Abstract
Physiological and biochemical responses, metal bioaccumulation and tolerance potential of Sphagnum squarrosum Crome Samml. to Cu and Cd were studied to determine its bioindication and bioremediation potential. Results suggest that glutathione treatment increases the metal accumulation potential and plays a definite role in heavy metal scavenging. High abundance of Sphagnum in metal-rich sites strongly suggests its high metal tolerance capabilities. This experiment demonstrates that S. squarrosum is able to accumulate and tolerate a high amount of metals and feasibility of its application as bioindicator and remediator test species of metal-contaminated environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals have been increasingly found in soil due to atmospheric deposition, sludge, sewage, agrochemicals, industrial, and mining processes resulting in potential risk to human health through biomagnification (Chen et al. 2011), and therefore, new economic plant-based remediation technologies are needed (Sharma 2011). Bryophytes lack roots and depend on precipitation, canopy leaching and wind-blown dust for their mineral nutrition. Owing to lack epidermis, the tissue readily absorbs heavy metals from the atmosphere and strongly binds to the organic matter. Furthermore, most bryophytes, including Sphagnum, lack conducting tissue, and little internal transport takes place (Blagnyte and Paliulis 2010). These characteristics make them an efficient accumulator and an ideal choice for biomonitoring and remediation studies.
The presence of metallic toxicants may induce a physiological response in the organism, often involving production of enzymes that are capable of metabolizing or degrading the toxicants (Srivastava et al. 2005). During the process, several physiological parameters such as protein, carbohydrate, proline, chlorophyll content, and some critical enzyme activities are influenced by the heavy metals (Godbold 1994; Syso 1998). Out of several proposed metal detoxification mechanisms, metal chelation through low-molecular weight peptides seems to be the most vital and widely accepted one (Inouhe 2005).
Cu is an essential micronutrient since it is the constituent of many metalloenzymes and proteins involved in e− transport, redox, and other important reactions. But Cu when present at higher levels in its free ionic form (Cu2+) is toxic to plant cell. Cd is widely and increasingly used in industries for corrosion-protecting coating, nickel–cadmium batteries, mining, coal utilization, and tobacco smoking. Cd is well known as a highly toxic environmental element due to its great toxicity and high mobility from soil to plant and further down the food chain (Vig et al. 2003).
The present study was undertaken to ascertain the phytotoxicity of Cu and Cd on Sphagnum squarrosum and its tolerance capabilities and mechanisms responsible for detoxification by analyzing its response to the metals.
Materials and methods
S. squarrosum Crome Samml. plants were collected from uncontaminated sites of Mukteswar (Kumaon hills), India, located at 2,380 msl. Identical sized, thoroughly washed, green young shoots (5 cm from apex) were incubated for 10 or 25 days in the 0.5 Hoagland medium containing varying concentrations (viz. 0.01, 0.1, 1.0, and 10 mM) of copper as copper sulfate or cadmium as cadmium chloride either alone or in combination with glutathione (1 mM) under laboratory conditions (light 60 μE2 s−1 and temperature 20 ± 2°C). Control plants were allowed to grow in 0.5 Hoagland medium without metals and glutathione.
For identification of metal-binding peptides, the method of Grill et al. (1991) was used. Five grams of fresh S. squarrosum tissue was cooled and frozen in liquid nitrogen, then homogenized in 0.5 ml of 1 N NaOH containing a freshly prepared solution of 1 mg/ml NaBH4. This was centrifuged at 11,000×g at 4°C for 15 min, and the precipitated portion was removed; it was again centrifuged at 13,000×g for 15 min, and the supernatant was used for HPLC. Phytochelatins were separated on reversed phase column (m Benapack 4 mm, C-18) with a linear gradient of 0.20% acetonitrile in 0.1% trifluroacetic acid at a flow rate of 0.5 ml/min. Detection of phytochelatin was performed at 220 nm. Simultaneously, known amount of standard protein samples were run under similar conditions.
