Abstract
Nitrogen (N) leaching has become a matter of worldwide concern. The objectives of this study were: (1) to use soil columns to investigate the leaching of nitrate (\( {\text{NO}}_3^{ - } \)), ammonium (\( {\text{NH}}_4^{ + } \)), and nitrite (\( {\text{NO}}_2^{ - } \)) from calcareous soils that had received an average of 200 kg−1 N ha−1 year−1 for the previous 30 years and (2) to determine the relationship between soil properties and \( {\text{NO}}_3^{ - } \), \( {\text{NH}}_4^{ + } \), and \( {\text{NO}}_2^{ - } \) leaching. The soils used in this study ranged in texture from clay to sandy loam. Leaching experiments were conducted under saturation conditions and consisted of the collection of 1,047–2,524 mL of leachate (12 pore volumes (PVs)), which was equivalent to 534–1,286 mm from rainfall or irrigation. Losses of \( {\text{NO}}_3^{ - } \) ranged from 62 to 437 kg ha−1, while losses of \( {\text{NH}}_4^{ + } \) and \( {\text{NO}}_2^{ - } \) ranged from 2.5 to 19.3 kg ha−1 and 0.1 to 10.6 kg ha−1, respectively. Leaching rates differed between soil samples. The initial and secondary rate of \( {\text{NO}}_3^{ - } \) leaching was determined using an exponential model, and it ranged from 2.8 to 14.7 mg kg−1 PV−1 and 0.11 to 0.32 mg kg−1 PV−1. Greater leaching rates in the initial period could be due to leaching of \( {\text{NO}}_3^{ - } \) in solution, while the secondary leaching might be attributable to the diffusion-controlled transfer of \( {\text{NO}}_3^{ - } \) between mobile and immobile liquid phases. Analysis of variance indicated that the effects of soil type on total \( {\text{NO}}_3^{ - } \) leaching were highly significant (p < 0.001). The results showed that soil \( {\text{NO}}_3^{ - } \) concentration was positively correlated with the peak concentration of \( {\text{NO}}_3^{ - } \) (r = 0.86; p < 0.01) and the total \( {\text{NO}}_3^{ - } \) leached (r = 0.93; p < 0.01). In addition, the total \( {\text{NH}}_4^{ + } \) leached was positively correlated with silt (r = 0.67; p < 0.05), clay (r = 0.61; p < 0.05), and pH (r = 0.77; p < 0.01), which suggests that soil parameters might be useful indicators of \( {\text{NO}}_3^{ - } \) and \( {\text{NH}}_4^{ + } \) leaching from calcareous soils. Nitrate leaching from soils could threaten groundwater supplies, so possible strategies for minimizing \( {\text{NO}}_3^{ - } \) leaching losses may need to be considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) is a key nutrient that influences soil fertility and crop production. Nitrogen is required in greater quantities by plants compared with other essential elements derived from the soil. The application of agricultural chemicals has increased the productivity of modern agriculture, but overfertilization with N does not lead to any extra yield of grain (Berenguer et al. 2009). Instead the excess N accumulates in the soil in the form of \( {\text{NO}}_3^{ - } \), and it can become a potential source of \( {\text{NO}}_3^{ - } \) in groundwater (Hayshi and Hatano 1999). Other researchers (e.g., Hallberg 1989) have reported that the application of fertilizer to irrigated crops is the most extensive human cause of \( {\text{NO}}_3^{ - } \) pollution of groundwater systems.
Nitrate is highly mobile in the soil, and it can readily move down into the subsoil and from there into groundwater. In certain soils, \( {\text{NO}}_3^{ - } \) movement could be as much as six times greater than \( {\text{NH}}_4^{ + } \).
Nitrate contamination of groundwater and surface water is a global agricultural and environmental issue (Böhlke 2002; Almasri and Kaluarachchi 2004; Beeson and Cook 2004; Wassenaar et al. 2006; Rivett et al. 2007; Roy et al. 2007). Nitrate pollution originating from fertilizers has been reported in groundwater from many parts of the world (Shomar et al. 2008; Angelopoulos et al. 2009). In Europe, \( {\text{NO}}_3^{ - } \) concentrations exceeding the World Health Organization (WHO) recommendations (1993) for drinking water (50 mg L−1) were found in groundwater beneath 22% of the cultivated land (Lægreid et al. 1999). Chen et al. (2007) reported that excessive chemical fertilizer application in the Tai Lake region led to an increase of \( {\text{NO}}_3^{ - } \) in groundwater, which in turn led to eutrophication of Tai Lake. Song et al. (2009) found large differences in leachate \( {\text{NO}}_3^{ - } \) concentrations (ranging from 17 to 457 mg N L−1) and \( {\text{NO}}_3^{ - } \) losses (152–347 kg N ha−1) from 1 m of soil profiles in the field, with conventional agronomic practices.
Nitrogen fertilization of agricultural crops is an important economic and environmental issue, especially in high-yielding irrigated Asian areas. The intensification of agriculture has contaminated groundwater with \( {\text{NO}}_3^{ - } \) in many parts of Iran. Producers have traditionally applied more N fertilizer than required, which has led to environmental problems in recent years. In some regions of Iran, groundwater is polluted with \( {\text{NO}}_3^{ - } \), often at levels exceeding 50 mg L−1 \( {\text{NO}}_3^{ - } \), which is the maximum permissible concentration recommended by the WHO. Jalali (2005) studied the \( {\text{NO}}_3^{ - } \) pollution of groundwater in Hamedan and found that 196 of 311 wells (63%) had \( {\text{NO}}_3^{ - } \) levels lower than 50 mg L−1, whereas the remaining 115 (37%) exceeded this level. The extent and intensity of agriculture in Hamedan, located in the west of Iran, has led to much higher annual rates of fertilizer application, and this has greatly affected water quality. Farmers in this area tend to apply high amounts of N fertilizer (ranging from 138 to 230 kg N ha−1) to their crops, which include potatoes, corn, tomatoes, and other vegetables (Jalali 2005). This high application rate resulted in a total loading that was much higher than the N demand of vegetables and other crops in fields (Jalali 2005). The contamination was detected in groundwater from wells in most of the potato and vegetable crop production areas, which exhibited \( {\text{NO}}_3^{ - } \) concentrations that were significantly elevated above the level that affects humans (13 mg L−1), while some exceeded the WHO limit of 50 mg L−1. Jalali (2008) also studied \( {\text{NO}}_3^{ - } \) concentrations in vegetables and soils in Hamedan, western Iran, and concluded that continuous vegetable cropping caused accumulation of \( {\text{NO}}_3^{ - } \) in the soil, with a mean N accumulation of 81 mg \( {\text{NO}}_3^{ - } \)–N kg−1.
