water upstream from the sampling point E1 was of good quality and could be used for human consumption. The waters at the sampling points E2, E3, E4 and E5 were very bad quality. They were unfit for human consumption. Sampling points E3 and E4 were the most contaminated points.
Conclusion
This study was carried out in the semi-mechanized gold mining site from the locality of Batouri (East Cameroon). The spatial and temporal variations of the physicochemical parameters, major ions and heavy metals were determined. By employing the heavy metal pollution index, the water quality index, the sodium adsorption rate and the percentage of sodium the overall water quality were assessed. The most important results of this study are as follows:
-
Visually, semi-mechanized gold mining activity has significant negative effects on surface water. The effects were manifested by the diversion of water courses and the turbid state of surface water.
-
The surface water in the study area was acidic to slightly basic, very weakly mineralized, very turbid, polluted by suspended solids and had high dissolved oxygen values.
-
The hydrochemical evaluation showed the dominance of calcium in these waters and these waters were suitable (excellent) for irrigation.
-
At certain sampling points, these waters were very bad quality and not suitable for human consumption.
References
Acheampong, M. A., Adiyiah, J., & Ansa, E. D. O. (2013). Physico-chemical characteristics of a gold mining tailings dam wastewater. Journal of Environmental Science & Engineering, 2, 469–475.
Akwinga, A. V. (2010). Lode gold mineralization in the Neoproterozoic granitoids of Batouri, south-eastern Cameroon. Doctoral Thesis (Dissertation).
Aranguren, S. M. M. (2008). Contamination en métaux lourds des eaux de surface et des sédiments du Val de Milluni (Andes Boliviennes) par des déchets miniers Approches géochimique, minéralogique et hydrochimique. Planète et Univers [physics]. Thèse de doctorat, Université Paul Sabatier – Toulouse III.
Aranguren, S. M. M., Probst, A., Roulet, M., & Isaure, M. P. (2008). Contamination of Surface Waters by Mining Wastes in the Milluni Valley (Cordillera Real, Bolivia): Mineralogical and Hydrological Influences. Applied Geochemestry, 23, 1299–1324.
Asamoah, E. (2012). The Impact of Small-Scale Gold Mining Activities on the Water Quality of River Birim in the Kibi Traditional Area. Unpublished Master’s Thesis. Kwame Nkrumah University of Science and Technology, Kumasi Ghana.
Asare-Donkor, N. K., Ofosu, J. O., & Adimado, A. A. (2018). Hydrochemical characteristics of surface water and ecological risk assessment of sediments from settlements within the Birim River basin in Ghana. Environmental Systems Research, 7, 9.
Ayiwouo, N. M., Mambou, L. L. N., Mache, J. R., Kingni, S. T., & Ngounouno, I. (2020). Waters of the Djouzami gold mining site (Adamawa, Cameroon): Physicochemical characterization and treatment test by Banasmectite (West, Cameroon). Case Studies in Chemical and Environmental Engineering. https://doi.org/10.1016/j.cscee.2020.100016.
Bamba, Y. (2012). Evaluation des impacts de l’exploitation de la mine d’or de Bonikro (Côte d’Ivoire) sur les ressources en eau. Master en ingénierie de l’eau et de l’environnement 2Ie Ouagadougou.
Boeglin, J. L., Ndam, J. R., & Braun, J. J. (2003). Composition of the different reservoir waters in a tropical humid area: example of the Nsimi catchment (Southern Cameroon). Journal of African Earth Sciences, 37, 103–110.
Brawer, J. R., McCarthy, G. F., Gornitsky, M., Frankel, D., Mechindate, K., & Schipper, M. (1998). Mercury chloride induces a stress response in calculated astrocytes characterized by mitochondrial uptake of iron. NeuroToxicology, 19(6), 767–776.
Chardot, V., Echevarria, G., Gury, M., Massoura, S., & Morel, J. (2007). Nickel bioavailability in an ultramafic toposequence in the Vosges Mountains (France). Plant and Soil, 293(1), 7–21.
