Abstract
Biocoenosis of macrobenthic communities in relation to environmental and mangrove flora was studied in mangrove ecosystem of Cochin backwaters during 2010–2012 period. A total of 48 species in 45 genera belonging to 38 families of macrobenthos were collected with a mean numerical density of 1628 ± 2283 ind m−2. Malacostraca was the dominant taxa in terms of benthic density (55%) and diversity with 17 spp., then polychaeta (11 spp.), mollusca (9 spp.) and others (9 spp.). The dominant species were amphipods Idunella sp. (40,170 ind m−2), Cheiriphotis geniculata (34,169 ind m−2), polychaete Dendronereis aestuarina (38,808 ind m−2), tanaid Ctenapseudes chilkensis (29,419 ind m−2), bivalve Indosphenia kayalum (23,835 ind m−2) and oligochaete Tubificoides psuedogaster (16,946 ind m−2). The PCA and redundancy analysis revealed that the nature of the substratum determined by sediment texture, organic matter, total organic carbon, sediment nutrients were found to be an influencing factor in the differential distribution and community structure of macrobenthic organisms in mangroves of Cochin. Mangrove plant density also exhibited significant correlation with the density of macrofauna. This study has noted a decline in species composition as well as diversity and richness of macrobenthic fauna compared to the previous study notably polychaetes reported in 1993 in Cochin mangroves. Moreover, community structure exhibited significant change with newer associations of species especially opportunistic oligochaetes, corophiids and tanaids that are tolerant of various anthropogenic stressors. This change in community assemblage and biodiversity, thus demanding efficient management strategies for mangrove ecosystem through integrated planning, rehabilitation and periodic benthic faunal surveillance coupled with mangrove floral assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The intertidal mangrove habitats are regions of remarkable biological productivity (Alongi 2018) along the continental margins where land, sea and atmosphere interact and interplay continuously. This marginal environment is well adapted to withstand the extreme winds, salinity variations, tidal actions, anaerobic soil, lower pH and higher temperature (Kathiresan 2010). The unique morphological and physiological characteristics such as pneumatophores, stilt roots, buttress roots, salt‐excreting leaves and viviparous propagules help them to adapt to the harsh environment and make them profusely rich in biodiversity compared to other coastal habitats (Alongi 2002). The habitat heterogeneity provided by mangroves attracted most of the species to this dynamic ecosystem (Lee 2008). However the mangrove destruction and deforestation due to increased population pressure in coastal areas is the most alarming reason for biodiversity loss (Duke et al. 2007).
According to Levinton (2001), the benthic fauna in mangroves composed of organisms that are associated with mangrove substrates, at least during part of their life cycle, where individuals usually attach to, move about or burrow into the substrate. These benthic epifauna and infauna occupies all the major and minor niches in the mangrove environment residing among the stilt roots, pneumatophores, barks, soft and hard substratum, as grazers, tube dwellers, nestlers, deposit feeders, shredders, scavengers, and predators (Lee 2008; Nagelkerken et al. 2008). They stabilise the mangrove sediment by maintaining the porosity, permeability, grain-size, water-content, organic-content and erosion-threshold by their bioturbation, productivity and carbon dynamics in the mangrove habitat (Austen et al. 1999; Tolhurst et al. 2003). Benthic functional efficacies not only restrict to mangrove habitats alone, instead have a profound influence on other associated coastal ecosystems (seagrass, estuaries, mudflats, coral reefs). They help in energy transfer through the nutrient outwelling, benthic-pelagic coupling, as indicators of pollution and sediment quality, trophic support and also to coastal communities as a major source of income (prawns, crabs) and livelihood support. Even though, the benthic fauna offers these multitudes of functions they are neglected due to our ignorance on their community ecology and taxonomic strength from various habitats.
Biocoenosis is the term introduced by Karl Mobius (1877) to include all organisms living in a particular habitat, which are closely linked to the environmental conditions prevailing in that habitat and maintain reciprocal interspecific functional relations (Basso and Corselli 2002). Pérès and Picard (1964) used the term biocoenosis to describe the distribution of benthic organisms, in relation to the environmental variables, within the framework of the benthic bionomy. The benthic fauna, unlike any other biota, because of their ubiquitous distribution and sedentary nature has a strong ecological relationship with mangroves and is an efficient assemblage to check the healthy status of this tropical ecosystem. Furthermore the structure of benthic assemblages and benthic production studies are a powerful tool in mangrove management (Ellison 2008).
Cochin mangroves along the Vembanad Lake (Ramsar site) of Kerala, located on the west coast of India is characterised by backwater estuarine type of mangroves experiencing intense upwelling associated with the south-west monsoon (Venkataraman and Wafar 2005; Asha et al., 2016). These habitats have reported a sharp loss in the mangrove area from 700 km2 (Ramachandran et al 1986) to about 9 km2 (India State of Forest report 2017) over the last three decades with 40% depletion in mangrove vegetation affecting many of the life forms (Satheeshkumar et al. 2011). Moreover, studies have reported that Cochin mangroves have been polluted by organic and inorganic contaminants, metal pollutants (Kumar et al. 2010; Joseph et al. 2019) from Eloor-Edayar industrial belt possessing more than 83 red category industries (KSPCB 2010). This study thus evaluated the habitat variability and ecosystem function of Cochin mangrove habitat, with respect to physicochemical nature of sediment, the floral structure and the resident benthic macrofaunal community. Moreover comprehensive studies pertaining to taxonomy and community structure of benthic macrofauna in the mangrove ecosystem is scanty in Kerala and in particular the interaction with mangrove vegetation and environmental factors has not been conducted so far. Accordingly, the biodiversity data of this study can be effectively used for mangrove management activities.
Materials and methods
Study site
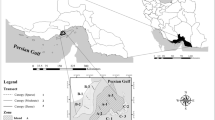
Cochin mangroves are located along the Vembanad Lake, a Ramsar site in central Kerala. Three mangrove zones (two stations each) with diverse mangrove vegetation, Aroor, Vypin and Valanthakad areas were chosen for study with a depth not more than 2 m (Fig. 1). Samples were collected from six stations on monthly intervals for two years (144 samples) from September 2010 to August 2012. Based on prevailing meteorological conditions, three seasons were distinguished, the pre-monsoon (PRM) (February–May), monsoon (MN) (June–September) and post-monsoon (PM) (October–January) period. Mangrove study stations were given in Table 1.
Mangrove plant density
Mangrove plant density in each site was taken once during sampling period using the quadrat method suggested by Cintron and Novelli (1984). Five quadrats of the size 5x5 m (25m2) were laid on each site considering the representativeness, importance and accessibility. The plant species in the quadrat were identified based on Tomlinson (1986) and counted to obtain the quantitative data and expressed in individuals per hectare (ind ha−1). The density of mangrove species was calculated as:
Density = number of individuals of a species / total area sampled.