The population is increasing in this region and farm land is limited, so it is important to understand the process of \( {\text{NO}}_3^{ - } \) movement in soil and to assess the consequences of new agricultural management practices. Identifying sites and soils that are prone to \( {\text{NO}}_3^{ - } \) leaching is necessary to ensure regularly monitoring and appropriate management.
A number of laboratory soil column studies on \( {\text{NO}}_3^{ - } \) leaching have been published in recent years (Azevedo et al. 1996; Chinkuyu and Kanwar 1999; Chen et al. 2007), but none report the effects of soil properties on N leaching in calcareous soils. Therefore, the objectives of this study were: (1) to examine leaching of \( {\text{NO}}_3^{ - } \), \( {\text{NH}}_4^{ + } \), and \( {\text{NO}}_2^{ - } \) in soil columns and (2) to determine the relationship between soil properties and the amount of \( {\text{NO}}_3^{ - } \), \( {\text{NH}}_4^{ + } \), and \( {\text{NO}}_2^{ - } \) leached.
Materials and methods
Experimental soils
Eleven soil samples were collected from the surface (0–30 cm) horizon of agricultural soils in Hamedan province, western Iran. The soils represented typical soils continuously cultivated for winter wheat (Triticum aestivum L.), potatoes (Solanum tubersum), garlic (Allium sativum), vegetables, pasture, and gardens. The soils were collected from fields with a history of 30 years continuous fertilization with 138 to 230 N kg−1 ha−1 year−1 (mean = 200 kg−1 N ha−1 year−1). Soils were air-dried and passed through a 2-mm mesh sieve to remove stones before analysis. Soil pH, organic matter, texture, electrical conductivity, and equivalent calcium carbonate were determined according to the methods of Rowell (1994). Mineral N (\( {\text{NO}}_3^{ - } \), \( {\text{NH}}_4^{ + } \), and \( {\text{NO}}_2^{ - } \)) was extracted from soils using 1 M KCl in a 1:10 solution ratio, with shaking for 1 h. Concentrations of \( {\text{NO}}_3^{ - } \), \( {\text{NH}}_4^{ + } \), and \( {\text{NO}}_2^{ - } \) were determined colorimetrically (Mulvaney 1996). Nitrate can be expressed as either \( {\text{NO}}_3^{ - } \) (nitrate) or \( {\text{NO}}_3^{ - } \)–N (nitrate–nitrogen). Maximum acceptable nitrate levels according to the U.S. Environmental Protection Agency (USEPA) are 10 mg L−1 \( {\text{NO}}_3^{ - } \)–N or 50 mg L−1 \( {\text{NO}}_3^{ - } \).
Soil column leaching experiment
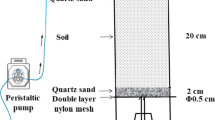
The leaching of native N was investigated using leaching columns, which consisted of Pyrex tubes that were 30 cm in length with an internal diameter of 5 cm. Soils of different textures were added to the columns to a height of 20 cm, with uniform tapping by a wooden rod to achieve a uniform bulk density of 1.2–1.7 g cm−3, which was dependent on field bulk densities. A gap of 10 cm remained above the soil in the columns. The soil was retained by a Whatman no. 42 filter paper, which was supported by a nylon mesh base. The soil was covered with a filter paper after packing to avoid dispersion of the surface soil. Columns were saturated with distilled water for 24 h, and the native N of soils was leached with distilled water. Distilled water was used to represent the relatively low salinity precipitation received from rainfall or snowmelt in arid and semi-arid regions (Rowell 1994; Al-Webel et al. 2002). The leaching trial resulted in the collection of 1,047–2,524 mL of leachate (12 pore volumes (PVs)), which was equivalent to 534–1,286 mm of rainfall or irrigation. The annual rainfall was assumed to equal 300 mm. The PV of soil columns was the volume of water-filled pores in the column, and it was calculated as 138–210 mL, based on the bulk density (1.23–1.73 g cm−3) and particle density (2.65 g cm−3) of the soil in the column (Rowell 1994). The PV enables comparisons of leaching studies. Leaching experiments were conducted under saturation conditions, with two replicates at room temperature (22–26°C). The leaching process was stopped after 7 PV with soil sample 4, due to blockage of the column. The solution level was maintained at approximately 5 ± 1 cm above the soil surface in the columns. Leachates from each leaching stage were collected at the following intervals: 1/8 PV until 1.5 PV; 2/8 PV until 7 PV; 4/8 PV until 10 PV; and every PV until 12 PV. Leachates were analyzed for pH, electrical conductivity (EC), \( {\text{NO}}_3^{ - } \), \( {\text{NO}}_2^{ - } \), and \( {\text{NH}}_4^{ + } \). Concentrations of \( {\text{NO}}_3^{ - } \) \( {\text{NO}}_2^{ - } \), and \( {\text{NH}}_4^{ + } \) were determined colorimetrically (Mulvaney 1996). The concentration change of solute in the leachate was determined using a breakthrough curve (BTC), and the observed concentration data were presented in a standard graphical form.
Cumulative leaching
To evaluate the cumulative leaching of N forms, two nonlinear regression mathematical models were used. The equations are as follows:
where Y is the cumulative \( {\text{NO}}_3^{ - } \) or \( {\text{NO}}_2^{ - } \) leached (mg kg−1); parameters a and c are initially (\( {a_{\text{NO}}}_3^{ - } \)), (\( {a_{\text{NO}}}_2^{ - } \)), and secondarily (\( {c_{\text{NO}}}_3^{ - } \)), (\( {c_{\text{NO}}}_2^{ - } \)), values for \( {\text{NO}}_3^{ - } \) or \( {\text{NO}}_2^{ - } \) leached (mg kg−1), respectively; parameters b and d are initially (\( {b_{\text{NO}}}_3^{ - } \)), (\( {b_{\text{NO}}}_2^{ - } \)), and secondarily (\( {d_{\text{NO}}}_3^{ - } \)), (\( {d_{\text{NO}}}_2^{ - } \)), values for the \( {\text{NO}}_3^{ - } \) or \( {\text{NO}}_2^{ - } \) leaching rate constant (mg kg−1 PV−1), respectively.