For cell fraction studies, the centrifugation method of Echols and Kisailus (1992) was adopted under chilled conditions. Carbohydrate content was estimated spectrophotometrically at 630 nm using anthrone reagent according to the method of Hedge and Hofreiter (1962). In vivo nitrate reductase (E.C.1.6.6.1) activity was measured in accordance to the method of Srivastava (1975) by spectrophotometrically quantifying the nitrite released into the incubation medium. The color is developed due to the formation of diazo compound with sulphanilamide and nitrite, which is coupled with NED. For peroxidase (E.C.1.11.1.7) estimation the method of Putter (1974) was followed using guaiacol as a dye. Protein was estimated following the Folin phenol method of Lowry et al. (1951) using bovine serum albumin as standard. Chlorophyll was estimated according to modified Arnon (1949) method by extracting the pigment in 80% acetone. Proline content was estimated following the method of Bates et al. (1973). For metal analysis the method of Shimwell and Laurie (1972) was adopted by digesting 1 g oven-dried S. squarrosum (80°C for 6 h) in concentrated (3:1) HCl to HNO3. The optical density was recorded at 228.8 nm for Cd and 324.7 nm for Cu using air acetylene oxidizing flame using atomic absorption spectrophotometer (EC India).
Each experiment was conducted thrice, each time in triplicate set. The data presented are the average of the treatments with standard error. For statistical analysis of data, first ANOVA is applied to identify whether the treatment had any significant influence on the parameters measured and followed by Duncan’s multiple range test (mean separation test).
Results
Effect of metal (Cu and Cd) on physiological responses
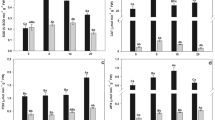
The physiological parameters of Sphagnum were affected sharply by treatment with various concentrations of copper and cadmium. Photosynthetic pigment (chlorophyll), biomass (data not shown), carbohydrate, and protein were affected adversely and showed a decreasing trend with the increasing concentration of metals, although not in a linear proportion. Decrease in nitrate reductase activity with concomitant increase in peroxidase activity upon treatment with metals suggests a stress condition in the plant, which is amply documented by increase in proline content (Table 1). Despite the moss having limited intake of Cd, its toxicity was more pronounced than that of Cu. The decrease in most of the metabolic parameters and nitrate reductase enzyme activity was negated when reduced glutathione was also added in the medium (Table 1). Similarly the heavy metal-induced increase in peroxidase activity was also arrested by glutathione.
Phytotoxicity of metals on S. squarrosum physiology upon prolonged treatment
When the treatment of the moss continued for 25 days, phytotoxicity of heavy metals was apparent, although at a magnitude lower than that observed at 10 days, especially at lower concentrations of the heavy metals (Table 2). However, at higher concentrations, there was an increased level of toxicity. It seems that there was some recovery during prolonged exposure at lower concentrations of the heavy metals. Exogenous supply of reduced glutathione has ameliorated the metal toxicity up to a certain extent.
Effect of metals (±glutathione) on bioaccumulation potentials of S. squarrosum
The moss Sphagnum, upon treatment with metal, accumulated a significant amount of heavy metals. After prolonged treatment (25 days) with metal, a decline in bioaccumulation rate was observed; however, the metal accumulation rate went down by 32–60% in all cases. Treatment with metals in combination with glutathione increased the bioaccumulation of S. squarrosum (Table 3). Cell fraction studies demonstrated that the heavy metals were accumulated mostly in the cell wall fraction (Table 4).
Effect of metal treatment on PCs synthesis
A considerable increase in PC level was observed with increase in copper concentration as 0.51 μM Glu Cys g−1 at 10.0 mM, 0.39 μM Glu Cys g−1 at 1.0 mM, 0.30 μM Glu Cys g−1 at 0.1 mM, and 0.12 μM Glu Cys g−1 at 0.01 mM concentration of copper (Fig. 1).
Discussion
The present study demonstrates the phytotoxic responses of 0.01–10.0 mM Cu or Cd on S. squarrosum, in terms of decline in chlorophyll and protein contents and nitrate reductase activity. The most sensitive parameter appeared to be the chlorophyll, which has been reported earlier also (Hou et al. 2007; Rau et al. 2007). The decline in chlorophyll may be due to reduced synthesis (Nag et al. 1981), which is the consequence of interaction of vital enzymes involved in chlorophyll synthesis (Stobart et al. 1985). The decreased chlorophyll content may lead to reduced photosynthesis and ultimately to reduced biomass. Inhibition of nitrogenous parameters such as protein and nitrate reductase activity has been observed in many higher plants (Bhandal and Kaur 1992; Kevresan et al. 1998) and even in Sphagnum (Saxena et al. 1999). It is well known that heavy metals can act at different sites to inhibit a large number of enzymes having functional sulfydryl groups, resulting in the disruption of protein synthesis pathways (Vallee and Ulmer 1972). Increased peroxidase activity upon metal treatment may be an indication of the induction of free radical-scavenging metabolism (Satyakala and Jamil 1997). Thus, the response of the moss S. squarrosum to Cu and Cd seems to be similar to the response of most of the higher plants to the heavy metals.
