Abstract
Cu, Cr, Fe, Mn, Ni, Pb, and Zn in the sediments of the Kabini River, Karnataka, India was studied to determine the association of metal with various geochemical phases by sequential extraction. The variations of heavy metal concentration depend on the lithology of the river basin and partly on anthropogenic activities. The Kabini River sediments are dominated by Sargur supracrustals with amphibolites, gneisses, carbonates, and ultrabasic rocks weathering into gneissic and serpentine soils carrying a natural load of cationic heavy metals. The source of heavy metals in the Kabini riverbed sediments is normally envisaged as additional inputs from anthropogenic over and above natural and lithogenic sources. Geochemical study indicates the metals under study were present mostly in the least mobilizable fraction in the overlying water and it is concluded that heavy metals in these sediments are to a great extent derived from multisource anthropogenic inputs besides geochemical background contributions The results show that lead and chromium have higher potential for mobilization from the sediment due to higher concentration at the exchangeable ion and sulfide ion bounded, also Cu and Pb have the greatest percentage of carbonate fraction, it means that the study area received inputs from urban and industrial effluents. Association of the Fe with organic matter fraction can be explained by the high affinity of these elements for the humic substances. Further, Zn and Ni reveal a significant enrichment in sediment and it is due to release of industrial wastewater into the river. These trace metals are possible contaminants to enter into aquatic and food chain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sediments function as sinks for various pollutants such as heavy metals and play a significant role in the remobilization of contaminants in the aquatic systems under favorable conditions. Such potential of sediment for being a sink as well as a source of contaminant can make sediment chemistry and toxicity key components of the quality of aquatic system. This could be particularly useful to investigate large riverine environments where the aqueous concentrations of pollutants are frequently close to the detection limits of chemical and toxicological methods. On the other hand, the ability of sediments to adsorb organic and inorganic contaminants makes sediment analysis a valuable tool to assess and monitor water quality and track contaminant transport in fluvial realms and mobilization in marine environments. Besides, chemical analysis of the natural matrices such as water, suspended particulate matter, and sediment is the most direct approach to reveal the heavy metal pollution status in the components of the environment, unless information on the extent of transfer of toxic metals on to the matrices of living beings an integrated multimatrix analytical data is made available.
Evaluated heavy metals concentrations in river systems are often considered as indicators of anthropogenic influence and they are a risk to the natural environment. Therefore, it needs to assess and track the concentration of these heavy metals. The assessment and the effects of sediment contamination can review through the geochemical partitioning of trace metals in sediment–water interface (Lu and Allen 2001).
The discharge of heavy metals from sediments into the water body will depend on the speciation (i.e., precipitated, complexed, adsorbed, or solubilized) of metals, sediment pH, and physical and chemical characteristics of the aquatic system (Morgan and Stumm 1991). Chemical partitioning can be defined as the identification and quantification of the different chemical species, forms, or phases present in sediment. Besides, heavy metals are associated with sediments in different ways, and their association determines mobility and availability (Ahumada et al. 1999). This type of association between metals and the sediments can be understood in detail by sequential extraction techniques. Heavy metals are distributed in sediments, as exchangeable bound, carbonate, iron–manganese oxide, organic matter, and residual fraction (Dean 2002).
A review of the earlier literature reveals that a good deal of attention has been paid to the heavy metal studies of sediment in various countries. A number of researches have been made in earlier publications on sediments contamination that caused by anthropogenic or natural activities (Aloupi and Angelidis 2001; Chiu-Wen et al. 2007; Evans et al. 2003;Liaghati et al. 2003; Karbassi and Shankar 2005; Santos et al. 2005; Matthieu et al. 2006; Jain et al. 2007).
The studies conducted in different locations in India have confirmed the serious contamination of river sediment by heavy metal (Sharma and Pandey 1998); Ganga River (Jain 2004), Yamuna River (Singh et al. 2005), Gomati river sediments (Jain et al. 2008), and Narmada river of Maharashtra state (Gholami and Srikantaswamy 2009) in Cauvery, India.
The Kabini River system in Mysore districts of Karnataka, India has a record of higher pollutants level than normal values. In this concern, this study attempts to apply the enrichment factor and geochemistry index to understand the degree of contamination by heavy metals and its sources.
Materials and methods
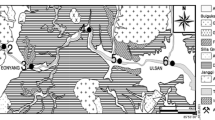
Riverbed sediments were collected from the surface along its main stream at 17 predetermined GPS recorded locations (Fig. 1). The sediments were stored in plastic vials and frozen at −20°C pending analytical procedures. In the laboratory, sediment samples were defrosted at room temperature, dried at 40°C up to a constant weight, ground, and homogenized in mortar to a fine powder.