Y is the cumulative \( {\text{NH}}_4^{ + } \) leached (mg kg−1), parameter a is the leached \( {\text{NH}}_4^{ + } \) as mg kg−1 (\( {a_{\text{NH}}}_4^{ + } \)), and parameter b is the \( {\text{NH}}_4^{ + } \) leaching rate constant as mg kg−1 PV−1 (\( {b_{\text{NH}}}_4^{ + } \)). The parameters of the models were obtained by fitting experimental data to the models using the nonlinear procedure with Sigmaplot statistical software package version 10.0.
Result and discussion
Soil characteristics
The chemical and physical properties of the soils studied are given in Table 1. The soils used in this study ranged in texture from clay to sandy loam. The \( {\text{NO}}_3^{ - } \) level extracted by KCl ranged from 18 to 180 g kg−1, indicating that application of N fertilizers over the past 30 years has increased the amount of \( {\text{NO}}_3^{ - } \) stored in the soil. The \( {\text{NH}}_4^{ + } \) level ranged from 2.7 to 15.9 g kg−1. The \( {\text{NO}}_2^{ - } \) concentration was below the instrument detection limit.
Electrical conductivity and pH
Breakthrough curves (BTCs) (plots of concentration as a function of PV) of EC for two soils are presented in Fig. 1. Other soils shared the same trends. The EC of leachates ranged from 0.72 to 3.82 dS m−1 at the beginning of the experiment. These values were in the usual EC range for irrigation water (0–3.0 dS m−1), according to the Food and Agriculture Organization of the United Nations (Ayers and Westcot 1985). However, the leachate EC values showed a marked reduction at the end of the experiment from 0.05 to 0.26 dS m−1. A similar trend was observed by Civeira and Lavado (2008) and Santibáñez et al. (2007). The drop in EC suggests that an initial high level of soluble salts was reduced by the leaching process (Civeira and Lavado 2008). The highest EC value during the initial leaching was found in soils 2 and 8, whereas the lowest value was found in soil 3.
Breakthrough pH curves for the two soils are presented in Fig. 2. The pH of the leachates ranged from 7.4 to 8 at the beginning of the experiment, and no variation was observed over time. These results can be attributed to the high calcium carbonate content of the soils (Table 1). The presence of calcium carbonate buffered the soil system and inhibited further pH decreases. A similar trend was reported by Qian and Cai (2007) and Santibáñez et al. (2007).
Nitrate leaching from soils
Breakthrough curves were used to identify processes that affected N leaching (Fig. 3). The BTCs were characterized by a peak concentration of \( {\text{NO}}_3^{ - } \) in the 0.125 PV leachates, followed by a sharp decline and extensive tailing in subsequent leachate fractions. The highest peak concentration of \( {\text{NO}}_3^{ - } \) was found in soil 8 (1,257 mg L−1), while the lowest was found in soil 5 (49 mg L−1) (Table 2). The \( {\text{NO}}_3^{ - } \) concentration in leachates from all soils (except soil 5) was greater than the drinking water limit of 50 mg L−1 after 0.125 PV, but declined to 8 to 40 mg L−1 after 1 PV. The results of leaching for these calcareous soils indicated that there was a high risk of \( {\text{NO}}_3^{ - } \) movement into groundwater at concentrations exceeding the groundwater quality standard. Behera et al. (2003) also studied the dynamics of water flow and fertilizer solute leaching in lateritic soils and reported an initial \( {\text{NO}}_3^{ - } \) concentration in the soil profile that exceeded the permissible limit for potable water.
Jiao et al. (2004) reported that the \( {\text{NO}}_3^{ - } \)–N concentration in leachate from intact soil cores exceeded the Canadian drinking water limit of 10 mg L−1 after the first leaching trial, but declined to 3,08 mg L−1 by the third leaching trial.
The differences in \( {\text{NO}}_3^{ - } \) movement and concentration in the leachate were probably attributable to the soil properties and soil \( {\text{NO}}_3^{ - } \).
The rapid decrease in EC and \( {\text{NO}}_3^{ - } \) concentration might be related to preferential (non-uniform) flow or bypass flow. Thus, the concentrations in the leachate were strongly not in equilibrium with the soil, and non-equilibrium transport was probably the most important processes during \( {\text{NO}}_3^{ - } \) transport in soil columns. Hendrickx and Flury (2001) defined preferential flow as all phenomena where water and solutes move along certain pathways, while bypassing a fraction of the porous matrix.
The high \( {\text{NO}}_3^{ - } \) concentration in the leachate could also be related to anion exclusion. Jury et al. (1991) defined anion exclusion as the process of high mobility of anions due to repulsion between anions and the negatively charged mineral surface. This process influences chemical transport through the soil because it enables anions to move faster than neutral ions moving with water.
Figure 4 shows the cumulative amounts of \( {\text{NO}}_3^{ - } \) leached from two soils. The cumulative \( {\text{NO}}_3^{ - } \) leaching BTC showed an initial rapid phase followed by a slow phase of \( {\text{NO}}_3^{ - } \) leaching. The rapid leaching continued for 0.375–0.500 PV. There was a slower continued leaching of \( {\text{NO}}_3^{ - } \) after 0.500 PV.
Cumulative leaching of \( {\text{NO}}_3^{ - } \) from two studied soils. Other soils had similar trends. (filled circle) measured, and (line) simulated using Eq. 1
Table 3 shows the total amount of \( {\text{NO}}_3^{ - } \) leached after up to 12 PV. Between 17.9 and 158.2 mg \( {\text{NO}}_3^{ - } \) kg−1 (average, 56.8 mg kg−1) were leached during the 12 PV leaching period (equivalent to 534–1,286 mm of water), which accounted for between 61.4% and 160.8% of \( {\text{NO}}_3^{ - } \). The total amount of \( {\text{NO}}_3^{ - } \) leached in soils 2, 5, 6, 7, 9 and 10 was higher than the initial \( {\text{NO}}_3^{ - } \) extracted by KCl. This may be attributed to the low ability of KCl in extracting \( {\text{NO}}_3^{ - } \) from soil compared with distilled water and nitrogen mineralization in the soil.
The results indicate that soils irrigated with good quality water can lose large amounts of \( {\text{NO}}_3^{ - } \) in field conditions, which ranged from 62 to 437 kg ha−1 (on a column surface area basis) after 12 PV leaching. Nitrate leaching loss mainly occurred with 1 PV of leaching (on average 92 mm of water), which accounted for between 20.7% and 98.4% of total \( {\text{NO}}_3^{ - } \) leached.