Glutathione (GSH) is ubiquitous in eukaryotes, and the tripeptide serves a plethora of physiological functions including redox regulation, conjugation of metabolites, and detoxification of xenobiotics. However, with the onset of environmental stresses, including the heavy metals, it plays a vital role in protecting plants. This is because of its role as an antioxidant, as it participates as a reactant in the Asada–Halliwell cycle of radical scavenging (Alscher 1989). Thus, in the present study, the supply of reduced glutathione has ameliorated Cu and Cd phytotoxicity as expressed by decline in chlorophyll, protein, and nitrate reductase activity (Table 1). Such a protective role of glutathione against heavy metal toxicity has been observed in Vigna (Bhattacharya et al. 1995), rice root growth (Chen and Kao 1995), sugar beet (Kevresan et al. 1998), Sorghum (Pandit and Prasannakumar 1999), lettuce (Maier et al. 2003), and in Fontinalis (Burns et al. 2001).
Field studies showed luxuriant growth of S. squarrosum along the roads at Cu and Cd rich sites (data not shown). This suggests that it has acclimated well to a metal-contaminated environment by developing some metal tolerance strategies viz. avoidance, protection, or detoxification. Being oxylophytic in nature, S. squarrosum grows in a slightly acidic environment. HSO3 and carbonic acids formed from SO2 and CO2 released from automobile exhaust may help in maintaining the slightly acidic pH of the cytosol needed for optimum physiological functions of S. squarrosum (Saxena and Saxena 1999). On the other hand, in a clean habitat, leafy liverworts and other sensitive bryophytes are more abundant, which may be due to minimum or no pollutant like that observed at Muketswar.
Bioaccumulation potential of the moss was determined to see whether the effects of Cu or Cd were topical or these elements were actually present in the cellular environment. The present study demonstrates a high accumulation potential which correlates to the environmental concentration of heavy metal. A significant accumulation in the cell wall fraction demonstrates that most of the metal was taken in by ion exchange process and was adsorbed by the cell walls. Sphagnum has far higher concentrations of polyuronic acids than other mosses, and therefore Sphagnum behaves as a cation exchanger with numerous sites where divalent ions bind to the cell walls (Glime and Keen 1984). This provides an extensive metal-binding capability of Sphagnum moss (Ruhling and Tyler 1973).
A greater increase in bioaccumulation potential of metals was observed upon treatment with metals in combination with GSH than with metals alone. The study supports the detoxification role of glutathione during accumulation when higher amount of metal is stored. It is likely that either glutathione itself or one of its metabolic products is able to bind the metal inside the cell. Decrease in accumulation rate during the last 15 days, in comparison to the first 10 days, could be due to several reasons: (1) decrease in concentration of metal in solution, (2) limited uptake and saturation of exchange sites, and (3) complete or partial depletion of exogenous GSH supplied during metal treatment.
When a moss sample is placed in solution, there is a swift (minutes) chemical equilibrium achieved between free metals and those bound to extracellular binding sites. The amount of metal remaining in the solution is then the concentration available to have a biological impact on the moss over the next days. Introducing glutathione to the solution establishes further chemical equilibria where the relative binding capabilities of the moss and glutathione for a particular chemical again determine how much of the metal remains available in solution in an unchelated form. Intracellular uptake of metals may involve a slower transmembrane-mediated process where interactions between different metal cations for the carrier (and their relative proportions) determine how much of an element may be incorporated to the metabolically active interior of the cell.
Similarities at the structural level between PC and GSH have suggested that synthesis of these molecules may be related (Gupta et al. 1999). The data presented herein support the hypothesis that GSH acts as a substrate for PC synthesis (Leopold et al. 1999).