Environmental parameters
Rainfall data was obtained from the India Meteorological Department (IMD) (www.imd.gov.in). Hydrological parameters such as salinity were measured by Mohr-Knudsen method (Strickland and Parsons 1972) and dissolved oxygen by the modified Winkler method (APHA 2005). Sediment samples were collected using van–Veen grab (0.04 m2) and temperature, pH and Eh were measured on site using a portable field analyser (Eutech ION 6 +). Homogenised and dried sediment was used for estimation of organic carbon, by modified wet oxidation method (El Wakeel and Riley 1957; Nelson and Sommers 1982; Trivedy and Goel 1986). Organic matter (OM) content of sediment was calculated by multiplying organic carbon values by Van Bemmelen factor of 1.724 (Trask 1939). The textural characteristics were determined by Pipette method (Folk 1974). Sediment nutrients such as total phosphorus and total sulphur were determined by digesting sediment using nitric acid and per-chloric acid in 5:1 ratio in KEL PLUS digestion unit (model KES 04L) and analysed by ICP-AES (Inductively Coupled Plasma Atomic Emission Spectrometer, Model: Thermo Electron IRIS INTREPID II XSP DUO) (AOAC, 1990) at DST-SAIF, Sophisticated Test and Instrumentation Centre, CUSAT.
Macrobenthic fauna
Monthly duplicate sediment samples were taken from each site by using standard van Veen grab of size 0.04 m2 and sieved for macrobenthic fauna onsite through a 0.5 mm mesh sieve. The samples were then preserved and stained in 5% neutral buffered formaldehyde containing Rose Bengal (Holme and McIntyre 1984; Eleftheriou and McIntyre 2005). The organisms were sorted into different taxonomic groups (Malacostraca, Polychaeta, Mollusca and Others) and identified up to the lowest possible taxonomic level and validated using World Register of Marine Species (WoRMS 2019) and numerical abundance was expressed in individuals per meter square (ind m−2). The wet weight of each macrofaunal group was determined by using a high precision electronic balance (Sartorius AG–ME215P, Germany with a precision of 0.01 mg). The biomass of macrofauna was expressed in g m−2. The wet weight of bivalve molluscs was determined with shells removed.
Statistical analysis
SPSS v.16 (Statistical Programme for Social Sciences) software was used for ANOVA and Pearson correlation analysis. ANOVA (Analysis of Variance) was computed to test statistically significant variability of macrobenthic faunal density, biomass and environmental factors on a spatio-temporal scale and Post-hoc comparisons were performed using Tukey HSD tests. The relationship between macrobenthic biomass and density with mangrove plants and environmental variables were estimated using Pearson’s correlation coefficient. Principal Component Analysis (PCA), an ordination technique was conducted to detect the habitat differences based on environmental data using PRIMER v.6. PRIMER v.6 software was also employed to analyse benthic community characteristics using univariate methods such as Shannon diversity index (H′), Margalef species richness index (d), Pielou's evenness index (J′) and Simpson's dominance index (λ) as well as multivariate methods such as Bray–Curtis hierarchical clustering and similarity profile (SIMPROF) permutation tests after square root transformation of abundance data of macrobenthic communities. A permutation-based hypothesis testing ANOSIM (one way) was performed to find out whether there was any significant variability in species between the clustered groups (Clarke and Warwick 2001). Similarity percentage (SIMPER) gives the average percentage contribution of individual species to the similarity or the dissimilarity of a clustered group of stations (Clarke and Gorley 2006). RDA (Redundancy Analysis) was performed to differentiate the species in each sampling site with respect to environmental variables using CANOCO v.4.5.
Results
Mangrove floral diversity and spatial variation in Cochin
In Cochin, 13 species of true mangroves belonging to 6 families and 8 genera were identified. The most diverse family was Rhizophoraceae with 6 species including Rhizophora apiculata Bl., R. mucronata Poir., Kandelia candel (L.) Druce., Bruguiera cylindrica (L.) Bl., B. gymnorrhiza (L.) Lamk., B. sexangula (L.) Bl., followed by Acanthaceae with Avicennia officinalis L., A. marina (Forssk.) Vierh., Acanthus ilicifolius L., Lythraceae family with Sonneratia caseolaris (L). Engler. and S. alba Griff., Euphorbiaceae with Excoecaria agallocha L., Pteridaceae with Acrostichum aureum L. The density of mangroves ranged from 80 to 50,000 ind ha−1, of which A. ilicifolius (14,729 ± 20,351 ind ha−1), E. agallocha (4293 ± 3674 ind ha−1) and A. aureum (3530 ± 3536 ind ha−1) were the densest species.
Valanthakad zone, S6 (6225 ± 14,726 ind ha−1) and S5 (3120 ± 4285 ind ha−1) have highest mangrove density followed by Vypin zone, S4 (1680 ± 2163 ind ha−1) and S3 (1124 ± 1094 ind ha−1) and least density in Aroor zone, S1 (818 ± 745 ind ha−1) and S2 (1453 ± 2719 ind ha−1). Even though Aroor has the lowest density, species diversity was maximum with 11 species in S1 of which A. officinalis (2080 ind ha−1) was the dominant vegetation however in S2 with 10 species, A. ilicifolius (9066 ind ha−1) dominated. Vypin has only seven species of mangroves, of which S3 is unique in having A. marina and S. alba and densest species was B. cylindrica (3467 ind ha−1), however, in S4 E. agallocha was predominant (6400 ind ha−1). Valanthakad, S5 and S6 have 9 and 10 species of true mangroves, respectively, both having the dominant mangrove species A. ilicifolius with a density of 12,500 ind ha−1 and 50,000 ind ha−1 respectively. Spatial variation in mangrove vegetation is plotted in Fig. 2. Avicennia officinalis, R. mucronata, E. agallocha and B. gymnorrhiza were common to all stations.
Environmental parameters
The monsoon season showed peak rainfall in Cochin linked to unique south-west monsoonal rains. During this season salinity and the temperature was lower, however significantly higher temperature (p < 0.01) and salinity (p < 0.01) was observed during PRM of the second year with a mean of 31.6 ± 2.2 °C and 15.2 ± 8.1 PSU respectively. Salinity was mixo-mesohaline (8.17 ± 7.19 PSU) with spatial variation (p < 0.05) that ranged between 0.12 to 35.9 PSU and was highest in Vypin zone due to proximity to Sea. A highly significant annual variability (p < 0.01) was observed in salinity values and was higher in the second year (10.8 ± 5.3 PSU) than first year (5.6 ± 6.2 PSU). Dissolved oxygen was moderate (3.8 ± 1.2 mg/L) in mangrove zones, however, it ranged between 0.79 to 9.84 and the lowest value was recorded in Aroor zone and highest in Valanthakad island, seasonally highest during PRM and MN. Turbidity (4.5 ± 5.7 NTU) showed significant spatiotemporal variation (p < 0.05) that ranged from zero to 39 NTU and Aroor, S1 (8.0 ± 9.8 NTU) were turbid especially during MN due to its relative shallowness and litter deposits. Sediment temperature was higher during PRM in both years as that of water temperature and spatially no significant variability, and mean value was in the range of 30 °C. The sediment pH exhibited slightly alkaline trend seasonally as well as spatially and was higher in Vypin zone, S3 (7.6 ± 0.4), while acidic in Aroor especially in S1 (6.9 ± 0.7) mostly in all months and even reached up to 4.6 during May 2012. Spatially, remarkable variation in sediment Eh with the highly reduced condition was seen in both sites of Aroor and Vypin zone, however island zone (Valanthakad) depicted comparatively oxidised condition. Seasonally significant variation (p < 0.05) observed with highest Eh in PM of first-year (-0.75 ± 46.7 mV) and lowest in second year PM (-189.9 ± 178.1 mV). Mangrove sediments were sandy in most of the selected sites, except in S1 which was silt dominated (56.28 ± 12.84%). Mangrove sediment usually exhibited a higher organic matter and in Cochin, it averages to 31.82 ± 23.09 g/kg. Spatially, Aroor, S1 has the highest organic matter (39.04 ± 8.83 g/kg), while lowest in island zone. Organic carbon in mangrove ecosystem of Cochin ranged between 0.78 to 54.21 with an average of 18.5 ± 13.4 gC.kg−1. Nutrients such as total phosphorus (581.88 ± 387.40 mg/kg) and total sulphur (6502.47 ± 5187.62 mg/kg) was also higher in Aroor zone (S1). Spatial variation in environmental parameters is given in Table 2.