The highest amount of leached \( {\text{NO}}_3^{ - } \) was found in soil 8 (437 kg ha−1), while the lowest was found in soil 1 (62 kg ha−1). Soil 8 came from a wheat field, where excessive amounts of N fertilizer may have been applied. This could have resulted in low N fertilizer efficiency because a large fraction of the applied N may have remained unutilized in the soil where it posed a potential risk for \( {\text{NO}}_3^{ - } \) leaching.
A long-term field study in England (over 140 years) reported leaching losses of \( {\text{NO}}_3^{ - } \) up to 442 kg ha−1 year−1 from silty clay loam soils (Johnston et al. 1989). Coarse-textured soils are particularly susceptible to leaching, and nearly 60% of the applied N fertilizer can be lost by leaching of these soils (Gaines and Gaines 1994). Rapid N leaching losses may be expected from soils with high permeability, high porosity, and a rapid infiltration rate.
Figure 4 shows plots of the cumulative \( {\text{NO}}_3^{ - } \) leaching data vs. the predicted leaching data for the two soils, using Eq. 1. The fit between the equation and the experimental data is indicated by the coefficient of determination and standard deviation, as shown in Table 4. Equation 1 provided a satisfactory prediction of the cumulative \( {\text{NO}}_3^{ - } \) because the r 2 values were high while the SE values were low.
The rate of leaching for the initial and secondary periods of leaching ranged from 2.8 to 14.7 mg kg−1 PV−1 and from 0.11 to 0.32 mg kg−1 PV−1, respectively. Greater leaching rates during the initial period could be due to the leaching of \( {\text{NO}}_3^{ - } \) in solution. Leaching in the secondary period could be due to diffusion-controlled transfer of \( {\text{NO}}_3^{ - } \) between mobile and immobile liquid phases in the soil (van Genuchten and Wierenga 1976; Nielsen et al. 1986).
Nitrate moves readily through the soil by diffusion and with the mass flow of water (Vitousek et al. 2002; Herrmann et al. 2005). Thus, there is the potential for a significant portion of the applied N fertilizer and naturally mineralized N to be leached from the root zone (Gulliam et al. 1985).
Ammonium leaching from soils
The results of \( {\text{NH}}_4^{ + } \) leaching for two soils are shown as a BTC in Fig. 5. The peak concentration of \( {\text{NH}}_4^{ + } \) in the leachate was observed at 0.125 PV, with the exception of soils 5 and 10, and it gradually decreased in subsequent leachate fractions. Ammonium concentration in soils 5 and 10 increased during 0.750 PV. The highest peak concentration of \( {\text{NH}}_4^{ + } \) was found in soil 11 (10.4 mg L−1), while the lowest was found in soil 9 (1.1 mg L−1). The concentration of \( {\text{NH}}_4^{ + } \) in leachates from soils 1 and 3 were lower than the detection limit after 7 and 11 PV, respectively, while in the other soils it decreased to between 0.2 and 1 mg L−1 at the end of the leaching experiment. The observed differences in \( {\text{NH}}_4^{ + } \) movement and concentration in the leachates may be related to levels of exchangeable and fixed \( {\text{NH}}_4^{ + } \) under nitrification conditions.
Exhaustion of oxygen occurs within several hours under conditions of soil flooding, which results in a variety of soil changes (Gliński and Stępniewski 1985; McKenney et al. 2001). In particular, the redox potential of the soil decreases, with a drop in Eh value to below +300 mV caused by the reduction of soil and successive activation of redox couples, other than the O2/H2O system, e.g., \( {\text{NO}}_3^{ - }-{\text{N}}/{\text{NO}}_2^{ - }-{\text{N}} \) (Gliński and Stępniewski 1985). Under favorable conditions (minimal Eh value and available organic matter), \( {\text{NO}}_3^{ - } \) (V) enters a path of dissimilative reduction, where the final product is the \( {\text{NH}}_4^{ + } \) ions, and its concentration gradually increases in the soil profile, especially in the lower horizons.
Furthermore, denitrification occurs when O2 is depleted by denitrifying microorganisms. The concentration of \( {\text{NO}}_3^{ - } \) (or nitrite) in the soil is another important regulator, and a third main controlling mechanism is the amount of available organic carbon (Tiedje 1988). Dissimilatory \( {\text{NO}}_3^{ - } \) reduction to \( {\text{NH}}_4^{ + } \) is an anaerobic microbial pathway in the N cycle that transforms \( {\text{NO}}_3^{ - } \) first to NO2, and then to \( {\text{NH}}_4^{ + } \).
Figure 6 shows the cumulative levels of \( {\text{NH}}_4^{ + } \) leached for two soils. Cumulative \( {\text{NH}}_4^{ + } \) leached from calcareous soils ranged from 0.85 to 7.40 mg kg−1 (mean, 3.1 mg kg−1) (Table 5). The highest and lowest amounts leached were in soil 9 (19.3 kg ha−1) and soil 4 (2.5 kg ha−1), respectively. All of the soil \( {\text{NH}}_4^{ + } \) was leached from soil 7 and soil 9 within 10 PV. The results show that soil irrigated with good quality water can lose a large proportion of \( {\text{NH}}_4^{ + } \), between 2.5 and 19.3 kg ha−1, within 12 PV.
Cumulative leaching of \( {\text{NH}}_4^{ + } \) from two studied soils. Other soils had similar trends. (Filled circle) measured and (lines) simulated using Eq. 2
The total amounts of \( {\text{NH}}_4^{ + } \) leached from soils 7 and 9 were in excess of the initial exchangeable \( {\text{NH}}_4^{ + } \) (approximately 118% and 144%, respectively), which indicates the release of \( {\text{NH}}_4^{ + } \) from nonexchangeable sites (Table 5). Mendoza et al. (2006) reported that cumulative \( {\text{NH}}_4^{ + } \) leaching from moderately alkaline and calcareous soils was 5.1 mg column−1. Yu et al. (2007) reported cumulative \( {\text{NH}}_4^{ + } \) losses of 1.2, and 0.9 mg column−1 from silty loam and heavy clay soils, respectively. Xiong et al. (2010) reported that cumulative \( {\text{NH}}_4^{ + } \) amounts leached from variable and permanent charge paddy soils during the drained season were 229.8 and 30.3 mg column−1, respectively, while during the flooded season the levels were 298.2 and 59.8 mg column−1, respectively.
