Abstract
The levels of 17 organochlorine pesticides residues (OCPs) in surface water and sediments from Tamiraparani river basin, South India were investigated to evaluate their potential pollution and risk impacts. A total of 96 surface water and sediment samples at 12 sampling stations were collected along the river in four seasons during 2008–2009. The ΣOCP concentrations in surface water and sediments were in the range of 0.1 to 79.9 ng l−1 and 0.12 to 3,938.7 ng g−1 dry weight (dw), respectively. Among the OCPs, the levels of dichlorodiphenyltrichloroethanes (DDTs), aldrin, dieldrin, cis-chlordane, trans-chlordane, and mirex were dominant in the sediments. The dominant OCPs in water samples are heptachlor, o,p′-DDE, dieldrin, o,p′-DDD, and mirex, which show different source of contamination pattern among sampling seasons. The distribution pattern of DDTs, hexachlorocyclohexane, and other OCPs in the present study shows heterogenic nature of nonpoint source of pollution. Notable contamination of water and sediment sample that was observed in upstream (S2) 58 ng l−1 and downstream (S11) 1,693 ng g−1 dw explains agricultural and municipal outfalls, whereas frequent damming effect reduces the concentration level in the midstream. The overall spatial–temporal distribution pattern of ΣOCP residues are illustrated by GIS package.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The environmental contaminations by pesticide residues are of great concern due to their toxicity, bioaccumulation, and persistent nature. Organochlorine pesticides such as hexachlorocyclohexane (HCH) and dichlorodiphenyltrichloroethane (DDT), endrin, lindane, and aldrin are among the most persistent and globally distributed organic pollutants. These pesticides are long-lived organic compounds that become concentrated as they move through the food chain and have toxic effects on reproduction and immunological function (Balakrishnan et al. 1985). Pesticides are unique in that they are purposefully released in to the environment to control the selected species. The chlorinated pesticides are now largely banned chemicals, which may still pose a threat to human health as well as the wider environment. Over the last 50 years, the increasing global use of toxic synthetic pesticides has seriously affected human health, environment, and agricultural sustainability. Pesticide use is a continuously increasing trend, especially in developing countries. It persists in the environment, and many are dispersed globally as a result of drift, volatilization from soil, and vaporization (Kurtz 1990). The chemical properties of OCPs such as low water and high fat solubility, stability to photo-oxidation, and low vapor pressure are the main elements not only in the efficacy of these compounds as pesticides but also in their persistence in the environment (Kim and Smith 2001). Further, combination of their physico-chemical properties and persistence in the environment make them capable of long-range transport (Singh et al. 2005). The input pathways of OCPs into the river environments include runoff from nonpoint sources, discharge of industrial waste, wet or dry deposition, and other means. Therefore, the residues of OCPs might ultimately pass onto people through consumption of agriculture food, fish, and drinking water (Iwata et al. 1993). Although bans and restriction on the usage of some OCPs in developed countries were enacted during the 1970s and 1980s, some developing countries are still using them for agricultural and public health purposes because of their low cost and versatility in controlling various pests (Xue et al. 2006).
India is one of the major producer and consumer of organochlorine pesticides, particularly DDTs and HCHs for agriculture and public health programs. Even in the 1990s, more than 70% of the gross tonnage of pesticides used in agricultural applications consisted of formulations which are banned or severely restricted in the east and west (Subramanian et al. 2007). Consumption of insecticide in agriculture has been increased more than 100% from 1971 to 1994–1995. For instance, insecticide consumption in India, which was to the tune of 22,013 tons, has increased to 51,755 tons by 1994–1995 (www.indiastat.com). The Indian pesticide industry with 82,000 metric tons of production for 2005–2006 is ranked second in Asia (behind China) and 12th on global market. According to Green Peace report, India is (2008) producing 90,000 metric tons of pesticides as the largest industry in the whole of Asia (http://www.greenpeaceindia.org/nopesti.htm). Agriculture sector is a very important part of the Indian economy and greatly contributes to overall food needs of the country and provides domestic industry with agricultural raw materials. In the thirst of modernization and industrialization, man has contributed pollution to the life and ecology of plants, animals, and microbes. Increased demand for food and fiber has lead to the chemicalization of agriculture, and we have reached on such a stage that modern agriculture is dependent on high-yielding varieties, which can only be grown under the influence of fertilizers and pesticides (Chauhan and Singhal (2006). These persistent organic compounds such as HCH, DDT, and its metabolites are the predominant chemical contaminants found along the Indian coast (Sarkar et al. 2008) and were reported in major rivers (Table 1).