PCA analysis
In PCA, the first five principal components accounted for 74.4% of the variability between stations (Fig. 3). The first PC accounted for 34.1% variability (eigenvalue of 5.12) and were determined by sediment parameters such as sediment texture, organic carbon, organic matter, total sulphur and total phosphorus. Here sand was the positive determinant while other parameters exhibited a negative correlation. The second PC accounted for 14% of the variability and was driven by sediment temperature. Hence the sediment variables were found to be an influencing factor in differentiating mangrove stations.
Two-dimensional Principal component analysis (PCA) ordination of selected normalized environmental variables in Cochin mangroves on a spatial basis during 2010–2012 period. (Sal-salinity, S.pH-sediment pH, DO-dissolved oxygen, Tur-turbidity, OC-organic carbon, OM-organic matter, T.pho-total phosphorus, T.sul-total sulphur, w.temp-water temperature, S.temp-sediment temperature, Eh-Redox potential, sand, silt, clay)
Macrobenthic standing stock in Mangrove habitat
About eleven diverse taxa (class) of macrobenthic fauna with a numerical density that ranged between zero to 11,223 ind m−2 with a mean of 1628 ± 2283 ind m−2 were encountered during the two-year sampling efforts. Out of the total 8437 organisms collected in the grab samples, the dominant taxa was malacostracans (55%), followed by polychaetes (23%), molluscans (13%) and few sporadic representatives pooled together as ‘others’ (9%), including oligochaetes, nemerteans, nematodes, gobiids, turbellarians, insects etc. Malacostracan crustaceans were dominant among other benthic representatives in numerical density. Amphipods were the prime representative of malacostracans with an overall numerical density of 87,507 ind m−2 (68.03%), then tanaids 39,364 ind m−2 (30.61%), together contributing to 99% of crustaceans in the mangrove ecosystem. The benthic biomass was contributed mainly by molluscs (64%), then polychaetes (19%), malacostracan crustaceans (15%) and others (2%) with a mean value of 20.85 ± 44.70 g m−2 and with a total biomass of 3003.15 g m−2. Significant spatial variation was observed in benthic density (F (5,144) = 16.54, p = 0.000) and biomass (F (5,144) = 15.240, p = 0.000). Open mangrove zones of Valanthakad island including S5 and S6 recorded highest density (3861 ± 2453 ind m−2) and (3015 ± 2265 ind m−2) as well as highest biomass (72.11 ± 71.90 g m−2) and (39.76 ± 50.94 g m−2), respectively. Vypin zone, in particular S4 documented lowest mean density (86 ± 326 ind m−2) and biomass (0.22 ± 0.78 g m−2) of macrobenthic fauna. Crustaceans, polychaetes as well as molluscs were abundantly seen in island mangroves (Valanthakad), however numerical density of ‘others’ particularly oligochaetes were higher in S1 of Aroor zone. Seasonally, highest mean numerical density (2247 ± 2509 ind m−2) as well as biomass (134.4 ± 193.5 g m−2) was recorded in PRM of second-year (2011–12) contributed mainly by crustaceans and molluscs, while the density (2019 ± 1805 ind m−2) and biomass (9.25 ± 10.6 g m−2) was lowest in first year PRM. Spatial and temporal variation in density and biomass of macrobenthos is given in Fig. 4a–d.
Community composition of macrofaunal species
Benthic Macrofauna of Cochin mangroves comprised of a total of 48 species in 45 genera belonging to 38 families (Table 3). Class Malacostraca (Crustacea) was represented by 17 spp. in 11 families and 4 orders. Family Penaeidae (4 spp.), Eriopisidae (3 spp.) and Corophiidae (2 spp.) have higher species richness with numerical dominance of the amphipods Idunella sp. (31.24%) and Cheiriphotis geniculata (26.57%). Polychaetes were represented by 11 species in 7 families and Nereididae (4spp.) and Capitellidae (2 spp.) were the most diverse family. Dendronereis aestuarina, alone contributed to 71.46% of polychaetes in mangroves and was seen abundantly in Valanthakad zone. Phylum Mollusca was represented by a total of 9 species in 9 families of which Indosphenia kayalum, was the most dominant (79.07%) molluscs in Cochin mangroves. The sporadic forms “Others” includes 11 species in 11 families, of which an oligochaete Tubificoides pseudogaster (79%) of family Tubificidae was predominant with respect to relative density in “others”.
Univariate diversity indices such as Shannon diversity index (H'[log2]) ranged from 0.41 to 3.10, and Margalef richness index(d) was 0.13 to 1.39, that of Pielou evenness index (J') was 0.36 to 0.98 and Simpson dominance index (1-λ’) was 0.15 to 0.86 in mangrove sites of Cochin. Spatially higher species diversity, species richness and species dominance was seen in S5 and S6 of Valanthakad zone and lowest in S4 of Vypin, however, Pielou evenness was highest in S4 in particular and lowest in Valanthakad zone. Seasonally higher species diversity, richness and dominance was recorded in PRM of second-year while species evenness in first year PRM, however all the diversity indices were lowest recorded in first year PM during the study period. Spatial and temporal variation in species richness and diversity is given in Fig. 5a–d.
The k-dominance curve of species abundance data also revealed high species diversity in Valanthakad with a similar assemblage pattern in S5 and S6 with the dominance of D. aestuarina, Idunella sp., C. geniculata, I. kayalum and C. chilkensis. S4, the least diverse station was characterised by dominance of chironomid larvae contributing to 60% of total fauna in the station, however in S3, pollution indicator, Capitella sp. and tanaids were predominant. Tubificid oligochaete (T. psuedogaster) was the most dominant macrofauna in S1 and S2 of Aroor zone during the study period. Seasonally in PRM, a different assemblage structure was seen during the first and second year of study. In first year, C.geniculata and C. chilkensis were the dominant fauna, whereas in second year Idunella sp. and I. kayalum dominated. In first year PM, T. pseudogaster and C. geniculata were predominant while in the second year D. aestuarina dominated replacing T. pseudogaster followed by C. geniculata. In MN both in the first and second year, D. aestuarina were the most dominant species in mangroves.
Multivariate analysis
Hierarchical cluster analysis and SIMPROF tests of six sampling sites were grouped into three significant clusters. First cluster between the stations S5 and S6 forming HDD (High Density and Diversity) group with 81.4% similarity (p = 100%) while S1, S2 and S3 form LDD (Low Density and Diversity) group with 53.4% similarity (p = 31.9%) and S4 is an outlier.
Analysis of Similarities with one way ANOSIM was applied to test the null hypothesis, that there was no significant difference in faunal composition between these clustered groups of stations. ANOSIM showed a significant difference between clustered stations where R value lies away from 95% confidence limit or null distribution (ANOSIM, Global R = 0.401, p = 0.1%). Pairwise test of ANOSIM gives the significant difference between HDD and LDD (ANOSIM, R = 0.311, p = 0.1%); HDD and Outlier (ANOSIM R = 0.845, p = 0.1%); LDD and Outlier (ANOSIM R = 0.395, p = 0.1%). The similarities or dissimilarities between the mangrove stations were due to difference in species assemblages, presence or absence of some unique species or the variation in abundance of predominant species.