The \( {\text{NH}}_4^{ + } \) concentration in soils was less than the \( {\text{NO}}_3^{ - } \) concentration. This clearly indicates a lower leaching rate of \( {\text{NH}}_4^{ + } \) compared with \( {\text{NO}}_3^{ - } \). The peak \( {\text{NO}}_3^{ - } \) concentration was about 100 times higher than the peak concentration of \( {\text{NH}}_4^{ + } \). However, the total leached \( {\text{NH}}_4^{ + } \) was less than the total leached \( {\text{NO}}_3^{ - } \). Electrostatic forces hold the cation, \( {\text{NH}}_4^{ + } \), to negatively charged clay particle surfaces and functional groups of soil organic matter. This binding is sufficient to limit \( {\text{NH}}_4^{ + } \) loss by leaching (Amberger 1993). Zhou et al. (2006) reported that the total \( {\text{NO}}_3^{ - } \) and \( {\text{NH}}_4^{ + } \) leached from soil columns in a drip fertigation system were 1.9 and 1.1 mg column−1, respectively. From an agricultural viewpoint, maintaining N in the form of \( {\text{NH}}_4^{ + } \) has the advantage of extending the time N remains in the rooting zone, which provides more time for plant absorption (Slangen and Kerkhoff 1984). Ammonium leaching depends on cation exchange, the fixing capacity of the soil, and the concentration of other cations in solution.
Most of the inorganic N is in the form of \( {\text{NO}}_3^{ - } \) in managed agricultural systems (Harmsen and Van Schreven 1955) and usually nitrification is very rapid in these systems (Huber and Watson 1974; Sahrawat 1980). However, poor quality groundwaters are a common feature of arid and semi-arid regions. When combined irrigation and rainfall exceeds the crop water requirement, excess soil water drains downward and soluble salts are carried with it, including \( {\text{NH}}_4^{ + } \). Soils containing CaCO3 can solubilize and supply Ca2+ to replace \( {\text{NH}}_4^{ + } \) ions. However, the extremely low solubility (0.0131 g L−1) (Qadir et al. 1996) of this native Ca2+ source means it makes an insignificant contribution to the replacement of \( {\text{NH}}_4^{ + } \). In the field, the release of CO2 in the root zone results from root and microbial respiration, and CO2 dissolution in water produces H2CO3, which in turn increases the dissolved CaCO3. Hydrogen ions released in the root zone also lower the soil pH and can dissolve further CaCO3. Thus, the dissolution of calcite can be enhanced when the CO2 concentration in the soil air is higher than that in the atmosphere. Increased Ca2+ concentrations would lead to increasingly rapid leaching of \( {\text{NH}}_4^{ + } \) and other cations with more realistic field flow rates and CO2 concentrations (Kolahchi and Jalali 2007).
Figure 6 shows plots of the cumulative \( {\text{NH}}_4^{ + } \) leaching data vs. the predicted leaching data for two soils, using Eq. 2. The fit between the equation and experimental data is indicated by the coefficient of determination and standard deviation, as shown in Table 4. With the exception of soil 9, Eq. 2 satisfactorily described the cumulative leaching of \( {\text{NH}}_4^{ + } \) because the r 2 values were high while the SE values were low.
The value of a ranged from 0.87 to 17.82 mg kg−1, whereas the value of b ranged from 0.03 to 0.46 mg kg−1 PV−1. Parameter b was less than 1 in all soils, indicating that \( {\text{NH}}_4^{ + } \) leaching rates decreased with PV. The difference between b values indicated that the \( {\text{NH}}_4^{ + } \) leaching rate was different for different soils. The high leaching rate for soil 5 could be due to its low CEC.
Nitrite leaching from soils
Figure 7 shows the results of \( {\text{NO}}_2^{ - } \) leaching for two soils. The results indicate that considerable amounts of \( {\text{NO}}_2^{ - } \) were leached from soils. A possible explanation is that \( {\text{NO}}_3^{ - } \) may be reduced to \( {\text{NO}}_2^{ - } \) during water saturation. The highest peak concentration of \( {\text{NO}}_2^{ - } \) was found in soil 3 (59.28 mg L−1), whereas the lowest was found in soil 5 (0.01 mg L−1) (Table 2). The \( {\text{NO}}_2^{ - } \) concentration at 0.125 PV exceeded the maximum concentration allowable by the Drinking Water Directive (USEPA 1986), i.e., a \( {\text{NO}}_2^{ - } \) level of 3 mg L−1, with the exception of soils 5, 7, 10, and 11. The concentration of \( {\text{NO}}_2^{ - } \) in soils 1, 2, 4, 5, 6, and 11 was lower than the detection limit after 1 PV, whereas it was detectable in soils 7, 8, 9, and 10 up to 5 PV. The concentration of \( {\text{NO}}_2^{ - } \) in soil 3 gradually decreased to 0.7 mg L−1 at the end of the leaching experiment. This can be explained by the relatively large content of organic matter in soil 3. The amount leached varied from 0.1 to 10.6 kg ha−1 when 12 PV had passed through the column (Table 6). High mobility is the characteristic of \( {\text{NO}}_2^{ - } \) in soil, and it tended to move downward as the wetting front moved through the profile.
The cumulative amounts of \( {\text{NO}}_2^{ - } \) leached for two soils are shown in Fig. 8. Cumulative \( {\text{NO}}_2^{ - } \) leached from calcareous soils was in the range of 0.04 to 3.94 mg kg−1 (mean, 1.45 mg kg−1) (Table 6).
Cumulative leaching of \( {\text{NO}}_2^{ - } \) from two studied soils. Other soils had similar trends. (Filled circle) measured and (lines) simulated using Eq. 1
Figure 8 shows plots of the cumulative \( {\text{NO}}_2^{ - } \) leaching data vs. the predicted leaching data for two soils, using Eq. 1. Equation 1 satisfactorily described the cumulative leaching of \( {\text{NO}}_2^{ - } \) for soils 2, 5, 6, 7, and 9 (Table 4). The \( {\text{NO}}_2^{ - } \) leaching BTC has two distinct sections: (1) the initial section and (2) the later section. The value of b ranged from 0.78 to 8.94 mg kg−1 PV−1, while d ranged from 0.78 to 4.84 mg kg−1 PV−1. The difference between b and d values indicate that \( {\text{NO}}_2^{ - } \) leaching rates differed between different soils. Nitrite leaching was initially rapid but decreased in subsequent leachate fractions.