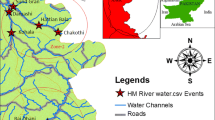
The river Tamiraparani is one of the few perennial rivers of the southern peninsular India, which originates on the eastern slopes of the Western Ghats at an altitude of 2,000 m, travels through about 125 km in the hills and plains, draining an area of 5,369 km2, and flows into the Gulf of Mannar (Ravichandran et al. 1996). The main cropping pattern in this river basin includes paddy, banana, groundnut, and coconut, which is regularly practiced through various irrigational (dams, check dams, weir, etc.) system. The cotton, sorghum, ragi, pulses, and ginger are the important varieties cultivated in the rain-fed lands. Interestingly, the river is the main source of drinking water and other industrial units of Tirunelveli and Tuticorin districts (Ravichandran 2003). Pesticide fate modeling, coupled with Geographical Information System (GIS) technologies for various data handling, spatial analyses, and visualization under variable inputs and spatial scales, is an innovative approach developed during recent years (Sood and Bhagat 2005). GIS is an automated approach to locational and nonlocational data synthesis, which combines a system capable of data capture, storage, retrieval, analysis, manipulation, and display (Burrough 1986). However, no report was available on OCPs in Tamiraparani river. In order to understand the pollution load of OCPs, a systematic survey has been carried out in the river basin from upstream to estuary region (Gulf of Mannar) during 2008–2009. The main objectives of this study are (1) to assess the levels with spatial and temporal distributions of OCPs in water and sediment and (2) to discuss their contamination profiles and possible sources of certain important OCPs in the river basin.
Material and methods
Chemicals
Standard solutions of organochlorine pesticides (99% purity) including aldrin, dieldrin, endrin, heptachlor, cis-chlordane, trans-chlordane, mirex, α-HCH, β-HCH, γ-HCH, p,p′-DDT (1,1,1,-tri-chloro-2,2-bis(p-chlorophenyl)ethane), o,p′-DDT (1,1,1,-tri-chloro-2(p-chlorophenyl)-2-(o-chloro-phenyl) ethane), p,p′-DDD (1,1-dichloro-2,2-bis(p-chlorophenyl)-ethane), o,p′-DDD (1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl)-ethane), p,p′-DDE (1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene), o,p′-DDE (1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl)-ethylene), and hexachlorobenzene (HCB) were purchased from the AccuStandard (USA). Burdick & Jackson Company, USA offered hexane (GC/GC-MS-graded), and acetone (HPLC-graded) was offered by Qualigens Fine Chemicals Company, India. Anhydrous sodium sulfate (Na2SO4, AR-graded, Hi-Media, Mumbai, India) and florisil (60–100 mesh, Hi-Media) were dried at 180°C using muffle furnace for overnight. The storage vials of 2 ml in capacity were purchased from Agilent Technologies, Germany.
Study area and samples
Tamiraparani river irrigation system is one of the oldest irrigation systems in the state of Tamil Nadu, South India extending between latitude 8°30′ to 9°15′ N and longitude 77°10′ to 78°10′ E. Water samples were collected in 2-L-capacity presterilized amber glass bottles for same selected location covering four seasons, i.e., premonsoon (July to August), monsoon (September to December), postmonsoon (January to February), and summer (March to May) during 2008–2009. Figure 1 shows the study area and sampling locations. A Global Position System (GPS, Leica G20, USA) was used to locate the sampling locations more precisely during the sampling. The water samples were brought into the laboratory using an icebox and stored at 4°C until further analysis. All water samples were filtered through 0.45-μm Millipore cellulose nitrate membrane filter paper to remove suspended and particulate matter and extracted for OCPs. The surficial bed sediment samples were collected in polyethylene bag using small plastic scoop at the same locations and were stored at 4°C until analysis (Ramesh and Anbu 1996). Sediments sample were air-dried in a clean laboratory condition at room temperature, homogenized and sieved through a 63-μm size, and then extracted for OCPs.