Commune de Batouri PNDP. (2012). Rapport de diagnostic de l’Espace Urbain Communal de la commune de Batouri.
Costa, M., Salnikow, K., Cosentino, S., Klein, C. B., Huang, X., & Zhuang, Z. (1994). Molecular Mechanisms of Nickel Carcinogenesis. Environmental Health Perspectives, 102(3), 127–130.
Cude, C. (2001). Oregon water quality index: A tool for evaluating water quality management effectiveness. Journal of American Water Resources Association, 3, 125–137.
Dictor, M. C., Baranger, P., Chéry, L., Michel, K., Barbier, J., Clozel, B., Touzé, S., Gall, A. C. L., & Brosselin, P. (2004). Synthèse des travaux de R&D en France (1999-2004) sur la thématique Arsenic. Rapport Final BRGM.
Dogaru, D., Zobrist, J., Balteanu, D., Popescu, C., Sima, M., & Yang, H. (2009). Community perception of water quality in a mining-affected area: A case study for the Certej Catchment in Apuseni Mountains in Romania. Environmental Management, 43, 1131–1145.
Emmanuel, K., Sundararajan, R., & Williams, J. (2004). Hurricanes and global warming, Results from Downscaling IPCC AR4 Simulations. Bulletin of the American Meteorological Society, 89(3), 347–368.
Fay, M., Wilbur, S., Abadin, H., Ingerman, L., & Swarts, S. G. (2005). Toxicological Profile for Nickel, U.S. Department of Health and Human Services, Public Health Service. Agency for Toxic Substances and Disease Registry (ATSDR).
FDEP. (2013). Derivation of dissolved oxygen criteria to protect aquatic life in Florida’s fresh and marine waters. DEP-SAS-001/13, 232.
Fifi, U. (2010). Impacts des eaux pluviales urbaines sur les eaux souterraines dans les pays en développement – mécanismes de transfert des métaux lourds à travers un sol modèle de Port-au-Prince, Haïti. Thèse de doctorat.
Gafur, N., Sakakibara, M., Sano, S., & Sera, K. (2018). A case study of heavy metal pollution in water of bone water by artisanal small-scale golg in the eastern part of gorontolo, Indonesia. Water, 10(11), 1507.
Garnels, R. M., & Mackenzie, F. T. (1971). Evolution of sedimentary rocks. New York: W. W Norton.
Goering, P. L., Waalkes, M. P., & Klaassen, C. D. (1995). Toxicology of cadmium. Handbook of Experimental Pharmacology, 115, 189–214.
Gould, D. W., King, M., Mohapatra, B. R., Cameron, R. A., Kapoor, A., & Koren, D. W. (2012). A critical review on destruction of thiocyanate in mining effluents. Mining Engineering, 34, 38–47.
Gourcy, L., Lions, J., Wyns, R., Dictor, M. C., Brenot, A., Crouzet, C., & Ghesten, J. P. (2011). Origine du sélénium et compréhension du processus dans les eaux du bassin Seine-Normandie. Rapport final BRGM/RP-59445-FR.
Hernandez, L. M. (2003). Dynamique des éléments traces métalliques dans les sols de différents écosystèmes français. Thèse de doctorat, Université Paul Sabatier Toulouse 280.
Jain, C. K., Bandhyopadhyay, A., & Bhadra, A. (2010). Assessment of ground water quality for drinking purpose, district Nainital, Uttarakhand, India. Environmental Monitoring Assessment, 166, 663–676.
Koupaee, M., & Wang, W. Y. (2018). Wikihow: A large scale summarization dataset. ArXiv preprint arXiv: 1810.09305.
Li, J., Li, F., Liu, Q., & Zhang, Y. (2014). Trace metal in surface water and groundwater and its transfer in a Yellow River alluvial fan: evidence from isotopes and hydrochemistry. Science of the Total Environment, 472, 979–988.