Total metals (Cu++, Cr+3, Fe+3, Mn++, Ni++, Pb++, and Zn++) were determined by absorption atomic spectrophotometer technique after acid digestion. For digestion, 2 g of dried sample was put into a polytetrafluoroethylene vessel with 4 ml of HNO3, 2 ml of HCl, and 2 ml of HF. For each digestion program, a blank was prepared with the same amount of acids.
The sequential extraction procedure is based on the methods of Tessier et al. (1979); 1 g sample was transferred to a Teflon vial for strong acid and high-temperature processing. Chemical partitioning studies were carried out in four sequential steps: (1) acetic acid 25 % v/v, (2) acetic acid 25 % v/v 0.1 M hydroxylamine hydrochloride, (3) 30 % H202 “extraction with 1 M ammonium acetate”, and (4) hot 50 % HCl (Chester and Hughes 1967; Gibbs 1977).
In this study, to identify the association between heavy metals, chromium, copper, iron, lead, manganese, nickel, and zinc analyses, statistical tools such as cluster analysis, principal component analysis (PCA), and correlation matrix were exploited on raw data through using computer software package SPSS 13.
Result and discussion
Concentration of copper, chromium, iron, manganese, lead, and zinc in bed sediments from sample station are presented in Table 1. The high value of chromium concentration at stations 5 and 6 seems due to textiles, paper mill, and leather industry in Nanajangud industrial area as well as domestic sewage. Since the pH was above 6, the chromium would probably be in the trivalent state and therefore be nontoxic. The high value of copper concentration at station 5 seems to be present at the pharmaceutical and electronic industry in Nanjangud industrial area; also at station 8, the industrial emission as well as Nanjangud municipal effluent that release to the Kabini River was attributed. The variation in copper at different station shows different sources of copper in the study area. The geochemistry index has been identified as the degree of contamination by heavy metals from lithogenic and anthropogenic sources.
Iron distribution in nature is in oxidation states at Fe2+ and Fe 3+. The ferric oxides are very fine grained and carried by rivers either as stabilized colloids as adsorbed coatings on particles. In addition, much of the Fe content are fixed within the crystalline structure of primary and secondary minerals and are totally nonreactive. A large portion may be soluble under reduced condition of typical anaerobic sediments, but essentially all of the potentially reactive Fe would be oxidized to sparingly soluble ferric oxyhydroxide under upland conditions (Gambrell et al. 1983). High concentration of iron in sediments could cause stress for bottom-dwelling organisms.
The high value of lead concentration at stations 5, 7, and 8 seems due to the presence of fuel combustion from industrial area and automobile emission of Nanjangud–Mysore road. Low hardness value in most stations of the Kabini River makes it more vulnerable to lead toxicity. Lead toxicity is a concern since the metal has a cumulative effect within an organism. Since low hardness values are evident in station 8, there is little dilution effect of the metal by Ca and Mg ions; therefore, more of the available lead can rapidly be taken by an organism. In this study, pH values were above 7, therefore the availability of Zn becomes very low and it precipitates.
The results of the PCA are presented in Tables 2 and 3. In the analysis, four principal components (PC) with eigenvalues higher than 1 were considered. PCA lead to a reduction of the initial dimension of the dataset to four components which explain 70.44 % of the data variation. Elements such as Zn and Ni were shown to be strongly associated in the first component, suggesting a natural geochemical association of major rock-forming elements in sediments; the rotated component matrix indicated that Cu and Pb were strongly associated in the second component (PC2). These elements may reflect anthropogenic contamination in the Kabini riverbed sediments. The third component included associated Cr and Mn which represented a natural input of these metals from parent rocks; iron was univocally isolated in the fourth component and has relatively weak association with all of the others. For understanding of the elemental mobility in the Kabini River, the chemical composition of surface sediments has to be determined.
Cluster analysis (CA) was applied on sediment samples in order to identify the degree of inter-element correlation. The clustering of elements indicates common anthropogenic sources. Heavy metal relationships were analyzed by correlation matrix (Table 4). The bed sediments result showed a significant positive correlation between Cu and Pb (r = 0.620) and it showed a positive correlation between Zn and Ni (r = 0.793). In general, correlation between metals agreed with the results obtained by CA. The influence of these interrelated elements was quantified by results of the PCA.