Extraction and cleanup
Water samples were extracted according to Veeraiah and Durga Prasad (1996) with minor modifications. Briefly, 1 L of the water sample from each location was twice extracted by liquid–liquid extraction in a glass separating funnel of 2 L in capacity using 50 ml of n-hexane. The combined solvent extract was demoisturized by anhydrous sodium sulfate (roasted at 180°C) and concentrated in a rotary evaporator to a final volume of 5 ml with n-hexane. Further, it was purified with glass column (12 mm i.d. - loaded with 5 g activated florisil) and was washed with 15 ml hexane. The eluents were concentrated to 1 ml under a gentle stream of pure nitrogen gas for gas chromatography–mass spectroscopy (GC-MS) analysis. The analytical procedure of OCPs residues in sediments are as follows. Initially, 10 g of homogenized, air-dried soil sample was extracted for 4 h in a Soxhlet apparatus with 140 ml of pesticide grade n-hexane/acetone 1:1 v/v (USEPA, Method 3630C 1996). The extract was concentrated up to 5 ml with a rotary vacuum evaporator (Buchi, Switzerland) at 40°C. A clean-up column (12 mm i.d.) containing 6 g activated florisil loaded with glass column (12 mm i.d.) was washed with 15 ml hexane. The eluents were concentrated to 1 ml under a gentle stream of pure nitrogen gas for GC/MS analysis.
Quality control
Analysis of blank levels of solvents, equipment and other materials, analysis of procedural blanks, recovery of spiked standards, monitoring of detector response, and linearity were strictly followed. All analyses were carried out in duplicate, and the recoveries of individual pesticides were determined through the spiked sample method, which were between 88.4% to 92% in water and 82.5% to 105% in sediment. Results are presented as range (minimum–maximum) and mean values with standard deviation (SD). Means were computed by treating the concentrations of the nondetected analyses as limit of detection (LOD). The LOD for α-HCH, β-HCH, HCB, γ-HCH, heptachlor, aldrin, trans-chlordane, o,p′-DDE, cis-chlordane, dieldrin, p,p′-DDE, o,p′-DDD, endrin, p,p′-DDD, o,p′-DDT, p,p′-DDT, and mirex was found to be 0.01, 0.05, 0.05, 0.06, 0.06, 0.02, 0.01, 0.01, 0.01, 0.01, 0.03, 0.02, 0.02, 0.02, 0.01, 0.01, and 0.01 ng l−1, respectively.
Instrumentation
Identification and quantification of aldrin, dieldrin, cis-chlordane, trans-chlordane, HCB, endrin, mirex, HCHs, and DDT compounds were made by using a gas chromatograph coupled to a quadrupole mass spectrometry (Shimadzu QP 2010, Japan). A solvent delay of 4 min was set to protect the filament from oxidation. Chromatographic separation was achieved using a DB-1 fused silica capillary column (30 m length × 0.32 mm i.d.; film thickness 0.25 μm; J&W Scientific, USA). The GC/MS was operated with an interface temperature of 270°C and an ionization source temperature of 230°C. The column oven temperature was programmed as 70°C (1.0 min) to 160°C at 20°C/min and from 160°C to 190°C at 2°C/min and finally increased to 320°C at 5°C/min, and the final temperature was held for 5 min. Helium was used as carrier gas (99.995% purity) at a flow rate of 2.25 ml/min. The gas chromatograph was equipped with splitless injector port operated at 250°C. The samples were injected in the splitless mode. The mass spectrometer was operated in the positive ion electron impact energy of 70 eV and an emission current 60 μA. Full scan data were obtained with a mass range of m/z 35–500. Scanning interval and SIM sampling rate were kept at 0.5 and 0.2 s, respectively (Iino et al. 2007).
Spatial distribution modeling
In the present work, the ArcGIS 9.2 software was used for spatial interpolation, and the technique applied has three main stages, i.e., (1) digitization, (2) GIS manipulation, and (3) GIS analysis. In the study area map, the sampling points were digitized using GIS software. All the feature of each digitized lines are taken as x, y coordinates, and the value (concentration) of each points were coded as z. The digitized points were stored as vector file of polygon format and finally transformed into raster format. Interpolation of the selected rasterized maps of ΣOCPs residues in surface water and sediment samples in different season were illustrated for the river basin.