Relationships between soil properties and N leaching
The influence of soil properties on N leaching was tested by calculating simple correlation coefficients and the results are shown in Table 7. Soil \( {\text{NO}}_3^{ - } \) concentration correlated with peak concentration of \( {\text{NO}}_3^{ - } \) (r = 0.86; p < 0.01) and total \( {\text{NO}}_3^{ - } \) amount leached (r = 0.93; p < 0.01). Jiao et al. (2004) found that the \( {\text{NO}}_3^{ - } \) load in leachates was positively related to soil \( {\text{NO}}_3^{ - } \) concentration in both intact soil cores and disturbed small soil columns, which indicated that soil \( {\text{NO}}_3^{ - } \) levels may be indicators of \( {\text{NO}}_3^{ - } \) leaching from top soils down to subsurface soils.
Total leached \( {\text{NH}}_4^{ + } \) was negatively correlated with sand (r = 0.75; p < 0.01). Clay soils contain more available sites compared with sandy soils, which enhances the retention of more \( {\text{NH}}_4^{ + } \). Ammonium ions present in exchangeable sites will be exchanged for ions such as Ca2+, Mg2+, and Na+ in poor quality waters, so leached \( {\text{NH}}_4^{ + } \) will exceed the amount evaluated in this study. Total \( {\text{NO}}_2^{ - } \) in leachates was positively correlated with soil \( {\text{NO}}_3^{ - } \) (r = 0.64; p < 0.05). Total leached \( {\text{NH}}_4^{ + } \) was positively correlated with clay (r = 0.61; p < 0.05). These results suggest that soil particle distribution might influence \( {\text{NH}}_4^{ + } \) leaching. There was no significant correlation between soil particle distribution and leached \( {\text{NO}}_3^{ - } \) or \( {\text{NO}}_2^{ - } \). Parameter \( {a_{\text{NH}}}_4^{ + } \) was positively correlated with pH (r = 0.80; p < 0.05) and CCE (r = 0.62; p < 0.05), while parameter \( {b_{\text{NH}}}_4^{ + } \) was negatively correlated with pH (r = –0.84; p < 0.05).
Table 8 shows that a multiple regression approach yielded better results. Multiple regression identified significant correlations between pH, EC, and silt and total leached \( {\text{NH}}_4^{ + } \), which explained 95% of the variability in total leached \( {\text{NH}}_4^{ + } \). There was also a significant correlation between sand and organic matter and total leached \( {\text{NO}}_2^{ - } \), which explained 78% of the variability in total leached \( {\text{NO}}_2^{ - } \). There was a significant correlation between the soil pH and the organic matter and \( {b_{\text{NH}}}_4^{ + } \) (r = 0.92; p < 0.01).
Overall, the N leaching results showed that leaching of N-rich soils resulted in huge \( {\text{NO}}_3^{ - } \) losses over the course of 12 PV of leaching. Approximately 118% (average of all soils) of the extractable soil \( {\text{NO}}_3^{ - } \) present was leached.
Nitrate losses via soil water drainage depend on the \( {\text{NO}}_3^{ - } \) content of the soil profile, the volume of leaching water, and the efficiency of leaching water exploring the pore volume (Magesan et al. 1996). Poor irrigation management is known to aggravate the polluting effects of fertilizers. Timing and method of both irrigation and fertilizer application are key factors that determine the leaching of chemical fertilizers during irrigated agriculture (Clothier and Green 1994). The amount of water applied in this study (534–1,286 mm) was approximately 1.8 to 4.3 times the average rainfall received in the study area. Therefore, less \( {\text{NO}}_3^{ - } \) will be leached on an annual basis. Significant leaching of \( {\text{NH}}_4^{ + } \) from soils raises a further concern in addition to the potential loss of a valuable nutrient. If the leached \( {\text{NH}}_4^{ + } \) subsequently emerges in a stream or other water course, it could have serious consequences for aquatic life. It is more harmful when the water pH is relatively high because this condition increases the proportion of \( {\text{NH}}_4^{ + } \) present as free NH3 (Qiang et al. 2004).
Intensive agricultural crop production in Iran is largely dependent on the application of N fertilizers. The average N fertilizer application in Iran currently exceeds 200 kg N h−1. Nitrogen fertilizer application typically exceeds 300 kg N ha−1 in high yielding crop regions of Iran (Jalali 2005). Agricultural systems in the study area received high amounts of inorganic N fertilizer in the form of \( {\text{NH}}_4^{ + } \), \( {\text{NO}}_3^{ - } \), or a combination of the two, which is expected to result in the accumulation of N in the soil profile.
The fertilizer application level for arable crops in the study area was higher (138–230 kg N ha−1 year−1) compared with the crop removal of N (29.4–154 kg N ha−1 year−1).
Farmers rarely take into account the N derived from soil organic matter, organic N application, and N in irrigation water when making decisions about N fertilizer applications, which often results in overfertilization with excess N being lost to the environment (Dinnes et al. 2002). Jalali (2008) reported that the total amount of N (N chemical fertilizer, N organic fertilizer, and N in irrigation water) supplied by the farmers to vegetable fields ranged from 375 to 965 kg ha−1 year−1. Nitrate leaching may also occur when the amount of water applied to irrigated soils in arid and semi-arid region exceeds evapotranspiration (Jalali and Rowell 2003). Application of large amounts of N fertilizer and inadequate management of N fertilization coupled with low irrigation efficiency are mainly responsible for the \( {\text{NO}}_3^{ - } \) concentrations in groundwater in the study area (Jalali 2005).
In a field situation, it is possible that actively growing plants would be capable of removing a substantial proportion of the N that was leached in this experiment, although perhaps not all. Minimizing the release of \( {\text{NO}}_3^{ - } \) from cropped fields to the environment can only be achieved by increased fertilizer efficiency. Agronomic practices such as cultivation, cropping, and irrigation water management may decrease the mean \( {\text{NO}}_3^{ - } \) and \( {\text{NH}}_4^{ + } \) concentration in water draining from the soil zone.