The SIMPER analysis was carried out to detect the fauna responsible for these clusters (Table 4). In HDD, 8 species were responsible for the formation of this group such as D. aestuarina (27.53%), Idunella sp. (21.83%), C. geniculata (16.93%), I. kayalum (9.67%) are major species, however in LDD (S1, S2, S3), 7 species of which C. chilkensis (34.15%), T. psuedogaster (18.69%), Idunella sp. (13.38%), V. chilkensis (10.53%) were showing maximum contribution. The dissimilarity between a clustered group of stations such as HDD and LDD were 89.85% that was mainly due to the difference in abundance pattern of species such as D. aestuarina (15.21%), C. geniculata (13.44%), Idunella sp. (12.71%), which were in higher abundance in HDD, furthermore I. kayalum, V. cusatensis, V. cyprinoides were absent in LDD similarly Capitella sp. was absent in HDD.
Dissimilarity between HDD and LDD with Outlier is 98% mainly due to the lower abundance or absence of species such as D. aestuarina, C. geniculata, V. chilkensis, and P. gymnophobia.
Interaction of environmental variables with macrobenthic fauna using RDA
RDA clearly defined spatial variations in environmental parameters along with their interaction and influence on structuring the benthic community (Fig. 6). In RDA triplot, vectors pointing in the same direction indicated a positive correlation between each other as between organic matter, total organic carbon, silt, clay texture, turbidity, total sulphur, total phosphorus pointing towards Aroor zone and opportunistic species which are tolerant to these variables dominantly oligochaetes, penaeids and tanaids were prevalent in this zone. The vector pointing opposite to these variables especially sand was negatively correlated and that point in a perpendicular direction (redox potential) indicated no correlation between the parameters. Most of the polychaetes, amphipods, isopods, mostly all insects, molluscs were seen in the Valanthakad island zone with higher redox potential and sandy texture implying higher species diversity. Vypin zone was characterised by higher salinity and alkalinity due to proximity to sea and species tolerant to salinity was seen here especially certain polychaetes (Ophelia sp., D. heteropoda), amphipod (Eriopisella sp., G. megnae), molluscs Donax pulchellus etc.
Discussion
Benthic standing stock in relation to environment and flora
The population density of macrofauna ranged from zero to 11,223 ind m−2 with an overall density of 234,381 ind m−2 in Cochin mangroves during the study period (2010–12). In the present study, benthic fauna exhibited comparatively higher density but lower diversity compared to the previous study by Sunil Kumar (1993) in Cochin mangroves who reported an overall density of 111,840 ind m−2 and range of 0–17,910 ind m−2. This increase in benthic density in the present study was attributed to the higher abundance of opportunistic species such as oligochaetes, amphipods and tanaids. The population density of macrofauna of Cochin mangroves was lower than that reported from other mangrove habitats of India such as Pichavaram (Pravinkumar et al. 2013) but higher than Kachchh mangroves (Saravanakumar et al. 2007). The malacostracan crustaceans (55%) were the dominant group during the entire study followed by polychaetes (23%), molluscs (13%) and ‘others’ (9%). However, in the previous study (Sunil Kumar 1993), polychaetes were predominant contributing to 51.7% followed by molluscs (26.23%), then crustacean (15.12%) and others (6.95%).
The biomass was also higher (3003.15 g m−2) with greater representation by molluscs (64%) compared to the previous study (424 g m−2), where polychaetes were predominant (51.44%) among other benthic macrofauna. Seasonally species density, as well as diversity, was highest in PRM of second year in Cochin estuarine-mangrove area (Sunil Kumar 1993; Sheeba 2000) however lower in first-year PRM which might be linked to the seasonal variability in environmental factors especially salinity regimes that exhibited highly significant variability, with higher salinity in the second year than the first year. Several studies have pointed out the influence of salinity on the seasonal distribution of macrobenthic fauna (Lui et al. 2002; Sivadas et al. 2011).
Generally, polychaetes are predominant taxa in estuaries (Jayachandran et al. 2019a), mangroves (Murugesan et al. 2016) and other marine systems (Jayaraj et al. 2007), however in the present study malacostracan crustaceans were the major group. Nordhaus et al. (2009) observed higher dominance of crustacea (43.3% of total) in mangrove-fringed Segara Anakan lagoon, Indonesia. The relative abundance of malacostracans usually increased in well-oxygenated sandy sediment, and they avoided organic matter accumulated sediment (Jayachandran et al. 2019b). In the present study, malacostracan density correlates negatively to clay (r = − 0.289), nutrients (r = − 0.291), organic matter (r = − 0.212) instead prefer sand dominated sediment with less organic matter. Molluscs species form the major biomass producers (64%), however, their density was comparatively lower (13%) in Cochin mangroves. They were predominant fauna in Australian mangroves (Kelehar et al. 1998). The spatial difference in biomass and density of macrofauna was highly significant and their standing stock was consistently higher in the sediment with larger grain size (r = 0.262) as sand possess more micro-habitats, excess of oxygen, food particles and good permeability to permanent burrowers (Sanders 1968), however, they exhibited a negative correlation to organic matter (r = − 0.304) and also to silt (r = − 253). Certain benthic organisms are transient visitors in mangroves mainly by tidal flow, in search of food and usually low in their density (Macintosh and Ashton 2002). These include mainly insects, turbellarians, nematodes, nemertines, oligochaetes and benthic fishes represented as ‘others’ in Cochin. Among them oligochaetes usually seen as swarms with a very higher density while other taxa were in lower numbers. Spatial variation was notable in the assemblage that correlates well with silty (r density = 0.170, r biomass = 0.211) and clayey (r density = 0.198, r biomass = 0.191) texture and also to total sulphur (r = 0.164) in the sediment. Giere and Pfannkuche (1982) observed higher densities of oligochaetes in sandy and detritus rich sediments; however, Schrijvers et al. (1995) accounted higher density of oligochaetes (94%) in mud and organic-rich sediment in Kenyan mangroves. The abundance of opportunistic species such as Tubificoides psuedogaster in Aroor (S1) indicated accumulation of organic matter (Pearson and Rosenberg 1978), with reduced macrofaunal species diversity, biomass and substantial increase in pollution tolerant forms such as C. chilkensis, P. gymnophobia (Albayrak et al. 2006).
One of the main goals of benthic ecology is to understand the mechanisms regulating relationships between environmental parameters and benthic fauna (Snelgrove and Butman 1994; Aller et al. 2001). The correlation of biomass and density of macrofauna with various physicochemical factors revealed that substratum with higher sediment particle usually sand (r density = 0.169; r biomass = 0.260) was suitable for macrofauna. Sasekumar (1974) also found the importance of sediment particle size in Malayan mangroves. As particle size decreases, the chemical contaminants such as heavy metals, pesticides got adsorbed on to fine-grained sediments making it unfavourable for benthic fauna (Zhang et al. 2013, 2014) thereby reducing the density (r = − 0.256) and biomass (r = − 0.311). It also implies that even though mangroves provides wider niches, majority of mangrove resident fauna opt for lower organic-rich (r density = − 0.293, r biomass = − 0.279), moderate nutrient (r density = − 0. 280, r biomass = − 0.320), less sulphidic (r density = − 0.223, r biomass = − 0.301) sediment with higher dissolved oxygen and salinity for their flourishing.