Results and discussion
Concentration of OCPs in water
The concentration of 17 organochlorine pesticides in surface water samples are ranged from 0.1 to 79.9 ng l−1 and given in Table 2. The concentrations of DDTs and other OCPs were much higher than that of HCHs. Figure 2a shows the distribution pattern of total OCPs residues in water sample for four seasons in the river. It clearly reveals that the OCP residues in monsoon and summer season were much higher than other two sampling seasons. This could be due to some OCPs being released from nonpoint sources and subsequent leaching processes during monsoon period throughout the basin and intense agriculture practices in summer season followed by southwest monsoon in the upper region. Higher concentrations of OCPs in upstream (S2 and S5) during monsoon season have probably resulted from farmland runoff (Tenkasi taluk) along with untreated municipal waste directly mixing into the river. At the same time, lower concentration of OCPs in the Servalar area explains dense forestation with less agriculture practice and midstream province (S9) influenced by man-made damming activities resulting subsequent soil deposition leads to decrease in pesticides load. The highest concentrations of 58.0 ng l−1 was observed at Papanasam lower dam (S2) site during monsoon season reveals the fact that it receive water from Western Ghats region where rubber cultivation dominates and used organochlorine insecticides. The organochlorine pesticides under different trade names as DDT are listed as (anofex, cezarex, chlorophenothane, clofenotane, Dicophane, Dinocide and Zerdane, etc.). Aldrin (ENT 15949 (compound 118), HHDN, Octalene, OMS 194) and dieldrin (ENT 16225 (compound 497), HEOD, alvit, octalox, OMS 18, Quintox) (http://www.inchem.org/documents/hsg/hsg/hsg021.htm.). In the downstream area of the river, Eral (S10) and Punnaikayal (S12) are also witnessed higher concentrations of OCPs during all the seasons as a result of collective down flow loads. In the four sampling periods, the highest concentration of HCHs in the river was 0.78 ng l−1 (S9) during premonsoon and 7.53 ng l−1 for dieldrin (S5) and 58.0 ng l−1 for endrin (S2) during monsoon seasons. This elevated concentration is comparable with other Indian and world rivers (Table 1).
Other OCPs including aldrin, endrin, dieldrin, heptachlor, trans-chlordane, cis-chlordane, and mirex were found in small amounts in all sampling sites and seasons, specifically endrin and dieldrin which were found in most of the locations in water as well as in sediment samples. Dieldrin was widely used as an effective insecticide around the world until the middle 1970s, mainly for the control of soil pests, like termites, grasshoppers, locusts, beetles, textile pests, and for the treatment of seeds. Additionally, it has also been used to control vectors of tropical diseases, including malaria and yellow fever, and also used for industrial purposes to protect electric and telephone cables and to preserve timber materials, etc. (Jong 1991). In the study region, dieldrin is effectively used as soil insecticide, especially for perennial crops, such as palm and banana. Also, aldrin and dieldrin were used to control termites in houses by direct soil injection in the past (ATSDR 2002). Aldrin is rapidly converted to dieldrin in the environment. The sums of aldrin concentrations were in the ranges of <0.02 to 4.0 ng l−1 in different seasons, and the same trend was observed for dieldrin in the range of <0.03 to 12.7 ng l−1. The present results showed that these compounds were probably originated from the above said industrial and public health practices. Pesticide pollution of surface water from agriculture use has been well documented around the world in recent years. But there are limited data available for these 17 OCPs, and hence, the presence study is the holistic approach towards comparison of Tamiraparani results with Indian and other world rivers (Table 1).
The heptachlor concentration was ranged between <0.06 and 2.10 ng l−1 during monsoon and <0.06 and 0.47 ng l−1 in postmonsoon season. Heptachlor is metabolized to heptachlor epoxides in the soils, plants, and animals, which are more stable in biological systems. In this study, heptachlor concentration is relatively lower than that reported by Zhang et al. (2002a) for Pearl river estuary (2.83–294 ng l−1). Similarly, the levels of ΣHCH (<0.01 to 0.78 ng l−1) were also found lower than Pearl river estuary (Zhang et al. 2002b) and Minjiang river estuary (Zhang et al. 2003). In contrary, the residual levels of aldrin and dieldrin were considerably higher (<0.02–4.0 ng l−1 and <0.03–12.7 ng l−1 respectively) than Gomti river (Malik et al. 2009). Aldrin has been used as a soil insecticide to control root worms, beetles, and termites. Dieldrin has been used in agriculture for soil and seed treatment as well as for control of mosquitoes and tsetse flies (Jensen 1983). Other uses for dieldrin include veterinary treatments for sheep, wood treatment against termites, and mothproofing of woolen products (ATSDR 1989). Endrin has been used as an agricultural insecticide on tobacco, apple trees, cotton, sugar cane, and grain, as well as to control rodents and birds (ATSDR 1997). Based on the water samples analysis for four seasons, the river basin is polluted in the order of endrin > dieldrin > aldrin > ΣDDT > heptachlor > mirex > cis-chlordane > ΣHCH > HCB > trans-chlordane.
