Abstract
In this study, the occurrence and sources of 16 priority polycyclic aromatic hydrocarbons (PAHs) in the water, surface sediment, and adjacent agricultural soil samples from the Wangyang River (WYR, a typical effluent-receiving river) were determined, and their ecological risks on the organisms were assessed using sediment quality guidelines. Results showed that the levels of 16 PAHs in the water, surface sediments, and soils ranged from 416.7–4907 ng/L, 545.1–134054.7 ng/g (dry weight, dw), and 297.4–10,849 ng/g dw, respectively. The molecular ratios, principal components analysis, and hierarchical cluster analysis were employed to differentiate PAHs sources, and results indicated that PAHs in WYR were mainly from pyrogenic sources which were attributed to the burning of corn straws as well as petroleum/coal combustion in the local industries in the region. The heavy PAH contamination in the sediments and soils might pose serious adverse ecological effects on the organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a group of carcinogenic persistent organic pollutants (Samia et al. 2013) that are highly stable in the environment. PAHs are the products of the incomplete combustion of organic materials, or from the spillage of crude oils and refined oil products (Lima et al. 2005; Al-Dabbas et al. 2013, 2014a; Jan et al. 2014). High emission of PAHs is usually associated with highly industrialized and urbanized activities which are mostly concentrated in the coastal areas and inland cities along major rivers (Yim et al. 2007). In China, the serious PAH pollution was due to the drastic increased use of fossil fuels in the past 30 years (Xu et al. 2006). It reported that biomass burning, domestic coal combustion, coking industry, and petroleum combustion accounted for 60, 20, 16, and 3 % of the total PAHs emissions in China, respectively (Xu et al. 2006). The diversity of PAHs sources results in their ubiquity and complexity of origin in various environmental media.
The Wangyang River (WYR) is situated in Shijiazhuang City, the capital of Hebei Province in north China. The river is 85 km long, and is the tributary of the Ziya River affiliated to the Haihe River Basin. Beijing and Tianjin also belong to the region of the Haihe River Basin. Wastewater, either after treatment or without treatment, is one of the main water sources of many rivers in this area, which is now facing crises for water resources due to rapid development and 30 years of drought recorded since the 1970s. High PAH levels have been previously reported in streams in Tianjin (Shi et al. 2005; Qi et al. 2011). The occurrence and characterization of PAHs have also been reported in urban river systems in Beijing (Zhang et al. 2004; Shen et al. 2009). Fuel and biomass combustion, coke ovens, etc., are clearly the primary sources of PAHs in the rivers in the Beijing and Tianjin area (Shi et al. 2005, 2007). However, for the rivers that receive treated and untreated wastewater as their main water sources, the contribution of wastewater to PAH content has not yet been determined.
WYR is the sole water body that receives treated and untreated wastewater from Shijiazhuang City and Hebei High-tech Industrial Development Zone via tributaries and the sewerage systems. In the past decades, local petroleum refining and smelting industries have contributed a lot to the contamination of WYR. In addition, the burning of straws in the area is also a significant pollution source (Streets et al. 2001; Zhang et al. 2007). The environmental problem in this region has drawn much concern by both scientific and regulatory communities. In this study, the occurrence and characterization of PAHs in WYR was determined and the potential PAHs sources were evaluated. The results will fill up the data gap of the occurrence of PAHs in the Haihe River Basin, and will also assess the ecological risk of PAHs to the organisms according to the sediment quality guidelines.
Materials and methods
Materials and reagents
Reference PAHs including naphthalene (NAP), acenaphthylene (ANY), acenaphthene (ANA), fluorine (FLU), phenanthrene (PHE), anthracene (ANT), fluoranthene (FLT), pyrene (PYR), benzo[a]anthracene (BaA), chrysene (CHR), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), indeno[1,2,3-cd]pyrene (IPY), dibenzo[a,h]anthracene (DBA), and benzo[ghi]perylene (BPE) were purchased from Dr. Ehrenstorfer Co. Ltd. (Germany). All solvents used for sample processing and analyses were HPLC grade from Beijing Chemical Factory (Beijing, China).