Conclusions
A similar pattern of N leaching was found in study soils, i.e., an initial rapid leaching followed by a slow, continuous leaching. The results of the present study indicated that 61.9–436.6, 2.5–19.3, and 0.1–10.6 kg ha−1 of \( {\text{NO}}_3^{ - } \), \( {\text{NH}}_4^{ + } \), and \( {\text{NO}}_2^{ - } \), respectively, were leached from these calcareous soils. Ammonium and \( {\text{NO}}_2^{ - } \) leaching from the profile was only detected in small quantities, whereas \( {\text{NO}}_3^{ - } \) was the predominant form of N that was transferred by vertical water movement. Leaching of mineral N under field conditions will differ from repacked soil columns. Losses are generally expected to be higher under field conditions because of the increased nitrification rates of organic N in soil under unsaturated conditions. Excessive use of readily available chemical fertilizers on agricultural land in the study area was the main source of groundwater contamination. Lack of vegetation and precipitation can accelerate the risk of N leaching and groundwater pollution. Poor quality groundwater is a common feature of arid and semi-arid regions. Excess soil water drains downward when the combined irrigation and rainfall exceeds the crop water requirement, which carries with it soluble salts including \( {\text{NO}}_3^{ - } \), \( {\text{NH}}_4^{ + } \), and \( {\text{NO}}_2^{ - } \). Regression of the leaching parameters of study soils on soil properties showed that soil \( {\text{NO}}_3^{ - } \), silt, and clay were the most important soil properties that influenced mineral N leaching from soils.
References
Almasri, M. N., & Kaluarachchi, J. J. (2004). Assessment and management of long-term nitrate pollution of groundwater in agriculture-dominated watersheds. Journal of Hydrology, 295, 225–245.
Al-Webel, M. A., Heil, D. M., Westfall, D. G., & Barbarick, K. A. (2002). Solution chemistry influence on metal mobility in biosolids-amended soils. Journal of Environmental Quality, 31(4), 1157–1165.
Amberger, A. (1993). Efficient management of nitrogen fertilization in modern cropping systems. In M. A. C. Fragoso & M. L. van Beusichem (Eds.), Optimization of plant nutrition. Dordrecht: Kluwer Academic Publishers.
Angelopoulos, K., Spiliopoulos, I. C., Mandoulaki, A., Theodorakopoulou, A., & Kouvelas, A. (2009). Groundwater nitrate pollution in northern part of Achaia Prefecture. Desalination, 248, 852–858.
Ayers, R. S., & Westcot, D. W. (1985). Water quality for agriculture. FAO Irrigation Drainage Paper 29 (p. 174). Rome: Food and Agriculture Organization of the United.
Azevedo, A. S., Kanwar, R. S., Singh, P., & Pereira, L. S. (1996). Movement of NO3-N and atrazine through soil columns as affected by lime application. Transactions of ASAE, 39(3), 937–945.
Beeson, S., & Cook, M. C. (2004). Nitrate in groundwater: a water company perspective. Quarterly Journal of Engineering Geology & Hydrogeology, 37(4), 261–270.
Behera, S., Jha, M. K., & Kar, S. (2003). Dynamics of water flow and fertilizer solute leaching in lateritic soils of Kharagpur region, India. Agricultural Water Management, 63(2), 77–98.
Berenguer, P., Santiveri, F., Boixadera, J., & Lloveras, J. (2009). Nitrogen fertilization of irrigated maize under Mediterranean conditions. European Journal of Agronomy, 30(3), 163–171.
Böhlke, J. K. (2002). Groundwater recharge and agricultural contamination. Hydrogeology Journal, 10, 153–179.
Chen, X., Wu, H., & Wo, F. (2007). Nitrate vertical transport in the main paddy soils of Tai Lake region, China. Geoderma, 142, 136–141.
Chinkuyu, A. J., & Kanwar, R. S. (1999). Effect of lime application on the movement of atrazine and nitrate-nitrogen through undisturbed-saturated soil columns. Water, Air, and Soil Pollution, 115, 371–384.
Civeira, G., & Lavado, R. S. (2008). Nitrate losses nutrients and heavy metal accumulation from substrates assembled for urban soils reconstruction. Journal of Environmental Management, 88(4), 1619–1623.
Clothier, B. E., & Green, S. R. (1994). Root zone processes and the efficient use of irrigation water. Agricultural Water Management, 25(1), 1–12.
Dinnes, D. L., Karlen, D. L., Jaynes, D. B., Kaspar, T. C., Hatfield, J. L., Colvin, T. S., & Cambardella, C. A. (2002). Nitrogen management strategies to reduce nitrate leaching in tile-drained midwestern soils. Agronomy Journal, 94(1), 153–171.
U. S. Environmental Protection Agency (1986). Quality criteria for water. EPA 440/5-86-001, Office of water Washington, DC: USEPA (pp. 189–194).
Gaines, T. P., & Gaines, S. T. (1994). Soil texture effect on nitrate leaching in soil percolates. Communications in Soil Science and Plant Analysis, 25, 2561–2570.
Gliński, J., & Stępniewski, W. (1985). Soil aeration and its role for plants (p. 229). Boca Raton: CRC Press.
Gulliam, J. W., Logan, T. J., & Broadbent, F. E. (1985). Fertilizer use in relation to the environment. In O. P. Engelstad (Ed.), Fertilizer technology and use (3rd ed., pp. 561–588). Madison: Soil Science Society of America.
Hallberg, G. B. (1989). Nitrate in groundwater in the United States. In R. F. Follet (Ed.), Nitrogen management and groundwater protection, developments in agricultural and managed-forest ecology. vol. 21 (pp. 35–74). New York: Elsevier.
Harmsen, G. W., & Van Schreven, D. A. (1955). Mineralization of organic nitrogen in soil. Advances in Agronomy, 7, 299–398.
Hayshi, Y., & Hatano, R. (1999). Annual nitrogen leaching to subsurface drainage water from a clayey aqiuc soil cultivated with onions in Hokkaido, Japan. Soil Science and Plant Nutrition, 45, 451–459.
Hendrickx, J. M. H., & Flury, M. (2001). Uniform and preferential flow, mechanisms in the vadose zone, conceptual models of flow and transport in the fractured vadose zone (pp. 149–187). Washington: National Research Council, National Academy Press.
Herrmann, M., Pust, J., & Pott, R. (2005). Leaching of nitrate and ammonium in heathland and forest ecosystems in Northwest Germany under the influence of enhanced nitrogen deposition. Plant and Soil, 273, 129–137.
Huber, D. M., & Watson, R. D. (1974). Nitrogen form and plant disease. Annual Review of Phytopathology, 12, 139–165.
Jalali, M. (2005). Nitrates leaching from agricultural land in Hamedan, western Iran. Agriculture, Ecosystems & Environment, 10, 210–218.
Jalali, M. (2008). Nitrate concentrations in some vegetables and soils in Hamedan, western Iran. Archives of Agronomy and Soil Science, 54(5), 569–583.
Jalali, M., & Rowell, D. L. (2003). The role of calcite and gypsum in the leaching of potassium in a sandy soil. Experimental Agriculture, 39(4), 379–394.