Residual levels of OCPs in sediments
The total OCPs concentrations in sediments were varied from 0.1 to 3938.7 ng g−1 dw. Table 3 and Fig. 2b illustrate the distribution of OCPs in surface sediments for four sampling seasons. The concentrations in sediments sample during summer season were in the range of 0.12 to 472.4 ng g−1 dw for ΣHCHs (α-, β-, and γ-HCH), 20.5 to 56.2 ng g−1 dw for DDTs (p,p′-DDT, o,p′-DDT, p,p′-DDD, o,p′-DDD, o,p′-DDE), and 12.6–421.5 ng g−1 dw for other ΣOCPs (HCB, heptachlor, aldrin, trans-Chlordane, dieldrin, and mirex). Like water samples, the concentrations of ΣDDTs and its isomer were much higher than those of ΣHCHs. This trend is consistent with the fact that the amount of technical DDT used was significantly higher than that of HCHs in the river basin (Table 3). The status of OCPs contamination in sediments was compared with other world rivers (Table 1). Among the Indian rivers, the levels of DDTs and other OCPs in sediments of Tamiraparani river ranked considerably higher (0.12 to 472.4 ng g−1 for HCHs, 37.8 to 857.4 ng g−1 for DDTs). In the present study, the ΣDDT concentrations are higher than those reported for Minjiang river estuary (1.6–13.1 ng g−1 dw; Zhang et al. 2003); Haihe and Dagu Drainage rivers (0.32–80.18 ng g−1 dw; 3.60–83.49 ng g−1 dw; Yang et al. 2005), and Taiwan rivers (0.21–8.81 ng g−1 dw; Doong et al. 2002). Among the DDT metabolites, o,p′-DDE, p,p′-DDE, o,p′-DDD, p,p′-DDD, o,p′-DDT, and p,p′-DDT were ranged from <0.02 to 34.9 ng g−1, <0.01 to 82.77 ng g−1, <0.02 to 50.2 ng g−1, <0.01 to 30.4 ng g−1, <0.01 to 252.7 ng g−1, and <0.01 to 460.8 ng g−1 dw, respectively. The levels of o,p′-DDT and p,p′-DDT in sediment are higher than those reported for Qiantang river (0.28–9.61 ng g−1; Zhou et al. 2006). In this study, the maximum percentages of p,p′-DDD and p,p′-DDT were detected in both water and sediments. The dominance of parent compound over their degradation products (metabolites) in the sediments suggested the recent use of DDT. The ΣHCH concentrations in sediments were twofold higher than Minjiang river (Zhang et al. 2003) and are comparable with river Cauvery, India (4.35–158.4 ng g−1 dw; Rajendran and Subramanian 1999); Qiantang river, East China (9.23–152.1 ng g−1; Zhou et al. 2006), and Dagu drainage river (33.24–141.03 ng g−1; Yang et al. 2005). HCB is a widespread contaminant that has entered the environment through their past production and its formation as by-products of a variety of chlorinated compounds. In aquatic systems, HCB is persistent in sediment and tends to accumulate in the tissues of organisms. In river sediments, the concentrations of HCB are ranged between <0.05 and 19.5 ng g−1 dw and are relatively higher than (0.14–3.96 ng g−1) Yangtse river (Xu et al. 2000), and trans-chlordane and cis-chlordane concentrations are ranged between <0.01 and 20.0 ng g−1 dw and <0.01 and 93.6 ng g−1 dw, respectively. The chlordane isomers are not frequently presented most of the sites for all four seasons in the river. It is notable that in India chlordane is completely banned on manufacturing, import and export (IPEP 2006).