The phytochelatin content in the present study increased with the increase in external Cu concentration, but not as much as the total Cu content of the moss (Table 3). For example, with an increase in external Cu concentration from 0.01 to 10.0 mM, the PC content increased by about 1.78-fold, while Cu accumulation increased by almost sixfold during a fivefold increase in external Cu concentration. Apparently induced synthesis of PC was unable to detoxify all the Cu accumulated; hence, with the increase in external Cu concentration, we observed the decline in chlorophyll and nitrate reductase activity.
References
Alscher, R. G. (1989). Biosynthesis and antioxidant function of glutathione in plants. Plant Physiology, 77, 457–464.
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiology, 24, 1–15.
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water stress studies. Plant Soil, 39, 205.
Bhandal, I. S., & Kaur, H. (1992). Heavy metal inhibition of nitrate uptake and in vivo nitrate reductase in roots of wheat, Triticum aestivum. Indian Journal of Plant Physiology, 35, 281–284.
Bhattacharya, M., Choudhuri, M. A., & Bhattacharya, M. (1995). Heavy metal (Pb2+ and Cd2+) stress induced damages in Vigna seedlings and possible involvement of phytochelatin like substances in mitigation of heavy metal stress. Indian Journal of Experimental Biology, 33(3), 236–238.
Blagnyte, R., & Paliulis, D. (2010). Research into heavy metals pollution of atmosphere applying moss as bioindicator: a literature review. Environmental Research, Engineering and Management, 4(54), 26–33.
Burns, L. A., Sutter, K., Menge, S., Neumann, D., & Krauss, G. J. (2001). Cadmium lets increase the glutathione pool in bryophytes. Journal of Plant Physiology, 158(1), 79–89.
Chen, S. L., & Kao, S. H. (1995). Glutathione reduces the inhibition of rice seedling root growth catalysed by cadmium. Plant Growth Regulator, 16, 249–252.
Chen, X., Wang, J., Shi, Y., Zhao, M. Q., & Chi, G. Y. (2011). Effect of cadmium on growth and photosynthetic activities in pakchoi and mustard. Botanical Studies, 52, 41–46.
Echols, R., & Kisailus, E. (1992). Cell fractionation in plants. In J. G. Chirikjian (Ed.), Biotechnology: theory and techniques. Plant biotechnology, animal cell culture and immunobiotechnology. London: Jones and Bartlett Publishers.
Glime, J. M., & Keen, R. E. (1984). The importance of bryophytes in a man-centered world. Journal of Hattori Botanical Lab, 55, 133–146.
Godbold, D. L. (1994). Aluminium and heavy metal stress: from the rhizosphere to the whole plant. In D. L. Godbold & Z. Zhutterman (Eds.), Effects of acid rain on forest processes (pp. 232–264). New York: Wiley-Liss.
Grill, E., Winnacker, E. L., & Zenk, M. H. (1991). Phytochelatins. In J. F. Riordon & B. L. Valle (Eds.), Methods in enzymology 205 (pp. 333–341). New York: Academic.
Gupta, M., Tripathi, R. D., Rai, U. N., & Haq, W. (1999). Lead induced synthesis of metal binding peptides (phytochelatins) in the submerged macrophyte Vallisneria spiralis L. Physiology and Molecular Biology of Plants, 5, 173–180.
Hedge, J. E., & Hofreiter, B. T. (1962). In R. L. Whistler & J. N. Be Miller (Eds.), Carbohydrate chemistry 17. New York: Academic.
Hou, W., Chen, X., Song, G., Wang, Q., & Chang, C. C. (2007). Effect of copper and cadmium on heavy metal polluted water body restoration by duckweed (Lemna minor). Plant Physiology and Biochemistry, 45(1), 62–69.
Inouhe, M. (2005). Phytochelatins. Brazilian Journal of Plant Physiology, 17(1), 65–78.
Kevresan, S., Papovic, M., Kandrac, J., & Petrovic, N. (1998). Effect of heavy metals on nitrate and protein metabolism in sugar beet. Biologia Plantarum (Czech Republic), 41(2), 235–240.
Leopold, I., Gunther, D., Schinidt, J., & Neumann, D. (1999). Phytochelatins and heavy metal tolerance. Phytochemistry, 50, 1323–1325.