Cluster analysis showed that Cu, Pb, Zn, and Ni formed cluster “A” which shows that Cu content in sediments was not only due to its presence in the parent rocks but also due to anthropogenic effluents of industrial areas, and confirms the combination of metal affiliation of varied origin. Lead and copper demonstrated similar behavior as did Zn and Ni as a result from a variety of industrial activities. Cr formed cluster “B” and Mn showed cluster “C”, finally Fe forms cluster “D” and joins cluster B and C; meaning, the sources of Fe are different (Fig. 2).
Chemical partitioning patterns for each metal and sampling point are shown in Table 5. The effects of heavy metals in the environment depend, to a large extent, on whether they occur in forms that can be taken up by plants or animals. A wide range of values for heavy metal concentrations was observed for the sediments in Fig. 3.
Percentile of elements in various sedimentary phases can be summarized as follows:
-
Exchangeable ion fractions: Pb (28 %) > Cr (22 %) > Cu, Mn (19 %) > Zn (18 %) > Ni (17 %) > Fe (15 %)
-
Carbonate fractions: Cu (21 %) > Pb (20 %) > Cr (19 %) > Mn (17 %) > Ni, Zn (14 %) > Fe (10 %)
-
Fe/Mn oxides fraction: Pb (18 %) > Cr (17 %) > Cu, Mn, Zn (13 %) > Ni (11 %) > Fe (3 %)
-
Organic matter fractions: Fe (24 %) > Mn, Cr, Cu (19 %) > Pb (11 %) > Zn (10 %) > Ni (8 %)
-
Residual fraction: Ni (50 %) > Fe (48 %) > Zn (45 %) > Mn (32 %) > Cu (25 %) > Cr, Pb (23 %)
Exchangeable species usually related to the adsorbed metals on the sediment surface. Lead and chromium in exchangeable forms can be easily remobilized into the Kabini River water and they are derived from natural and anthropogenic sources. High concentration in exchangeable fraction indicates high bio-availability of Pb.
The results from carbonate fractions reveal 21 % Cu and 20 % Pb. The study area receives inputs from urban and industrial effluents, particularly at stations 6 and 9. The significant concentration of Pb present in the bioavailable fraction is due to the influence of anthropogenic material. Relative concentration of Pb and Cr in Fe/Mn oxides fraction may be attributed to the flocculation of colloids on Pb and Cr in the Kabini River system. Relative concentration of Pb and Cr associated with this fraction are caused by the adsorption of these elements by the colloids of Mn and Fe (Jenne 1968).On the other hand, association with hydroxides of Fe and Mn acts to dissipate trace metals, especially Pb, due to large adsorption in surface areas. Significant associations between trace metals and Fe and Mn oxides in sediments have been recorded in regions that receive discharges of industrial effluents (Relic et al. 2005; Sakan et al. 2007).
Association of the metals with organic matter can be explained by the high affinity of the elements for the humic substances that comprise of these materials. This is due to the formation of the stable metal complexes and insoluble metal sulfides that are important sinks for trace metals in sediments (Chandra Sekhar et al. 2004). Fe showed the highest content in the organic matter fraction.
These metals are strongly complexed in sediments and are released following degradation of the organic matter or oxidation of sulfides to sulfates. The observed behavior was probably due to the affinity of the metals for the organic matter present in water, given the high anthropogenic loadings in the study region (Alves et al. 2007).
It has been suggested that residual fraction is most important for uncontaminated sediments. In the present study, Cu, Cr, and Pb were associated with the lowest portion of residual fraction. In uncontaminated settings, the residual fraction is a significant geochemical phase for trace metal retention. Ni and Zn have 50 % and 45 %, respectively, in this fraction and attributes in less concentration in aquatic fauna and food chain than the other metals. The relative content of a metal in the residual phase can be used as a measure of the contribution of natural sources and also of the degree of contamination of the fluvial system, with a higher percentage indicative of lower levels of pollution (Singh et al. 2005).
With reference to the above study result, that bioavailability is related to solubility, metal bio-availability decrease in order of:
Exchangeable > sulfide ions > organic > residual (Ma and Rao 1997; Xiangdong et al. 2000).
Among the nonresidual fractions, the ion exchange fraction was much higher than other fractions in all stations. The percentage of lead, chromium, and copper in the nonresidual fractions was greater than the residual fraction. About 77 % of the lead and chromium in sediment was associated with the exchangeable, carbonate, organic matter, and oxidation fractions. These results indicate that lead and chromium have greater potential for mobilization from sediments to water because of their higher concentration at the ion exchangeable fraction.