Jiao, Y., William, H. H., & Joann, K. W. (2004). Agricultural practices influence dissolved nutrients leaching through intact soil cores. Soil Science Society of America Journal, 68(6), 2058–2068.
Johnston, A. E., McGrath, S. P., Poulton, P. R., & Lane, P. W. (1989). Accumulation and loss of nitrogen from manure, sludge, and compost: Long-term experiments at Rothamsted andWoburn. In J. A. Hansen & K. Henriksen (Eds.), Nitrogen in organic wastes applied to soils (pp. 126–139). London: Academic Press.
Jury, W. A., Gardner, W. R., & Gardner, W. H. (1991). Soil physics. John Wiley and Sons, Inc.
Kolahchi, Z., & Jalali, M. (2007). Effect of water quality on the leaching of potassium from sandy soil. Journal of Arid Environments, 68, 624–639.
Lægreid, M., Bøckman, O. C., Kaarstad, O. (1999). Agriculture, fertilizers and environment. Oslo, CAB International. Wallingford & Norsk Hydro: ASA.
Magesan, G. N., White, R. E., & Scotter, D. R. (1996). Nitrate leaching from a drained, sheep-grazed pasture. 1. Experimental results and environmental implications. Australian Journal of Soil Research, 34(1), 55–56.
McKenney, D. J., Drury, C. F., & Wang, S. W. (2001). Effects of oxygen on denitrification inhibition, repression, and derepression in soil columns. Soil Science Society of America Journal, 65, 126–132.
Mendoza, C., Assadian, N. W., & William, L. (2006). The fate of nitrogen in a moderately alkaline and calcareous soil amended with biosolids and urea. Chemosphere, 63(11), 1933–1941.
Mulvaney, R. L. (1996). Nitrogen-inorganic forms. In D. L. Sparks (Ed.), Methods of soil analysis—part 3. Chemical methods—SSSA Book Series No. 5 (pp. 1123–1184). Madison: Soil Science Society of America and American Society of Agronomy.
Nielsen, D. R., van Genuchten, M. T., & Biggar, J. W. (1986). Water flow and solute transport processes in the unsaturated zone. Water Resource Research, 22(10S), 89S–108S.
Qadir, M., Qureshi, R. H., & Ahmad, N. (1996). Reclamation of a saline-sodic soil by gypsum and Leptochloa fusca. Geoderma, 74, 207–217.
Qian, C., & Cai, Z. (2007). Leaching of nitrogen from subtropical soils as affected by nitrification potential and base cations. Plant and Soil, 300, 197–205.
Qiang, Z., Speir, T. W., & Schaik, A. P. V. (2004). Leaching of nutrients from soil cores treated with a single large dose of digested sewage sludge. Biology and Fertility of Soils, 40(4), 284–289.
Rivett, M. O., Smith, J. W. N., Buss, S. R., & Morgan, P. (2007). Nitrate occurrence and attenuation in the major aquifers of England and Wales. Quarterly Journal of Engineering Geology & Hydrogeology, 40(4), 335–352.
Rowell, D. L. (1994). Soil science: Methods and applications. Harlow: Longman Group.
Roy, S., Speed, C., Bennie, J., Swift, R., & Wallace, P. (2007). Identifying the significant factors that influence temporal and spatial trends in nitrate concentrations in the Dorset and Hampshire Basin Chalk aquifer of Southern England. Quarterly Journal of Engineering Geology & Hydrogeology, 40(4), 377–392.
Sahrawat, K. L. (1980). Nitrification in some tropical soils. Plant and Soil, 65(2), 281–286.
Santibáñez, C., Ginocchio, R., & Varnero, M. T. (2007). Evaluation of nitrate leaching from main tailing amended with biosolids under Mediterranean type climate conditions. Soil Biology and Biochemistry, 39(6), 1333–1340.
Shomar, B., Osenbrück, K., & Yahya, A. (2008). Elevated nitrate levels in the groundwater of the Gaza Strip: distribution and sources. Science of the Total Environment, 398, 164–174.
Slangen, J., & Kerkhoff, P. (1984). Nitrification inhibitors in agriculture and horticulture: a literature review. Nutrient Cycling in Agroecosystems, 5(1), 1–76.
Song, X.-Z., Zhao, C.-X., Wang, X.-L., & Li, J. (2009). Study of nitrate leaching and nitrogen fate under intensive vegetable production pattern in northern China. Comptes Rendus – Biologies, 332(4), 385–392.
Tiedje, J. M. (1988). Ecology of denitrification and dissimilatory nitrate reduction to ammonium. In A. J. B. Zehnder (Ed.), Biology of anaerobic microorganisms (pp. 179–244). New York: Wiley.
Van Genuchten, M., & Wierenga, P. J. (1976). Mass transfer studies in sorbing porous media I. Analytical solutions. Soil Science Society of America Journal, 40(4), 473–480.
Vitousek, P. M., Hättenschwiler, S., Olander, L., & Allison, S. (2002). Nitrogen and nature. Ambio, 31(2), 97–101.
Wassenaar, L. I., Hendry, J. M., & Harrington, N. (2006). Decadal geochemical isotopic trends for nitrate in a transboundary aquifer and implication for agricultural beneficial management practices. Environmental Science and Technology, 40(15), 4626–4632.
WHO. (1993). Guidelines for drinking water quality. 1. Recommendations (2nd ed.). Geneva: World Health Organization.
Xiong, Z. Q., Huang, T. Q., Yu-Chun, M. A., Xing, G. Xi, & Zhu, Z. L. (2010). Nitrate and ammonium leaching in variable- and permanent-charge paddy soils. Pedosphere, 20(2), 209–216.
Yu, Q., Chen, Y., Ye, X., Zahng, Q., Zhang, Z., & Tian, P. (2007). Evaluation of nitrification inhibitor 3,4-dimethyl pyrazole phosphate on nitrogen leaching in undisturbed soil columns. Chemosphere, 67(5), 872–878.
Zhou, J. B., Xi, J. G., Chen, Z. J., & Li, S. X. (2006). Leaching and transformation of nitrogen fertilizer in soil after application of N with irrigation: a soil column study. Pedosphere, 16(2), 245–252.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zarabi, M., Jalali, M. Leaching of nitrogen from calcareous soils in western Iran: a soil leaching column study. Environ Monit Assess 184, 7607–7622 (2012). https://doi.org/10.1007/s10661-012-2522-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2522-3