Lugo and Snedaker (1974) mentioned the existence of a causal association between fauna and type of mangroves. It was also mentioned the importance of structural complexity of mangrove roots, type and density that provides excellent shelter from predators for benthic invertebrate species (Kon et al. 2009). In the current study, 13 true mangroves were identified from Cochin out of the total 18 species reported in Kerala (Sreelekshmi et al.2018). Significant correlation was seen between macrobenthic biomass and density with mangrove plants especially to Acrostichum aureum (r biomass = 0.899; r density = 0.903) and Rhizophora apiculata (r biomass = 0.910; r density = 0.894). The mangrove fern (A.aureum) grow on the landward side of the mangrove, provide shade for other plants and at the same time they take over an area with low mangrove trees so rapidly that they form impenetrable thickets which prevent other plants from taking root. These thickets provide safety and shelter for invertebrates (http://www.wildsingapore.com). The soil around the roots of plants (rhizosphere) harbours microbes (Rahaman et al. 2018) that promote the abundance of macrofauna for feeding. Acrostichum, the mangrove fern is densely seen in Valanthakad, S5 (6200 ind ha−1) and S6 (6960 ind ha−1). Similarly, R. apiculata have higher density in Valanthakad zone compared to other mangrove sites, where both benthic biomass and density were higher. Sasekumar and Chong (1998) also reported higher density and biomass of epifaunal taxa in mature R. apiculata forest in Malaysia and Kon et al. (2007, 2010) also reported higher benthic stock in the mangrove forest in Thailand with R. apiculata as the dominant vegetation. The activities of R. apiculata roots are known to lower the pH and alkalinity of sediments (Kristensen et al. 1991), furthermore physical structure of R. apiculata facilitates benthic fauna, with canopy shade providing a cool, moist surface layer.
Multitude of factors govern differential production pattern and spatial variability of macrobenthic fauna in mangrove habitats, however, in Cochin, plant density as well as substratum is the most influential factors. Alfaro (2006) also reported the influence of mangrove plant types as well as density in macrofaunal standing stock in New Zealand mangroves while the influence of sediment variables in mangroves was reported in several mangrove habitats (Satheeshkumar and Khan 2012; Lacerda et al.1995).
Community composition of macrobenthic fauna
Biodiversity of a benthic community can be measured by relative species richness in a particular area at a particular time (Clarke 1990). Benthic diversity is relatively poor comprised of 48 species and with lower species richness (Margalef index, 0.13 to 1.39) and Shannon diversity (0.41 to 3.10) compared to the previous study (Sunil Kumar 1993) that comprised of 54 species with species richness index and Shannon diversity index that ranged between 0.38 to 4.5 and 0.52 to 3.03, respectively, in Cochin mangrove zones indicating biodiversity loss within two decades. Moreover, a severe decline or disappearance of polychaetes was noted in the present study in contrast to earlier studies in Cochin mangroves by Sunil Kumar (1993, 1995), from 33 to 11 species while malacostracans were increased from 9 species (Sunil Kumar 1993) to 17 species especially amphipods, Idunella sp. and C. geniculata dominated the system replacing the polychaete communities of Nereis glandicincta-Dendronereis aestuarina—Marphysa gravelyi. Generally, mangrove habitats have reported to have a higher composition of polychaete species rather than crustaceans (Metcalfe and Glasby 2008; Samidurai et al. 2012). According to Pocklington and Wells (1992), polychaetes respond to disturbance induced by different kinds of pollution exhibiting quantifiable changes in community structure which was evident in the current study. Higher rate of pollutants discharged from nearby industries (Salas et al. 2017; Joseph et al. 2019) may induce sensitive species to respond either by their mortality or by community shift. It was also noticed that temperature exhibited a substantial increase in the current study which ranged from 27–35 °C compared to 26–33.5 °C reported in the previous study (Sunil Kumar 1993) which may be linked to global climatic variation. The temperature rise has a severe effect on polychaetes (Simonini and Prevedelli 2003) which leads to their decline. Furthermore, the pH was alkaline in mangroves of Cochin generally (Sunil Kumar 1993), but in the present study acidic condition was observed infrequently due to discharge of acidic and chemical pollutants, especially heavy metals from the industrial sectors upstream Cochin mangrove-estuarine area which was recently documented in our study (Joseph et al. 2019).
Dendronereis aestuarina is the only abundant polychaete in Cochin mangrove area with a numerical density (38,809 ind m−2) during the two-year study. Previous reports also suggested a higher abundance of D. aestuarina in Cochin estuarine region (Jayachandran et al. 2015). Among Crustaceans, family Eriopisidae represents the maximum number of species (3 species), two from Victoriopisa genus (Joseph et al. 2018) and one Eriopisella. The family is characterized by marine, epigean and hypogean fauna with cosmopolitan distribution (Lowry and Myers 2013). Victoriopisa chilkensis (previously under genus Eriopisa) occurred in large numbers in the organically enriched sediments of the Cochin mangrove especially in S1 and it can be regarded as a tolerant species of organic pollution (Aravind et al. 2007) as they are filter feeders feeding on organic-rich nutrients (Asari 1983). In mangroves of Cochin, Idunella sp. of Liljeborgiidae family attained total density of 40,170 ind m−2, highest of all macrobenthic species. The algal association of Idunella was reported by Rabindranath (1971), the pneumatophores and stilt roots of mangroves are algal rich zone which enhances the abundance of Idunella in mangrove habitats and in present study Valanthakad attained maximum density. Corophid C. geniculata also adds to the density of macrofauna along with Idunella sp. together contributing to 58% of malacostracans. The recent study by Asha (2017) in Vembanad backwaters also listed the abundance of C. geniculata especially in the oligohaline zone, having higher organic carbon in the sediment. However, in mangrove stations, they were observed in Valanthakad region, a pristine mangrove zone and preferred sandy sediment with low organic matter and lower salinity. Tanaids was represented by Ctenapseudes chilkensis and Pagurapseudopsis gymnophobia, are opportunistic forms and their abundance indicated organic matter accumulation in an area (Pearson and Rosenberg 1978). Tanaids have a varied preference for the substrate as some preferred muddy and fine sand shell deposition for its proliferation (Priya et al. 2014) while some found in marine caves (Guţu and Iliffe 2001) and some even been found in sulfurous anoxic environments (Sieg and Heard 1985). In Cochin mangrove, these opportunistic species were present in all stations due to their tolerance to environmental variables but attained maximum density in polluted fine sediments of S1 with higher nutrients and organic content and even anoxic at times (Joseph et al. 2019). They directly feed on mangrove leaves and help in nutrient recycling. Among the molluscan fauna, Myidae represented the single dominant family with Indosphenia kayalum, the new bivalve identified from Vembanad Lake recently (Oliver et al. 2018) with a numerical density of 23,835 ind m−2 during two year study, followed by Villorita cyprinoides. The soft-shell clams, Myidae (Indosphenia) are suspension feeders with two fused siphons that make burrows and lead a sessile lifestyle (Oliver et al. 2018). Siphoning activities may create water current that has a profound effect on sediment biochemistry (Hansen et al. 1996). Villorita is of great importance in fisheries and source of protein and is adapted to low to high saline conditions and thrive at salinities as high as 15 ppt (Sheeba 2000). Their diversity was also higher in S5 and S3 that may be based on salinity gradient. Molluscs are least sensitive to organic matter enrichment and H2S accumulation (Kuk-Dzul and Dıaz-Castaneda 2016) but intolerant to increased siltation (Alexander et al.1993). The absence of molluscs in S1 may be due to silty sediment texture of the station associated with higher organic enrichment. Oligochaetes in mangroves mostly belong to the family Tubificidae (synonymised to Naididae) and also to Enchytraeidae (Erseus 2002). Similarly in the present study, these families were encountered and tubificid oligochaete worms were the dominant taxa in the infauna of mangrove sediments represented by Tubificoides psuedogaster contributed to 80% to “others” density while Enchytridae were least represented. Mangrove oligochaetes are opportunistic and largely represent genera adapted to low salinity and organically enriched sediments (Giere 2006), both conditions being characteristic of S1 where they present in larger densities.