The extent of sediment contamination was assessed using the enrichment factor (EF) and geo-accumulation index (Igeo; Table 6). Enrichment factor is a geochemical approach to discriminate anthropogenic and natural sources and determine with confidence the degree of enrichment/contamination of given metals. It can be calculated as the ratio between the sample and the natural background
The mean EF values for Cr recorded at 52.44 while the highest EF value was found to be 102.52 at station 6. The mean EF values for Cu and Pb measured at 63.52 and 2.7, respectively, while the highest EF values were found to be 100.11 and 8.98 at station 7 which received a huge amount of effluents from Nanjangud industrial area and sewage discharges. Extremely high enrichment of Cu and Cr was recorded in sediments from stations close to pulp and paper mills indicating a point source. The results also show that minor enrichment of Pb is from the effluents released due to industrial activities around the Kabini River.
The mean EF values for Ni and Zn was determined at 9.40 and 9.56, respectively, while the highest EF values are 36.13 and 21.55 at station 11. The results from these calculations show that Zn and Ni reveal significant enrichment in the sediment and that enrichment is due to the release of industrial wastewater to the river.
Table 6 shows the calculated values for the Kabini River sediments (Mueller 1979). Zn and Ni remain in class 1 (unpolluted), along the course of the Kabini River, suggest that there is no pollution with respect to Zn and Ni metals and Cu, Cr in class 2 attribute that the Kabini sediments are moderately polluted except in three stations (11, 12, and 13) which were unpolluted.
Conclusions
The major sources of pollution of the Kabini River are the industrial effluents (return flows), agricultural runoff, and domestic and municipal sewage besides pedogenic background contributions. The source of heavy metals in the Kabini riverbed sediments is envisaged as additional inputs from anthropogenic sources over and above lithogenic sources. The heavy metal averages of riverbed sediments are above and more concentrated than the combined averages contributed by lithogenic sources.
The present study indicates that the heavy metals are in the least mobilizable fraction in the overlying water and in these sediments are to a great extent derived from multisource anthropogenic inputs besides geochemical background contributions . Assessment of sediment pollution by total heavy metal concentrations values, might not firmly guarantee the occurrence of deleterious ecological effects. Lead and chromium have higher potential for mobilization from the sediment because of their higher concentration at the exchangeable ion and sulfide ion bounded. Therefore, geoaccumulation index based on total concentration affirm the intensity of pollution.
A higher geoaccumulation index is to Cu and Cr concentration. Igeo value indicated that the sediment at Kabini River was moderately polluted by Cu and Cr. This contamination can possibly enter into aquatic and solid food chain. It may, however, be added that higher metal values reflect to adsorbed metals in the deposited sediments due to turbulence generated by scavenging organisms at the sediment–water interface. The research result of the study reveal that to protect the river from further contamination, periodical monitoring of the level of pollution, control the mixing of effluent of the concentration of heavy metals in the region, environmental remediation, treatment of industrial effluent and municipal wastewater is warranted. It is essential to prevent direct input of agricultural runoff in minimizing metal remobilization impacts by improving the quality of water through sediment dredging.
References
Ahumada, I., Mendoza, J., & Ascar, L. (1999). Sequential extraction in soils irrigated with wastewater. Communications in Soil Science and Plant Analysis, 30, 1057–1519.
Aloupi, M., & Angelidis, M. O. (2001). Geochemistry of natural and anthropogenic metals in the coastal sediments of the island of Lesvos. Aegean Sea. Environment Pollution, 113(2), 211–219.
Alves, J. P. H., Passos, E. A., & Garcia, C. A. B. (2007). Metals and acid volatile sulde in sediment cores from the Sergipe River Estuary, Northeast Brazil. Journal of the Brazilian Chemical Society, 18, 748–758.
Chandra Sekhar, K., Chary, N. S., Kamala, C. T., Suman Raj, D. S., & Sreenivasa Rao, A. (2004). Fractionation studies and bioaccumulation of sediment-bound heavy metals in Kolleru lake by edible fish. Environment International, 29(7), 1001–1008.
Chester, R., & Hughes, M. J. (1967). A chemical technique for the separation of ferromanganese minerals, carbonate minerals and adsorbed trace element from pelagic sediments. Chemical Geology, 2, 249–262.
Chiu-Wen, C., Chih-Ming, K., Chih-Feng, C., & Cheng-Di, D. (2007). Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan. Chemosphere, 66(8), 1431–1440.
Dean, J. R. (2002). Methods for environmental trace analysis. New York: Wiley.
Evans, G., Howarth, R. J., & Nombela, M. A. (2003). Metals in the sediments of Ensenada de San Simo´n (inner Rı´a de Vigo), Galicia, NW Spain. Journal Applied. Geochemistry, 18, 973–996.
Gambrell, R. P., Reddy, C. N., & Khalid, R. A. (1983). Characterization of trace and toxic materials in sediments of a lake being restored. Journal of Water Pollution Control Federation, 55(9), 121–1210.