Sample collection and preparation
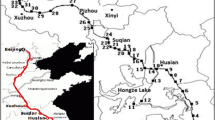
Water, sediment, and soil samples were collected in May 2013 from 13 sites in the Wangyang River, as illustrated in Fig. 1. Triplicate samples were collected from each site. Water samples were collected with a stainless steel bucket and stored in brown glass bottles at 4 °C, in which dichloromethane was added to prevent bacterial degradation. Surface sediment samples (0–5 cm) were collected from the same sites using a grab sampler. Top soil (0–10 cm) was collected from the river bank using a stainless steel scoop that had been prewashed with acetone. Samples were transported to the laboratory in ice box, and treated immediately.
Before filtration, naphthalene-d8, acenaphthene-d10, phenanthrene-d10, chrysene-d12, and perylene-d12 were added in the water samples (2 L). Filtered water samples were passed at the flow rate of 5 mL/min through solid-phase extraction (SPE) cartridges (C18 cartridges, 500 mg/6 mL, Thermo Electron Corporation) which were pre-conditioned with 5 mL ethyl acetate, 5 mL methanol, and 5 mL ultra-pure water, respectively. PAHs were eluted from the cartridge using 4 mL hexane-dichloromethane mixture (1/1, v/v) and 4 mL hexane to 10 mL glass tube with stoppers. The eluate was passed through the anhydrous sodium sulfate (600 °C, 4 h) to remove the water content and then exchanged solvent to hexane phase and evaporated under a stream of purified N2 in a water bath to 200 μL for GC-MS analysis.
Frozen sediment samples were dried using a freeze-drying apparatus and grinded through a 40-mesh sieve. The sediment samples were extracted by accelerated solvent extraction (ASE) technique (Zhang et al. 2011). Briefly, a mixture of deuterated PAH compounds (naphthalene-d8, acenaphthene-d10, phenanthrene-d10, chrysene-d12, and perylene-d12) as recovery surrogate standards was added to the samples prior to extraction; 5 g of each sediment sample combined with 5 g of copper powder and 2 g of diatomite were extracted with an automated Dionex ASE 200 (Sunnyvale, CA, USA), using hexane-dichloromethane mixture (1/1, v/v) as extraction solvent with extraction temperature at 100 °C and extraction pressure at 1500 psi. Both preheating time and static time were set to 5 min. A total flush volume of 100 % the cell volume and a purge time of 60 s with nitrogen was used. The final extraction volume was approximately 20 mL with two extraction cycles. The extracts were completely condensed to approximately 2 mL, followed by solid-phase extraction (SPE) for clean-up, and concentrated to 1 mL for analysis.
Instrumental analysis
All the water and sediment samples were analyzed using an Agilent 6890N gas chromatograph with 5975C mass selective detector (GC/MSD), equipped with an Agilent 7683B automatic liquid sampler and a DB-5 MS capillary column (30 m, 0.25 mm i.d., 0.25-μm film thickness). Helium was used as the carrier gas, with a column flow rate of 1.2 mL/min in constant-flow mode. Injector, ion source, and transfer line temperatures were set at 250, 230, and 260 °C, respectively. The GC oven temperature was programmed from 60 (1 min) to 160 °C (8 min) at 10 °C/min, then to 300 °C at 6 °C/min and held for 8 min. The electron impact energy was set at 70 eV; 2 μL of samples was injected in splitless mode. PAHs were analyzed with the selected ion monitoring (SIM) mode. Quantification of samples was performed using an internal standard method.
Quality control
The PAHs in the sediment and water blanks were not detected or much lower than the detection limits of the method, which were 0.13–2.2 ng/g for the sediment and 0.4–1.3 ng/L for the water. Mean recoveries and the relative standard deviation of surrogates in the sediment and water extracts were 70.2 ± 7.5 and 63.4 ± 6.8 % (naphthalene-d8), 84.5 ± 5.6 and 78.4 ± 9.2 % (acenaphthene-d10), 92.5 ± 5.6 and 94.1 ± 13.2 % (phenanthrene-d10), 92.4 ± 10.2 and 90.5 ± 10.5 % (chrysene-d12), and 89.0 ± 9.4 and 84.7 ± 8.8 % (perylene-d12), respectively. For statistical analysis, two-tailed t test was used to compare the average concentrations between samples in the present study.