Lowry, O. H., Rose, N. J., Brough, A. L., & Randall, N. R. J. (1951). Protein measurement with foline phenol reagent. J Biol Chem, 193, 265–275.
Maier, E. A., Matthews, R. D., McDowell, J. A., Walden, R. R., & Ahner, B. A. (2003). Environmental cadmium level increase phytochelatin and glutathione in lettuce grown in a chelator-buffered nutrient medium. Journal of Environmental Quality, 32(4), 1356–1364.
Nag, P., Paul, A. K., & Mukherjee, S. K. (1981). Heavy metal effects in plant tissues involving chlorophyll, chylorophyllase, Hill reaction activity and gel electrophoretic patterns of soluble proteins. Indian Journal of Experimental Biology, 19, 702–706.
Pandit, B. R., & Prasannakumar, P. G. (1999). Effect of metals of Jowar (Sorghum bicolor L.) seedling growth—II, biochemical changes. Pollution Research, 18(4), 483–488.
Putter, J. (1974). Methods of enzymatic analysis 2 (Ed Bergmeyer) (p. 685). New York: Academic.
Rau, S., Miersah, J., Neumann, D., Weber, E., & Krauss, G. J. (2007). Biochemical responses of the aquatic moss Fontinalis antipyretica to Cd, Cu, Pb and Zn determined by chlorophyll fluorescence and protein levels. Environmental and Experimental Botany, 59(3), 299–306.
Ruhling, A., & Tyler, G. (1973). Heavy metal deposition in Scandinavia. Water, Air, and Soil Pollution, 2, 445–455.
Satyakala, G., & Jamil, K. (1997). Studies on the effect of heavy metal pollution on Pistia statiotes L. (water lettuce). Indian Journal of Environmental Health, 39(1), 1–7.
Saxena, D. K., & Saxena, A. (1999). Biomonitoring of SO2 phytotoxicity on Sphagnum squarrosum Cram. Samml. Journal of Indian Botanical Society, 78(3 and 4), 367–374.
Saxena, D. K., Saxena, A., & Srivastava, H. S. (1999). Heavy metal accumulation and in vivo nitrate reductase activity in the Sphagnum squarrosum Cram. Samml. Proceedings of National Academy of Science, India, 69(B) III and IV, 307–312
Sharma, H. (2011). Metal hyperaccumulation in plants: a review focusing on phytoremediation technology. Journal of Environmental Science and Technology, 4, 118–138.
Shimwell, D. W., & Laurie, A. E. (1972). Lead and zinc contamination of vegetation in the Southern Pennines. Environmental Pollution, 3, 291–301.
Srivastava, H. S. (1975). Distribution of nitrate reductase in ageing bean seedlings. Plant and Cell Physiology, 16, 995–999.
Srivastava, M., Ma, L. Q., Singh, N., & Singh, S. (2005). Antioxidant responses of hyper-accumulator and sensitive fern species to arsenic. Journal of Experimental Botany, 56(415), 1335–1342.
Stobart, A. K., Griffiths, W. T., Ameen-Bukhari, I., & Sherwood, R. P. (1985). The effect of Cd2+ on the biosynthesis of chlorophyll in leaves of barley. Plant Physiology, 63, 293–298.
Syso, A. S. (1998). Using the Cr, Ni relationship for monitoring environmental pollution. Agrokhimiya, 4, 76–83.
Vallee, B. L., & Ulmer, D. D. (1972). Biochemical effects of mercury, cadmium and lead. Annual Review of Biochemistry, 41, 91.
Vig, K., Megharaj, M., Sethunathan, N., & Naidu, R. (2003). Bioavailability and toxicity of cadmium to microorganisms and their activities in soil: a review. Advances in Environmental Research, 8, 121–135.
Acknowledgments
The Visiting Fellowship to AS from the Indian National Science Academy, New Delhi, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to the late Professor H. S. Srivastava.
Rights and permissions
About this article
Cite this article
Saxena, A., Saxena, A. Bioaccumulation and glutathione-mediated detoxification of copper and cadmium in Sphagnum squarrosum Crome Samml.. Environ Monit Assess 184, 4097–4103 (2012). https://doi.org/10.1007/s10661-011-2246-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-011-2246-9