Spatial and temporal distribution
The two highest concentrations of total OCPs including DDT, endrin, and dieldrin were found in stations S8 and S10 during monsoon season, which is agreed with the distribution pattern of surface water. It may be due to similar chronological input and source of OCPs in this region. The third highest concentration is observed at Punnakayal station (S12), located in the end part of river before confluence to estuary region. As a whole, OCP concentration in sediments were higher in downstream than upstream explains heavy rainfall in association with water leaching processes, adsorption and resuspension could cause source and sink for pollution material. For some OCPs, chemical transformation, and microbial decomposition may occur, e.g., DDT is transforming to DDE and δ-HCH to α-HCH. However, complete mineralization of organochlorine compounds does not usually take place either or is an extremely slow process (Falandysz et al. 2001). The differing composition of DDT metabolites and HCH isomers in the environment could indicate their different sources of contamination (Yang et al. 2005). The present result revealed that the physiochemical and biosolubility properties of HCHs and DDTs differ since HCHs having higher water solubility vapor pressure, and biodegradability than DDTs while lower lipophilicity and particle affinity as compared to DDTs (Loganathan and Kannan 1994), owing multi- level entry of contaminants and nonbiodegradability in the environment. Mirex has been observed in most of the sampling sites for all the four seasons. It is mainly used as a stomach insecticide, usually formulated into baits, for the control of ants, especially fire ants and harvester ants. We could conclude that mirex is recently used for agricultural purposes in the river basin, whereas it is not registered pesticide in India (IPEP 2006).
The percentage composition of DDT isomers measured in the sediment samples with reference to four different seasons are o,p′-DDE, 32.7%; o,p′-DDD, 19.3%; p,p′-DDD, 19.3%; p,p′-DDE, 17.2%; p,p′-DDT, 7.13%; and o,p′-DDT, 4.23% (Fig. 3a), with the dominance of the parent compound over their degradation products (metabolites) in the sediments suggests for the recent use of DDT along with degradation of DDT used earlier. It is evident that India has banned DDT for agricultural use in 1989, although it is still used in the public health sectors for malaria control (UNEP 2003). The relative concentration of the parent DDT compound and its metabolites, DDD and DDE, can be used as indicative indices for assessing the possible pollution sources. Since DDT can be biodegradable to DDE (aerobic condition) and DDD (anaerobic condition) (Bossi et al. 1992), the ratio of (DDE+DDD)/DDTs less than 0.5 indicate that long-term weathering process (Doong et al. 2002). Figure 3b shows the relationship between the (DDE + DDD)/DDTs and DDD/DDE in the surface sediments collected for monsoon and summer season. Ratios of (DDE + DDD)/DDTs were in the range of 0.1 to 0.86 with most values being less than 0.5 (Fig. 3b). This indicates that in the river sediments, source of DDT contamination is from the aged and weathered agricultural soils with signature of recently used DDT in Tamiraparani river catchment area.
In order to assess any adverse effect of persistent organic pesticides (POPs) contamination in the freshwater quality, criteria of maximum residue limit (MRL) by Food and Agricultural Organization (FAO) and WHO recommended certain limits for aldrin (0.1–180 μg l−1), chlordane (1.5–6.0 μg l−1), dieldrin (0.1–1.8 μg l−1), and DDT (1.0 μg l−1). Also, the levels of endrin, heptachlor, HCB, and mirex even in low concentration could be toxic to fish, aquatic invertebrates, and phytoplankton (IPEP 2006). In the present study, though the concentration levels of aldrin, chlordane, dieldrin, and DDT were well below FAO/ WHO values (Table 2), trace amount of endrin, heptachlor and mirex may contaminate the freshwater ecosystem of Tamiraparani river to become a toxic source and sink.
GIS interpolation
In order to compare seasonal distribution pattern of OCPs for the entire river basin, two analogous periods (monsoon and summer) were plotted in Arcview 9.2 GIS software for both water and sediment samples (Fig. 4a–d). In the upstream region, though it was dominated by forest land, agriculture area occupies about 3,000 ha, whereas midstream region on witnessed double the amount of land (6,000 ha). Consequently, the GIS distribution pattern shows heterogeneous type for upstream and monotonous type for mid- and downstream regions on monsoon season for water samples. Contrary during summer season, the mid- and downstream regions exhibit heterogenic nature due to lack of water flow, smaller check dams, and accumulation with evaporation effect. At the same time, sediment samples show heterogenic distribution pattern for mid- and downstream regions irrespective of both seasons. The land use pattern, urbanization and accumulation of sediments within smaller check dams could be the reason and also, increasing trend towards downstreams were observed for both season.