Results and discussion
Occurrence and distribution of PAHs
The levels of 16 PAHs in the surface water, sediments, and soil from WYR were summarized in Table 1. In the river water, all 16 PAHs were detected, with a mean concentration of 1566.9 ng/L and the total concentration (ΣPAH) ranging from 416.7 to 4907 ng/L. Concentrations of ΣPAH varied spatially between sampling sites. PHE and NAP were the predominate compound detected in the water samples, while BaP, the most carcinogenic PAH, was detected in most of the surface water samples with a mean value of 8.1 ng/L. The level of BaP was three times higher than the guideline value of 2.8 ng/L by the Environmental Quality Standard for Surface Water in China (GB3838-2002). The contamination of PAHs in WYR surface water was relatively severe. The concentration was comparable to the levels in Taihu Lake (238–7422 ng/L, mean concentration of 1592 ng/L) in Zhejiang Province, east China (Zhang et al. 2011), and the Wuhan section of the Yangtze River (321.8–6234.9 ng/L), south China (Feng et al. 2007), much higher than that in the middle and lower reaches of the Yellow River (179–369 ng/L) (Middle China) in the dry season (Li et al. 2006) and in the Mississippi river (62.9–144.7 ng/L) (USA) in the dry season (Mitra and Bianchi 2003), and Seine River (4–36 ng/L) (France) in the normal season (Fernandes et al. 1997).
The levels of ΣPAH in the sediments ranged from 545.1 to 134054.7 ng/g (dry weight, dw), with a mean concentration of 14,872.7 ng/g (Table 1). The median concentration of ΣPAH (4177 ng/g dw) was much lower than the mean, which indicated there were some sites containing relatively high concentrations. FLT, PYR, CHR, and BbF were found to be the four most dominant compounds in the sediment samples. The mean concentrations were 1716.5 ng/g dw for three-ring PAHs, 1302.5 ng/g dw for four-ring PAHs, 548.8 ng/g dw for five-ring PAHs, and 676 ng/g dw for six-ring PAHs. The compositional pattern of PAHs in the sediment was different from that in the water samples (Fig. 2). Four-ring PAHs were the most abundant in the sediment, accounting for 43.8 % of ΣPAHs, which was comparable with the abundance of three-ring PAHs in the water. The proportion of six-ring PAHs in the sediment was also higher than in the water (Fig. 2). This different compositional pattern can be explained by the low vapor pressure and low water solubility for high-ring PAHs (Sun et al. 2009; Al-Dabbas et al. 2014b), which tend to be adsorbed more readily to the particulates and sediment and are resistant to degradation (Zhu et al. 2008).
Concentrations of PAHs in the soil samples were also summarized in Table 1. All 16 PAHs were detected, with PHE, FLT, BbF, and PYR being the most dominant compounds, accounting for 43.7 % of ΣPAH. Concentrations of ΣPAH varied from 297.4 to 10,849 ng/g dw, with mean and median concentrations of 1683.1 and 686.1 ng/g dw, respectively. The maximum concentration was almost 36 times higher than the minimum concentration, indicating different anthropogenic activities among sampling sites. The compositional pattern of PAHs in soil was similar to that in the sediment. Concentrations of three-ring PAHs and four-ring PAHs were 148.3 and 118.5 ng/g dw, accounting for 26.4 and 35.2 % of ΣPAH, respectively. Totally, PAHs in soil had a common characteristic with high-ring PAHs (4 to 6) accounting for more than 76.9 % of ΣPAH. All the soil PAHs levels in this study were much higher than the target value set by the Dutch government for unpolluted soil (20–50 ng/g dry weight) (Netherlands Ministry of Housing 1994). The concentrations of BaP were below the US control standard (110 ng/g) in soil; however, the levels of BbF/BkF (161 and 88 ng/g) and CHR (113.5 ng/g) measured in the present study far exceeded the US control standard levels (22 ng/g for B(b/k)F, 4 ng/g for CHR) (New York State Department Conservation 2003).