Loi n°2016–17 du 14 décembre (2016) portant Code minier.
Longchamp, M. (2012). Etude biogéochimique du transfert du sélénium dans un système eau-plante atmosphère: conséquences sur la physiologie du Zea mays subsp. mays (L.). Biodiversité et Ecologie. Université Pierre et Marie Curie - Paris VI.
Lundgren, D. G., & Silver, M. (1980). Ore leaching by bacteria. Annual Review of Microbiology, 34, 263–283.
Lyon, J. (2010). Interview. Washington D.C.: Minera Policy Center.
Mambou, N. L. L., Mache, J. R., Ayiwouo, N. M., Takougang, K. S., Abende, S. R. Y., & Roukaiyatou, S. (2020). Physicochemical characterization of mining waste from the Betare-Oya gold area (East Cameroon) and an adsorption test by Sabga smectite (North-West Cameroon). Scientifica, 2020, 12.
Milési, J. P., Toteu, S. F., Deschamps, Y., Feybesse, J. L., Lerouge, C., Cocherie, A., et al. (2004). Geology and major ore deposits of Central Africa: Orléans. Paris: BRGM.
Mohan, S. V., Nithila, P., & Reddy, S. J. (1996). Estimation of heavy metal in drinking water and development of heavy metal pollution index. Journal of Environmental Science and Health, A31, 283–289.
NEPIC. (1996). Normes environnemtales et procédures d’inspection au Cameroun. Redcar: NEPIC.
Ngon Ngon, G. F., Yongué, F. R., Bitom, D. L., & Bilong, P. (2009). A geological study of clayey laterite and hydromorphic material of the region of Yaoundé (Cameroun): a prerequisite for local material promotion. Journal of African Earth Sciences, 55, 69–78.
Ngoupayou, N. J. R., Dzana, J. G., Kpoumie, A., Ghogomu, T. R., Takounjou, F. A., Braun, J. J., et al. (2016). Present-Day Sediment Dynamics of the Sanaga Catchment (Cameroon): From the Total Suspended Sediment (TSS) to Erosion Balance. Hydrological Sciences Journal, 61, 1080–1093.
Nollet, M. L. (2007). Hand book of water analysis (2nd ed., p. 786). London: CRC Press.
Odoulami, L. (2009). La problématique de l’eau potable et la santé humaine dans la ville de Cotonou (République du Bénin). Thèse de Doctorat, Université d’Abomey-Calavi.
Oze, C., Fendorf, S., Bird, D. K., & Coleman, R. G. (2004). Chromium geochemistry in serpentinized ultramafic rocks and serpentine soils from the Franciscan complex of California. American Journal of Science, 304, 67–101.
Pahimi, H., Panda, C. R., Ngassoum, M. B., & Tchameni, R. (2015). Environmental impacts of mining in the volcano-sedimentary basins of cameroon: Case study of artisanal gold mine tailings (BetareOya, East-Cameroon). International Journal of Energy and Environmental Engineering, 2, 5–15.
Pichard, A., Bisson, M., Diderich, R., Houeix, N., Hulot, C., Lacroix, G., Lefevre, J. P., Leveque, S., Magaud, H., Morin, A., Rose, M., & Pepin, G. (2005).Chrome et ses dérivés, INERIS—Fiche de données toxicologiques et environnementales des substances chimiques (https://www.ineris.fr).
Piper, A. M. (1944). A graphic procedure in the geochemical interpretation of water analyses. Transactions of the American Geophysical Union, 25, 914–923.
Rakotondrabe, F., Ngoupayou, J. R. N., Mfonka, Z., Rasolomanana, E. H., Abolo, A. J. N., Asone, B. L., et al. (2017a). Assessment of surface water quality of Bétaré-Oya gold mining area (East-Cameroon). Journal of Water Resources Protection, 9, 960–984.