Conclusions
In the study, the presence of OCPs in both water and sediments indicates previous usage on agriculture, pest control, and household activities of pesticides in the river basin. Dieldrin and DDTs are the dominant OCPs present in water and sediment samples, and it reflects the previous and recent usage on agriculture activities. Higher concentration of DDT metabolites in the study area indicates that the aged and weathered agricultural soils were retained under anaerobic conditions in the sediment. The hydrological characteristic of the river basin, such as seasonal variation of flow and damming activities, can influence the spatial and temporal distribution of OCPs in water and sediments. Assessment of the freshwater contamination using the MRL of the FAO/WHO permissible limits for water quality criteria reflects that water is contaminated with OCPs and toxic effects may affect the biota in the river. The results indicate that there is an existence of certain potential health hazards for drinking water consumers and organisms in the river water, and further work is needed to assess the bioaccumulation of POPs in the food web and associated risks to the ecosystem and human health.
References
Ahmed, A., Barbary, El, Yehia, M. M., Mohamed, M., & Bouraie, El. (2008). Evaluation of organochlorine pesticides (OCPs) in surface water and bed sediment samples from the river Nile at Rosetta Branch, Egypt. Journal of Applied Sciences Research, 4, 1985–1993.
ATSDR. (1989). Public health statement for aldrin and dieldrin. Atlanta: US Department of Health and Human Services, Public Health Service.
ATSDR. (1997). Tox FAQs for endrin. Atlanta: US Department of Health and Human Services, Public Health Service.
ATSDR. (2002). Toxicological profile for aldrin/dieldrin. Atlanta: US Department of Health and Human Services, Public Health Service.
Awofolu, R. O., & Fatoki, O. S. (2003). Persistent organochlorine pesticide residues in freshwater systems and sediments from the Eastern Cape, South Africa. Water SA, 29, 323–330.
Balakrishnan, G., Ramachandran, M., Banerjee, B. D., & Hussain, Q. Z. (1985). Effect of dietary protein, dichlorodiphenyltrichloroethane (DDT) and hexachlorocyclohexanes (HCH) on hepatic microsomal enzyme activity in rats. British Journal of Nutrition, 54, 563–566.
Bossi, R., Laesen, B., & Premazze, G. (1992). Polychlorinated biphenyl congeners and other chlorinated hydrocarbons in bottom sediment cores of Lake Garden (Italy). Science of the Total Environment, 121, 77–93.
Burrough, P. A. (1986). In principles of geographical information systems for land resource assessment (pp. 104–156). Oxford: Clarendon.
Chauhan, R. S., & Singhal, L. (2006). Harmful effects of pesticides and their control through cowpathy. International Journal of Cow Science, 2, 61–70.
Darko, G., Akoto, O., & Oppong, C. (2008). Persistent organochlorine pesticide residues in fish, sediments and water from Lake Bosomtwi, Ghana. Chemosphere, 72, 21–24.
Doong, R. A., Sun, Y. C., Liao, P. L., Peng, C. K., & Wu, S. C. (2002). Distribution and fate of organochlorine pesticide residues in sediments from the selected rivers in Taiwan. Chemosphere, 48, 237–246.
Doong, R. A., Lee, S. H., Lee, C. C., Sun, Y. C., & Wu, S. C. (2008). Characterization and composition of heavy metals and persistent organic pollutants in water and estuarine sediments from Gao-ping river, Taiwan. Marine Pollution Bulletin, 57, 846–857.
Falandysz, J., Brudnowska, B., Kawano, M., & Wakimoto, T. (2001). Polychlorinated biphenyls and organochlorine pesticides in soils from the southern part of Poland. Archives of Environmental Contamination and Toxicology, 40, 173–178.
Iino, F., Sebesvari, Z., Renaud, F., Kitsuwa, T., Morita, M., Shibata, Y., et al. (2007). Pops analysis and monitoring in the Asian coastal hydrosphere. Organohalogen Compounds, 69, 1406–1409.
IPEP (2006). Country situation on persistent organic pollutants (POPs) in India, 1–57.
Iwata, H., Tanabe, S., Sakai, N., & Tatsukawa, R. (1993). Distribution of persistent organochlorines in the oceanic air and surface seawater and the role of ocean on their global transport and fate. Environmental Science and Technology, 27, 1080–1098.
Jensen, A. A. (1983). Chemical contaminants in human milk. Residue Reviews, 89, 1–128.
Jong, G.D. (1991). Long-term health effects of aldrin and dieldrin, a study of exposure, health effects and mortality of workers engaged in the manufacture and formulation of the insecticides aldrin and dieldrin. Toxicology Letters, 1–206.
Kim, J. H., & Smith, A. (2001). Distribution of organochlorine pesticides in soils from South Korea. Chemosphere, 43, 137–140.