Source apportionment
Several methods were commonly used to identify the potential sources of PAHs in the environment, such as isomer ratios, principal component analysis (PCA), and hierarchical cluster analysis (HCA). Yunker et al. (2002) suggested that the FLU/(FLU + PYR) ratio <0.4 indicated the petroleum input, the ratio between 0.4 and 0.5 indicated the liquid fossil fuel (vehicle and crude oil) combustion, and the ratio >0.5 indicated the grass wood or coal combustion. Similarly, an ANT/(ANT + PHE) ratio >0.1 represented combustion processes, whereas lower values represented petroleum contamination (Maioli et al. 2011). The data for the ratios of these compounds in the water, sediment, and soil samples in WYR were presented in Fig. 3. It showed that ANT/(ANT + PHE) ratios for all samples were >0.2, suggesting that combustion may be the predominant source of PAHs in the WYR region. It was confirmed by the FLU/(FLU + PYR) ratio that in all sediments, one water sample and two soil samples in the study area, FLU/(FLU + PYR) ratios were <0.4, indicating that combustion and petroleum were the major PAH sources. Other twelve water (92.3 %) and eleven soil (84.6 %) samples with typical PAH isomer ratios of FLU/(FLU + PYR) indicated the predominance of coal or wood combustion around the study area.
Plot of PAHs with the ratio of ANT/(ANT + PHE) plotted against that of FLU/(FLU + PYR). A FLU/(FLU + PYR) ratio <0.4 indicates petroleum input, ratio >0.5, grass, wood, or coal combustion and ratio between 0.4 and 0.5, mixed sources. An ANT/(ANT + PHE) ratio >0.1 indicates combustion processes, whereas lower values represented petroleum contamination
PCA was performed using a VARIMAX rotation with principal components (factors) with eigenvalues >1 to identify possible sources. The selected factors from PCA were summarized in Table 2. For the water, three factors were extracted, which accounted for 96.1 % of the total variance (36.5, 35.7, and 23.9 %, respectively). Factor 1 (PC 1) was predominantly loaded on PYR, PLT, BkF, and DBA, which was associated with coal combustion (Harrison et al. 1996). Factor 2 (PC 2) was predominantly loaded on BbF, BaP, and BPE. As PHE, BkF, IPY, and BPE are regarded as tracers of vehicle exhaust emissions (Harrison et al. 1996), it was therefore suggested that PC 2 was pyrogenic sources (vehicle emission). Factor 3 (PC 3) was enriched in ANA, NAP, and ANY, which were tracers of coke oven source (Simcik et al. 1999), indicating that PC 3 might be related to coke oven emission.
For the soil and sediments, two factors were extracted. PC 1 and PC 2 accounted for 99.1 % in both the sediment and soil samples (Table 2). These two factors had similar loading characteristics of individual PAHs, indicating the similar sources. PC 1 was mostly associated with high-ring PAHs like PYR, BaA, CHR, BbF, BkF, BaP, DBA, IPY, and BPE. This profile of PAHs was consistent with the emission profile of PAHs from coal combustion in residential areas of North China (Shi et al. 2007). PC 2 had high loading on ANA, NAP, ANY, and FLU, accounting for 14.6 and 22.6 % for the sediment and soil samples, respectively. As a result, this factor identified the source as coke oven emission. Overall, pyrogenic sources (including combustion of coal and biomass, vehicle emissions) were the main sources of PAHs in the WYR region, with coke oven emission identified as a specific source of PAHs in northeastern China, which was consistent with the results of an atmospheric PAHs study in the same region (Wang et al. 2008).
The PAH concentration data set was also analyzed by HCA. The two-dimensional HCA heat map was depicted in Fig. 4. Three groups were discriminated in the soil and sediment samples, respectively. The species which cluster together in one group implies that they might come from a common source (Garcı́a et al. 2004). In soil samples, FLU, PHE, ANA, and NAP formed a group, which were associated with the coke/coal combustion (Sofowote et al. 2008), and these species might come from the pyrogenic source. Some high-ring PAHs including IPY, BaP BPE, BkF, and BaA were clustered in the other group, which were associated with vehicular emissions sources (Harrison et al. 1996). Similar clustering of PAHs in the sediment samples was also observed (Fig. 4), which was consistent with the results from the PCA method, that in the WYR region PAHs were originated mainly from pyrogenic sources.