Macrofaunal assemblages in the mangroves were significantly separated based on the spatial distribution and diversity by hierarchical clustering and SIMPROF and also based on species composition by similarity percentage analysis (SIMPER). The higher similarity in macrofauna in HDD (81.4%) was due to similar ecological condition with moderate organic matter, higher redox potential and sandy texture of these island mangroves with a pristine ecology compared to LDD (53.4%). LDD characterised by lower sediment grain size, higher nutrients entailed the colonization of opportunistic benthic forms. They have a varied preference for substrate and observed in strongly eutrophicated areas (Jorissen et al. 2018). The first order opportunists observed in LDD were T. psuedogaster, Capitella sp. Enchytraeids and third-order opportunists were C. chilkensis, P. gymnophobia and Chironomids. S4 is an outlier perhaps due to their irregular assemblage pattern and lower diversity of species. The higher density of mangrove plant E. agallocha rich in diterpenoids, flavonoids, phenolic acids, sterols, tannins, and triterpenoids cause toxicity effects to microbial and other faunal components (Chan et al. 2018) in S4 reducing species diversity. RDA summarises the linear relationships of both the species and the environmental matrices and attempt to explain variability in species composition and indicated that the changes in the macrobenthic fauna in mangroves were associated with edaphic factors.
Conclusion
Cochin mangroves are floristically diverse zone; however, the anthropogenic perturbations and pollution impacts had led to the severe decline in benthic stock and species composition. The variation in benthic assemblage in multiple spatial scales corroborates with the sediment structure and flora. Sandy substratum supports higher species density and biomass with an assemblage of crustaceans, while opportunistic forms at certain sites imply polluted status with lower diversity. Cochin mangroves are home for newer and newer species of crustaceans, molluscs and hence biodiversity assessment and taxonomic studies of benthic fauna have to be strengthened along with periodic benthic faunal surveillance coupled with mangrove floral assessment and proper rejuvenation measures to conserve the existing mangroves. This change in community assemblage and biodiversity, thus demanding efficient management strategies for mangrove ecosystem through integrated planning, rehabilitation and conservation of its biodiversity and minimising anthropogenic stress factors, pollutants and initiating public participation in afforestation of mangroves thus preserving these ecological marvels for future.
References
Albayrak S, Balkis H, Zenetos A, Kurun A, Kubanc C (2006) Ecological quality status of coastal benthic ecosystems in the Sea of Marmara. Mar Pollut Bull 52:790–799
Alexander RR, Stanton Jr RJ, Dodd JR (1993) Influence of sediment grain size on the burrowing of bivalves: correlation with distribution and stratigraphic persistence of selected neogene clams. Palaios, pp. 289–303.
Alfaro AC (2006) Benthic macro-invertebrate community composi- tion within a mangrove/seagrass estuary in northern New Zealand. Estuar Coast Shelf Sci 66:97–110
Aller JY, Woodin SA, Aller RC (2001) Organism Sediment Interactions. University of South Carolina Press, Columbia
Alongi DM (2002) Present state and future of the world’s mangrove forests. Environ Conserv 29(3):331–349
Alongi DM (2018) Impact of global change on nutrient dynamics in mangrove forests. Forests 9(10):596
AOAC (1990) AOAC official methods of analysis, 15th edn. Association of Official Analytical Chemists, Arlington
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington DC, p 1220p
Aravind NP, Sheeba P, Nair KKC, Achuthankutty CT (2007) Life history and population dynamics of an estuarine amphipod, Eriopisa chilkensis Chilton (Gammaridae). Estuar Coast Shelf Sci 74(1–2):87–95
Asari KP (1983) On two species of gammarids (Amphipoda, Crustacean) from Andaman and Nicobar Islands, India. Bulletin de Museum National d‘Histoire Naturelle Paris, Series 4, 5. Section A 2:641–649
Asha CV (2017). Assessing the structural and functional ecology of benthic fauna in Vembanad estuarine system, India. Doctoral dissertation, Cochin University of Science & Technology.
Asha CV, Retina IC, Suson PS, Bijoy Nandan S (2016) Ecosystem analysis of the degrading Vembanad wetland ecosystem, the largest Ramsar site on the South West Coast of India- Measures for its sustainable management. Reg Stud Mar Sci 8:408–421. https://doi.org/10.1016/j.rsma.2016.06.003
Austen I, Andersen TJ, Edelvang K (1999) The influence of benthic diatoms and invertebrates on the erodibility of an intertidal mudflat, the Danish Wadden Sea. Estuar Coast Shelf Sci 49(1):99–111
Basso D, Corselli M (2002) Community versus biocoenosis in multivariate analysis of benthic molluscan thanatocoenoses. Riv Ital Paleontol Stratigr 108(1):153–172
Chan EWC, Oshiro N, Kezuka M, Kimura N, Baba K, Chan HT (2018) Pharmacological potentials and toxicity effects of Excoecaria agallocha. J App Pharm Sci 8(05):166–173
Cintron G, Novelli YS (1984) Methods for studying mangrove structure. In:Mangrove ecosystem: research methods (pp. 91–113). UNESCO.
Clarke KR (1990) Comparisons of dominance curves. J Exp Mar Biol Ecol 138(1–2):143–157
Clarke KR, Gorley RN (2006) PRIMER v6: User manual/tutorial.Plymouth, UK: PRIMER-E.
Clarke KR, Warwick RM (2001). Change in marine communities. An approach to statistical analysis and interpretation, 2.
Duke NC, Meynecke JO, Dittmann S, Ellison AM, Anger K, Berger U, Koedam N (2007) A world without mangroves? Science 317(5834):41–42
El Wakeel SK, Riley JP (1957) The determination of organic carbon in marine muds. ICES J Mar Sci 22(2):180–183
Eleftheriou A, McIntyre AD (2005) Methods for the study of marine benthos, 3rd edn. Blackwell Science Publication, Oxford, p 418
Ellison AM (2008) Managing mangroves with benthic biodiversity in mind: moving beyond roving banditry. J Sea Res 59:2–15
Erséus C (2002) Mangroves and marine oligochaete diversity. Wetlands Ecol Manage 10(3):197–202
Folk RL (1974) The petrology of sedimentary rocks. Hemphill Publishing Company, Austin, Texa S50
Giere O, Pfannkuche O (1982) Biology and ecology of marine Oligochaeta, a review. Oceanogr Marine Biol Ann Rev 20:173–308
Giere O (2006) Ecology and biology of marine oligochaeta–an inventory rather than another review. Hydrobiologia 564(1):103–116
Gutu MODEST & Iliffe TM (2001) Grallatotanais antipai, a new genus and species of the family Leptocheliidae Lang, 1963 from a marine cave in the Bahamas (Crustacea: Tanaidacea, Tanaidomorpha). Travaux du Muséum National d’Histoire Naturelle “Grigore Antipa, 43, 93–100.