Rakotondrabe, F., Ngoupayou, J. R. N., Mfonka, Z., Rasolomanana, E. H., Abolo, A. J. N., Asone, B. L., et al. (2017b). Water quality assessment in the Bétaré-Oya gold mining area (East-Cameroon): Multivariate statistical analysis approach. Science of the Total Environment, 610–611, 831–844.
Rieuwerts, J. (2015). The elements of environmental pollution. Abingdon: Routledge.
Save Drinking Water Foundation. (2017). Exploitation minière et la pollution de l’eau. https://www.safewater.org.
Segalen, P. (1967). Les sols et la géomorphologie du Cameroun. Cahier ORSTOM series pédologique, 2, 137–187.
Singh, G., & Rakesh, K. K. (2016). Heavy metal contamination and its indexing approach for groundwater of Goa mining region. India. Applied Water Science. https://doi.org/10.1007/s13201-016-0430-3.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemestry, 17(5), 517–568.
Su, J. Y., & Chen, W. J. (1979). The effects of methylmercury on isolated cardiac issues. American Journal of Pathology, 95(3), 753–764.
Suh, C. E. (2008). Sulphide Microchemistry and Hydrothermal Fluid Evolution in Quartz Veins, Batouri Gold District (Southeast Cameroon). Journal of the Cameroon Academy of Science, 8, 19–30.
Tay, C., Asmah, R., & Biney, C. A. (2008). Trace metal concentrations in commercially important fishes from some coastal and inland waters in Ghana. West African Journal of Applied Ecology, 13, 27–38.
Toteu, S. F., Penaye, J., Deloule, E., Van Schmus, W. R., & Tchameni, R. (2006). Diachronous evolution ofvolcanosedimentary basins north of the Congo craton: Insights from U-Pb ion microprobe dating of zircons from the Poli, Lom and Yaoundé Groups (Cameroon). Journal of African Earth Sciences, 44, 428–442.
Toteu, S. F., Van Schmus, R. W., Penaye, J., & Michard, A. (2001). New U-Pb and Sm-Nd Data from North Central Cameroon and Its Bearing on the Pre-Pan-African History of Central Africa. Precambrian Research, 108, 45–73.
Umer, A., Assefa, B., & Fito, J. (2020). Spatial and seasonal variation of Lake water quality: Beseka in the Rift Valley of Oromia region, Ethiopia. International Journal of Water Energy and Resources, 4, 47–54.
UNEP. (2002). Chemicals, Inter-Organization Programme for the Sound Management of Chemicals. Global mercuryassessment. Nairobi: UNEP Chemicals.
Wanélus, F. (2016). Caractérisation physico-chimique de l’eau destinée à la consommation humaine dans la Région Métropolitaine de Port-au-Prince. Haïti: Master, Université de Lyon France.
WHO (World Health Organization). (2008). Guidelines for drinking-water quality incorporating first addendum to Third Edition. Recommendations Geneva, 1, 595.
WHO World Health Organization. (2011). World Health Organization Guidelines for Drinking-Water Quality. Recommandations. 4th Edition, Geneva.
Zobrist, J., Sima, M., Dogaru, D., Senila, M., Yang, H., Popescu, C., et al. (2009). Environmental and socioeconomic assessment of impacts by mining activities—a case study in the Certej River catchment, Western Carpathians, Romania. Environmental Science and Pollution Research, 16, 14–26.
Acknowledgements
We thank Mr. NSOE MENGUE and of Water Treatment and Industrial Filtration of the IUT of Ngaoundéré. We thank Dr KAYO head of Laboratory Hydrobiology and Environment of the University of Yaoundé I, We also thank Mr. MIMBANG Aristide, the Coordinator of CAPAM. We thanks Pr Ngounouno, Director of School of Geology and Mining Engineering.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mambou Ngueyep, L.L., Takougang Kingni, S., Ayiwouo Ngounouno, M. et al. The impact of gold mining exploitation on the physicochemical quality of water: case of Batouri (Cameroon). Int J Energ Water Res 5, 159–173 (2021). https://doi.org/10.1007/s42108-020-00106-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42108-020-00106-0