Assessment of potential ecological risk
The potential ecological risks of PAHs in the sediments were assessed using the sediment quality guidelines according to Long et al. (1995), and the results were summarized in Table 1. The effect range low (ERL) and effect range median (ERM) have been used to assess the aquatic sediment quality with a ranking of low to high impact values. ERL represents the concentration below which an adverse effect would rarely be observed. ERM represents the concentration above which adverse effects would frequently occur. Table 1 showed that all the maximum concentrations exceeded their respective ERL values of individual PAHs in the sediment and soil samples, and except NAP and ANY, measured maximum concentrations were higher than the ERM value in the sediment. It indicated that PAHs in the WYR region probably had posed an adverse ecological effect to organisms.
Conclusion
PAHs in the surface water, sediment, and soil of a typical effluent-receiving river, North China, were comprehensively investigated in this study. Results demonstrated that PAH levels and composition patterns varied in different environment matrices due to different transportation and transformation processes. Two- and three-ring PAHs were abundant in the water samples with PHE as its dominant PAHs, whereas high-ring PAHs were major species in the sediment samples with BbF as its dominant PAHs. PAH concentrations in the WYR water and sediment were close to or much higher than those of some other rivers in China, and higher than some rivers in foreign countries. According to the isomer ratios, principal component analysis and hierarchical cluster analysis, PAHs mainly originated from combustion process, such as coal/petroleum and biomass burning. As to the ecological risk assessment, the concentrations of most PAHs in the WYR sediments were much higher than guideline values such as the ERL and ERM. It may be concluded that PAHs would cause immediate biological effects in sedimentary environment in the WYR region.
References
Al-Dabbas MA, Ali LA, Afaj AH (2013) Determination of total suspended particles and the polycyclic aromatic hydrocarbons concentrations in air of selected locations at Kirkuk. Iraq Arab J Geosci. doi:10.1007/s12517-013-1229-2
Al-Dabbas MA, Ali LA, Afaj AH (2014a) Determination of heavy metals and polycyclic aromatic hydrocarbon concentrations in soil and in the leaves of plant (Eucalyptus) of selected locations at Kirkuk—Iraq. Arab J Geosci. doi:10.1007/s12517-014-1454-3
Al-Dabbas MA, Ali LA, Afaj AH (2014b) Comparison of the polycyclic aromatic hydrocarbons and heavy metal concentrations in soil and leaves of eucalyptus plants at Kirkuk. Iraq Arab J Geosci. doi:10.1007/s12517-014-1520-x
Feng C, Xia X, Shen Z, Zhou Z (2007) Distribution and sources of polycyclic aromatic hydrocarbons in Wuhan section of the Yangtze River, China. Environ. Monit Assess 133:447–458
Fernandes M, Sicre M-A, Boireau A, Tronczynski J (1997) Polyaromatic hydrocarbon (PAH) distributions in the Seine River and its estuary. Mar Pollut Bull 34:857–867
Garcı́a JH, Li W-W, Arimoto R, Okrasinski R, Greenlee J, Walton J (2004) Characterization and implication of potential fugitive dust sources in the Paso del Norte region. Sci Total Environ 325:95–112
Harrison RM, Smith D, Luhana L (1996) Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, UK. Environ Sci Technol 30:825–832
Jan FA, Khan S, Ishaq M, Naeem M, Ahmad I, Hussain S (2014) Brick kiln exhaust as a source of polycyclic aromatic hydrocarbons (PAHs) in the surrounding soil and plants: a case study from the city of Peshawar, Pakistan. Arab J Geosci 7(1):13–19
Li G, Xia X, Yang Z, Wang R, Voulvoulis N (2006) Distribution and sources of polycyclic aromatic hydrocarbons in the middle and lower reaches of the Yellow River, China. Environ Pollut 144:985–993
Lima ALC, Farrington JW, Reddy CM (2005) Combustion-derived polycyclic aromatic hydrocarbons in the environment—a review. Environ Forensic 6:109–131
Long ER, MacDonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97