Gholami, S., & Srikantaswamy, S. (2009). Statistical multivariate analysis in the assessment of river water quality in the vicinity of KRS Dam, Karnataka, India. Natural Resources Research. doi:10.1007/s11053-009-9096-y.
Gibbs, R. J. (1977). Transport phases of transition metals in the Amazon and Yukon river. Geological Society of America Bulletin, 88, 829–843.
Jain, C. K. (2004). Metal fractionation study on bed sediments of River Yamuna. India, Water Research, 38, 569–578.
Jain, C. K., Malik, D. S., & Yadav, R. (2007). Metal fractionation study on bed sediments of Lake Nainital, Uttaranchal, Índia. Environmental Monitoring and Assessment, 130, 129–139.
Jain, C. K., Harish, G., & Chakrapani, G. J. (2008). Enrichment and fractionation of heavy metals in bed sediments of River Narmada. India. Environmental Monitoring Assessment, 141(1–3), 35–47.
Jenne, E. A. (1968). Controls on Mn, Fe, Co, Ni, Cu and Zn concentrations in soils and water: the significant role of hydrous Mn and Fe oxides. Advances in Chemistry Series, 73, 337–388.
Karbassi, A. R., & Shankar, R. (2005). Geochemistry of two sediment cores from the west coast of India. International Journal of Environmental Science and Technology, 1(4), 307–316.
Liaghati, T., Preda, M., & Cox, M. (2003). Heavy metal distribution and controlling factors within coastal plain sediments, Bells Creek catchment, southeast Queensland, Australia. Environment International, 29(7), 935–948.
Lu, Y., & Allen, H. E. (2001). Portioning of copper onto suspended particulate matter in river waters. Science Total Environment, 277(1–3), 119–132.
Ma, L. Q., & Rao, G. N. (1997). Chemical fractionation of cadmium, copper, nickel, and zinc in contaminated soils. Journal of Environmental Quality, 26, 259–264.
Matthieu, M., Gérard, B., & Jörg, S. (2006). Geochemical signals and source contributions to heavy metal (Cd, Zn, Pb, Cu) fluxes into the Gironde Estuary via its major tributaries. Science of the Total Environment, 370(1), 133–146.
Morgan, J. J., & Stumm, W. (1991). Chemical processes in the environment, relevance of chemical speciation. In E. Merien (Ed.), Metals and their compounds in the environment (pp. 67–103). Germany: VCH.
Mueller, G. (1979). Schwermetalle in den sediments des Rheins- Veranderungen Seitt. mschan, 79, 778–783.
Relic, D., Dordevic, A., & Popovic Blagojevic, T. (2005). Speciation of trace metals in the Danube alluvial sediments within an oil refinery. Environmental Pollution, 3, 661–669.
Sakan, S., Grñeti, I., & Worpevi, D. (2007). Distribution and fractionation of heavy metals in the Tisa (Tisza) River sediments. Environmental Science and Pollution Research, 14, 229–236.
Santos, I. R., Silva-Filho, E. V., Schaefer, C. E. G. R., Albuquerque-Filho, M. R., & Campos, L. S. (2005). Heavy metal contamination in coastal sediments and soils near the Brazilian Antarctic Station, King George Island. Marine Pollution Bulletin, 50(2), 185–194.
Sharma, S. D., & Pandey, C. P. (1998). Pollution studies on Ramaganga river at Moradabad physico-chemical characteristics and toxic metals. Pollution Research, 17(2), 201–209.
Singh, K. P., Mohan, D., Singh, V. K., & Malik, A. (2005). Studies on distribution and fractionation of heavy metals in Gomti river sediments—a tributary of the Ganges, India. Journal Hydrology, 312, 14–27.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of heavy metals. Analytical Chemistry, 51(7), 844–861.
Xiangdong, L., Zhenguo, S., Onyx, W. H. W., & Yok-sheung, L. (2000). Chemical partitioning of heavy metal contaminants in sediments of the Pearl River Estuary. Chemical Speciation and Bioavailability, 12, 17–25.
Acknowledgments
The authors are thankful to Mr. Poolad Daneshvar for help in statistical data treatment in this research work. The authors also thank Prof. S. Sathyanarayan and Dr. A. Karbassi for their guidance and useful discussion during the course of the preparation of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hejabi, A.T., Basavarajappa, H.T. Heavy metals partitioning in sediments of the Kabini River in South India. Environ Monit Assess 185, 1273–1283 (2013). https://doi.org/10.1007/s10661-012-2631-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2631-z