Hansen DJ, Berry WJ, Mahony JD, Boothman WS, Di Toro DM, Robson DL, Ankley GT, Yan Ma D, Pesch CE (1996) Predicting the toxicity of metal contaminated field sediments using interstitial concentration of metals and acidvolatile sulfide normalizations. Environ Toxicol Chem 15:2080–2094. https://doi.org/10.1016/j.rsma.2018.100444
Holme NA, McIntyre AD (eds) (1984) Methods for the study of marine benthos. Blackwell Scientific Publications, Oxford, pp 1–387
India State of Forest Report (2017) Forest Survey of India. Ministry of Environment and Forests, Dehradun
Jayachandran PR, Bijoy Nandan S, Jima M, Sreedevi OK, Philomina J, Prabhakaran MP (2019a) Bioecology of macrobenthic communities in the microtidal monsoonal Kodungallur-Azhikode Estuary, southwest coast of India. Lakes Reserv Res Manage 24(4):372–390. https://doi.org/10.1111/lre.12292
Jayachandran PR, Bijoy Nandan S, Jima M, Philomina J, Don Xavier ND, Sreedevi OK, Prabhakaran MP, Joseph KJ (2019b) Macrobenthic functional feeding groups in a microtidal monsoonal estuary (Kodungallur–Azhikode estuary, India). Regional Stud Mar Sci 25:100444
Jayachandran PR, Prabhakaran MP, Asha CV, Bijoy Nandan S, Vijay A (2015) First report on mass reproductive swarming of a polychaete worm, Dendronereis aestuarina (Annelida, Nereididae) Southern 1921, from a freshwater environment in the south west coast of India. Int J Mar, Sci, p 5
Jayaraj KA, Jayalakshmi KV, Saraladevi K (2007) Influence of environmental properties on macrobenthos in the northwest Indian shelf. Environ Monit Assess 127(1–3):459–475. https://doi.org/10.1007/s10661-006-9295-5
Jorissen F, Nardelli MP, Almogi-Labin A, Barras C, Bergamin L, Bicchi E, El Kateb A, Ferraro L, McGann M, Morigi C, Romano E (2018) Developing Foram-AMBI for biomonitoring in the Mediterranean: species assignments to ecological categories. Mar Micropaleontol 140:33–45
Joseph P, Bijoy Nandan S, Adarsh KJ, Anu PR, Rani V, Sreelekshmi S, Preethy CM, Jayachandran PR, Joseph KJ (2019) Heavy metal contamination in representative surface sediments of mangrove habitats of Cochin. Southern India Environ Earth Sci 78(490):1–11
Joseph P, Bijoy Nandan S, Jayachandran PR (2018) New species of Victoriopisa Karaman & Barnard, 1979 (Crustacea: Amphipoda: Eriopisidae) from Vembanad backwaters. Southwest Coast India Zootaxa 4433(1):69–70
Móbius K (1877) Die Auster und die Austernwirtschaft. Wiegundt. Hampel Ec Parey, Berlin. English translation in Rep. U.S. Fish Comm 1880:683–751
Kathiresan K (2010) Importance of mangrove forests of India. J Coastal Environ 1(1):11–26
Kelehar BP, Underwood AJ, Chapman MG (1998) Effect of boardwalks on the semaphore crab Heloecius cordiformis in temperate urban mangrove forests. J Exp Mar Biol Ecol 227:281–300
Kon K, Kurokura H, Hayashizaki K (2007) Role of microhabitats in food webs of benthic communities in a mangrove forest. Mar Ecol Prog Ser 340:55–62
Kon K, Kurokura H, Tongnunui P (2009) Do mangrove root structures function to shelter benthic macrofauna from predators? J Exp Mar Biol Ecol 370(1–2):1–8
Kon K, Kurokura H, Tongnunui P (2010) Effects of the physical structure of mangrove vegetation on a benthic faunal community. J Exp Mar Biol Ecol 383(2):171–180
Kristensen E, Holmer M, Bussarawit N (1991) Benthic metabolism and sulphate reduction in a Southeast Asian mangrove swamp. Mar Ecol Progr Ser 73:93–103
KSPCB (2010) Action plan for greater Kochi area. Kerala State Pollution Control Board, Kerala, pp 1–89
Kuk-Dzul JG, & Díaz-Castañeda V (2016) The relationship between mollusks and oxygen concentrations in Todos Santos Bay, Baja California, Mexico. J Mar Biol 2016.
Kumar R, Joseph MM, Gireesh Kumar TR, Renjith KR, Manju MN, Chandramohanakumar N (2010) Spatial variability and contamination of heavy metals in the inter-tidal systems of a tropical environment. Int J Environ Res 4(4):691–700
Lacerda LD, Ittekkot V, Patchineelam SR (1995) Biogeochemistry of Mangrove soil organic matter: a comparison between Rhizophora and Avicennia soils in southeastern Brazil. Estuar Coast Shelf Sci 40:713–720
Lee SY (2008) Mangrove macrobenthos: assemblages, services, and linkages. J Sea Res 59(1–2):16–29
Levinton JS (2001) Marine biology: function biodiversity, ecology, 2nd edn. Oxford University Press, New York
Lowry JK, Myers AA (2013) A phylogeny and classification of the Senticaudata subord. Nov. (Crustacea: Amphipoda). Zootaxa 3610(1):1–80
Lugo AE, Snedaker SC (1974) The ecology of mangroves. Annu Rev Ecol Syst 5(1):39–64
Lui TH, Lee SY, Sadovy Y (2002) Macrobenthos of a tidal impoundment at the Mai Po marshes nature reserve. Hong Kong Hydrobiologia 468(1–3):193–211
Macintosh DJ, Ashton EC (2002) A Review of Mangrove Biodiversity and Conservation. University of Aarhus, Denmark, Centre for Tropical Ecosystem Research
Metcalfe KN, Glasby CJ (2008) Diversity of Polychaeta (Annelida) and other worm taxa in mangrove habitats of Darwin Harbour, northern Australia. J Sea Res 59:70–82
Murugesan P, Pravinkumar M, Muthuvelu S, Ravichandran S, Vijayalakshmi S, Balasubramanian T (2016) Benthic biodiversity in natural vis-a-vis artificially developed mangroves of south east coast of India. Indian J Geo-Mar Sci 45(8):1049–1058
Nagelkerken ISJM, Blaber SJM, Bouillon S, Green P, Haywood M, Kirton LG, Somerfield PJ (2008) The habitat function of mangroves for terrestrial and marine fauna: a review. Aquat Bot 89(2):155–185
Nelson DW, Sommers LE (1982) Total carbon, organic carbon, and organic matter vol 2. In: Page AL, Miller RH, Keeney DR (eds) Method of soil analysis, Part 2, 2nd ed, Agronomy 9. American Society of Agronomy, Madison
Nordhaus I, Hadipudjana FA, Janssen R, Pamungkas J (2009) Spatio-temporal variation of macrobenthic communities in the mangrove-fringed Segara Anakan Lagoon, Indonesia, affected by anthropogenic activities. Reg Environ Change 9:291–313
Oliver GP, Hallan A, Jayachandran PR, Philomina J, Sanu VF, Bijoy Nandan S (2018) Taxonomy of myid bivalves from fragmented brackish water habitats in India, with a description of a new genus Indosphenia (Myida, Myoidea, Myidae). ZooKeys 799:21–46
Pearson TH, Rosenberg R (1978) Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr Mar Biol Annu Rev 16:229–311
Pérès JM, Picard I (1964) Nouveau manuel de bionomie bentique de la Mer Méditerranée. Rec. Trar. St. Mar. Endowme, 31/47: 1–137, Marseille
Pocklington P, Wells PG (1992) Polychaetes: Key taxa for marine environmental quality monitoring. Mar Pollut Bull 24:593–598
Pravinkumar M, Murugesan P, Prakash RK, Elumalai V, Viswanathan C, Raffi SM (2013) Benthic biodiversity in the Pichavaram mangroves, Southeast Coast of India. J Oceanogr Mar Sci 4(1):1–1. https://doi.org/10.5897/JOMS12.004
Priya A, Sesh Serebiah J, Gomathy R and Moses R (2014) New records of tanaids in Pulicat lake ecosystem In: Ecology, environment and conservation paper. Special Issue-; Pg.107–116).