Maioli OL, Rodrigues KC, Knoppers BA, Azevedo DA (2011) Distribution and sources of aliphatic and polycyclic aromatic hydrocarbons in suspended particulate matter in water from two Brazilian estuarine systems. Cont Shelf Res 31:1116–1127
Mitra S, Bianchi T (2003) A preliminary assessment of polycyclic aromatic hydrocarbon distributions in the lower Mississippi River and Gulf of Mexico. Mar Chem 82:273–288
Netherlands Ministry of Housing (1994) Environmental quality objective in the Netherlands. Ministry of Housing, The Hague
New York State Department Conservation (2003) SVOCs soil cleanup objectives. Appendix A of TAGM#4046
Qi WX, Liu HJ, Qu JH, Hu CZ, Lan HC, Berg M, Ren HM, Xu W (2011) Polycyclic aromatic hydrocarbons in effuents from wastewater treatment plants and receiving streams in Tianjin, China. Environ Monit Assess 177:467–480
Samia K, Abdelkarim C, Yassin H, Teruo H (2013) The effect of long-term soil irrigation by wastewater on organic matter, polycyclic aromatic hydrocarbons, and heavy metals evolution: case study of Zaouit Sousse (Tunisia). Arab J Geosci 6(11):4337–4346
Shen Q, Wang YK, Zhang W, Zhang CS, Wang JX (2009) Characterization and sources of PAHs in an urban river system in Beijing, China. Environ Geochem Health 31:453–462
Shi Z, Tao S, Pan B, Fan W, He XC, Zuo Q, Wu SP, Li BG, Cao J, Liu WX, Xu FL, Wang XJ, Shen WR, Wong PK (2005) Contamination of rivers in Tianjin, China by polycyclic aromatic hydrocarbons. Environ Pollut 134:97–111
Shi Z, Tao S, Pan B, Liu WX, Shen WR (2007) Partitioning and source diagnostics of polycyclic aromatic hydrocarbons in rivers in Tianjin, China. Environ Pollut 146:492–500
Simcik MF, Eisenreich SJ, Lioy PJ (1999) Source apportionment and source/sink relationships of PAHs in the coastal atmosphere of Chicago and Lake Michigan. Atmos Environ 33:5071–5079
Sofowote UM, McCarry BE, Marvin CH (2008) Source apportionment of PAH in Hamilton Harbour suspended sediments: comparison of two factor analysis methods. Environ Sci Technol 42:6007–6014
Streets DG, Gupta S, Waldhoff ST, Wang MQ, Bond TC, Yiyun B (2001) Black carbon emissions in China. Atmos Environ 35:4281–4296
Sun J-H, Wang G-L, Chai Y, Zhang G, Li J, Feng J (2009) Distribution of polycyclic aromatic hydrocarbons (PAHs) in Henan reach of the Yellow River, Middle China. Ecotoxicol Environ Saf 72:1614–1624
Wang Z, Zhang L, Zhang Y, Zhao Z, Zhang S (2008) Morphology of single inhalable particle in the air polluted city of Shijiazhuang, China. J Environ Sci 20:429–435
Xu S, Liu W, Tao S (2006) Emission of polycyclic aromatic hydrocarbons in China. Environ Sci Technol 40:702–708
Yim UH, Hong S-H, Shim W-J (2007) Distribution and characteristics of PAHs in sediments from the marine environment of Korea. Chemosphere 68:85–92
Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33:489–515
Zhang ZL, Huang J, Yu G, Hong HS (2004) Occurrence of PAHs, PCBs and organochlorine pesticides in the Tonghui River of Beijing, China. Environ Pollut 130:249–261
Zhang Y, Tao S, Cao J, Coveney RM (2007) Emission of polycyclic aromatic hydrocarbons in China by county. Environ Sci Technol 41:683–687
Zhang Y, Lu Y, Xu J, Yu T, Zhao W (2011) Spatial distribution of polycyclic aromatic hydrocarbons from Lake Taihu, china. Bull Environ Contam Toxicol 87:80–85
Zhu L, Chen Y, Zhou R (2008) Distribution of polycyclic aromatic hydrocarbons in water, sediment and soil in drinking water resource of Zhejiang Province, China. J Hazard Mater 150:308–316
Acknowledgments
The authors would like to acknowledge the financial support by the Major Science and Technology Program for Water Pollution Control and Treatment No.2012ZX07203-003 in China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Yu, H., Xi, B. et al. Levels, sources, and potential ecological risks of polycyclic aromatic hydrocarbons (PAHs) in a typical effluent-receiving river (Wangyang River), North China. Arab J Geosci 8, 6535–6543 (2015). https://doi.org/10.1007/s12517-014-1695-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12517-014-1695-1