Rabindranath P (1971) A New Liljeborgiid Amphipod (Crustacea) from Kerala. India Biol Bull 140(3):482–488
Rahaman S, Bera AR, Vishal V, Singh PK, Ganguli S (2018) A phylogenetic insight into the fern rhizosphere of Acrostichum aureum Linn. Int J Pharm Biol Sci 8:452–456
Ramachandran KK, Mohanan CN, Balasubrarnanian G, Kurian J, Thomas J. (1986). The mangrove ecosystem of Kerala, its mapping inventory and some environmental aspects. Thiruvananthapuram: State Committee on Science, Technology and Environment Project Report (1985–86).
Salas PM, Sujatha CH, Ratheesh Kumar CS, Cheriyan E (2017) Heavy metal distribution and contamination status in the sedimentary environment of Cochin estuary. Mar Pollut Bull 119:191–203. https://doi.org/10.1016/j.marpolbul.2017.04.018
Samidurai K, Saravanakumar A, Kathiresan K (2012) Spatial and temporal distribution of macrobenthos in different mangrove ecosystems of Tamil Nadu Coast. India Environ Monitor Assess 184(7):4079–4096
Sanders HL (1968) Marine benthic diversity: a comparative study. Am Nat 102(925):243–282
Saravanakumar A, Serebiah JS, Thivakaran GA, Rajkumar M (2007) Benthic macrofaunal assemblage in the arid zone mangroves of gulf of Kachchh-Gujarat. J Ocean Univ China 6(3):303–309
Sasekumar A, Chong VC (1998) Faunal diversity in Malaysian mangroves. Glob Ecol Biogeog Lett 7:57–60
Sasekumar A (1974) Distribution of macrofauna on a Malayan mangrove shore. J Anim Ecol 43:51–69
Satheeshkumar P, Manjusha U, Pillai NG (2011) Conservation of mangrove forest covers in Kochi coast. Curr Sci 101(10):1400
Satheeshkumar P, Khan AB (2012) Identification of mangrove water quality by multivariate statistical analysis methods in Pondicherry coast, India. Environ Monitor Assess 184(6):3761–3774
Schrijvers J, van Gansbeke D, Vincx M (1995) Macrobenthic infauna of mangroves and surrounding beaches at Gazi Bay, Kenya. Hydrobiologia 306:53–66
Sheeba P (2000) Distribution of benthic infauna in the Cochin backwaters in relation to environmental parameters, Doctoral dissertation. Cochin University of Science and Technology, Kochi, p. 241.
Sieg J, Heard RW (1985) Tanaidacea (Crustacea: Peracardia) of the Gulf of Mexico. IV. On Nototanoides trifurcatus Gen. Nov., Sp. Nov., with a Key to the Genera of the Nototanaidae. Gulf Caribbean Res 8(1):51–62
Simonini R, Prevedelli D (2003) Effects of temperature on two Mediterranean populations of Dinophilus gyrociliatus (Polychaeta: Dinophilidae): I. Effects on life history and sex ratio. J Exp Mar Biol Ecol 291(1):79–93
Sivadas S, Ingole B, Nanajkar M (2011) Temporal variability of macrofauna from a disturbed habitat in Zuari estuary, west coast of India. Environ Monit Assess 173(1–4):65–78
Snelgrove PVR, Butman CA (1994) Animal-sediment relationships revisited: cause versus effect. Oceanogr Mar Biol 32:111–177
Sreelekshmi S, Preethy CM, Varghese R, Philomina J, Asha CV, Bijoy Nandan S, Radhakrishnan CK (2018) Diversity, stand structure, and zonation pattern of mangroves in southwest coast of India. J Asia-Pacific Biodiv 11(4):573–582
Strickland JD, Parsons TR (1972) A practical handbook of seawater analysis. Fisheries Research Board of Canada, Otawa, p 357
Sunil Kumar R (1993) Studies on the benthic fauna of the mangrove swamps of Cochin area (Ph. D thesis, Cochin University of Science & Technology).
Sunil Kumar R (1995) Macrobenthos in the mangrove ecosystem of Cochin backwaters, Kerala (Southwest coast of India). Indian J Mar Sci 24:56–61
Tolhurst TJ, Jesus B, Brotas V & Paterson DM (2003) Diatom migration and sediment armouring—an example from the Tagus Estuary, Portugal. In Migrationsand Dispersal of Marine Organisms (pp. 183–193). Springer, Dordrecht
Tomlinson PB (1986) The botany of mangroves. Cambridge tropical biology series. El Wakeel, S. K., & Riley, J. P. (1957). The determination of organic carbon in marine muds. ICES J Mar Sci 22(2):180–183
Trask PD (1939) Recent marine sediments. American Association of Petroleum Geologists Publication, Tulsa, p 736
Trivedy PK, Goel PK (1986) Chemical and biological methods for water pollution studies. Series in Methodology. Environmental Publications, Karad, pp 1–220
Venkataraman K, Wafar MVM (2005) Coastal and marine biodiversity of India. Indian J Mar Sci 34(1):57–75
WoRMS (2019) World Register of Marine Species. Accessed Jan 4, 2019. Available from http://www.marinespecies.org at VLIZ. https://doi.org/https://doi.org/10.14284/170.
Zhang C, Yu ZG, Zeng GM, Jiang M, Yang ZZ, Cui F, Hu L (2014) Effects of sediment geochemical properties on heavy metal bioavailability. Environ Int 73:270–281. https://doi.org/10.1016/j.envint.2014.08.010
Zhang Y, Lv Z , Guan B, Liu Y, Li F, Li S, ... & Li Y (2013) Status of macrobenthic community and its relationships to trace metals and natural sediment characteristics. CLEAN–Soil, Air, Water, 41(10), 1027–1034. https://doi.org/10.1002/clen.201200575
Acknowledgements
The first author is thankful to UGC MANF (MANF-2013-14-CHR-KER-23851) for the financial assistance. This work forms part of the research project entitled ‘‘Studies on mangrove ecosystem of south-west coast of India in the context of sustainable livelihood objectives’’ funded by Directorate of Environment and Climate change (DOECC). Authors are also thankful to the Head, Department of Marine Biology, Microbiology and Biochemistry, Cochin University of Science and Technology for providing necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Joseph, P., Nandan, S.B., Sreelekshmi, S. et al. Benthic biocoenosis: influence of edaphic factors in the tropical mangroves of Cochin, Southern India. Trop Ecol 62, 463–478 (2021). https://doi.org/10.1007/s42965-021-00162-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42965-021-00162-5