Kurtz, D. A. (Ed.). (1990). Long range transport of pesticides. Chelsea: Lewis.
Loganathan, B. G., & Kannan, K. (1994). Global organochlorine contamination trends: an overview. Ambio, 23, 187–191.
Malik, A., Ojha, P., & Singh, K. P. (2009). Levels and distribution of persistent organochlorine pesticide residues in water and sediments of Gomti river (India)—a tributary of the Ganges river. Environmental Monitoring and Assessment, 148, 421–435.
Rajendran, R.B., & Subramanian, A.N. (1999). Chlorinated pesticide residues in surface sediments from the River Kaveri, South India. Journal of Environmental Science & Health Part B: Pesticides, Food Contaminants, and Agricultural Wastes (USA), 269–288.
Ramesh, R., & Anbu, M. (1996). Chemical methods for environmental analysis. Chennai: Macmillan India Ltd. 210.
Ravichandran, S., Ramanibai, R., & Pundarikanthan, N. V. (1996). Ecoregions for describing water quality patterns in Tamiraparani basin, South India. Journal of Hydrology, 178, 257–276.
Ravichandran, S. (2003). Hydrological influences on the water quality trends in Tamiraparani basin, South India. Environmental Monitoring and Assessment, 87, 293–309.
Rissato, S. R., Galhiane, M. S., Ximenes, V. F., de Andrade, R. M. B., Talamoni, J. L. B., Libanio, M., et al. (2006). Organochlorine pesticides and polychlorinated biphenyls in soil and water samples in the Northeastern part of Sao Paulo State, Brazil. Chemosphere, 65, 1949–1958.
Samoh, A. N. H., & Ibrahim, M. S. (2008). Organochlorine pesticide residues in the major rivers of Southern Thailand. Malaysian Journal of Analytical Sciences, 12, 280–284.
Sarkar, S. K., Bhattacharya, B. D., Bhattacharya, A., Chatterjee, M., Alam, A., Satpathy, K. K., et al. (2008). Occurrence, distribution and possible sources of organochlorine pesticide residues in tropical coastal environment of India: an overview. Environment International, 34, 1062–1071.
Singh, K. P., Malik, A., Mohan, D., & Takroo, R. (2005). Distribution of persistent organochlorine pesticide residues in Gomti river, India. Bulletin of Environmental Contamination and Toxicology, 74, 146–154.
Sood, C., & Bhagat, R. M. (2005). Interfacing geographical information systems and pesticide models. Current Science, 89, 8–25.
Subramanian, A., Ohtake, M., Kunisue, T., & Tanabe, S. (2007). High levels of organochlorines in mother’s milk from Chennai (Madras) city, India. Chemosphere, 68, 928–939.
UNEP. (2003). Global report on regionally based assessment of persistent toxic substances. Geneva: UNEP Chemicals.
USEPA. (1996). Method 3630, Revision C, EPA-737-F-96-005, Research Triangle Park, NC 27711.
Veeraiah, K., & Durga Prasad, M. K. (1996). A study on the organochlorine pesticides residue input into Kolleru through Tammieleru river. Ecology Environmental and Conservation, 2, 83–86.
Xue, N., Zhang, D., & Xu, X. (2006). Organochlorinated pesticide multiresidues in surface sediments from Beijing Guanting reservoir. Water Research, 40, 183–194.
Xu, S., Dong, Y., Sun, C., Fenf, J., & Wang, L. (2000). Polychlorinated organic compounds in Yangtse river sediments. Chemosphere, 41, 1897–1903.
Yang, R., Ji, G., Zhoe, Q., Yaun, C., & Shi, J. (2005). Occurrence and distribution of organochlorine pesticides (HCH and DDT) in sediments collected from East China Sea. Environmental International, 31, 799–804.
Zhang, Z., Hong, H., Zhou, J., Yu, G., Chen, W., & Wang, X. (2002). Transport and fate of organochlorine pesticides in the River Wuchuan, Southeast China. Journal of Environmental Monitoring, 4, 435–441.
Zhang, Z., Dai, M., Hong, H., Zhou, J., & Yu, G. (2002). Dissolved insecticides and polychlorinated biphenyls in the Pearl river estuary and South China sea. Journal of Environmental Monitoring, 4, 922–928.
Zhang, Z. L., Hong, H. S., Zhou, J. L., Huang, J., & Yu, G. (2003). Fate and assessment of persistent organic pollutants in water and sediment from Minjiang river estuary, Southeast China. Chemosphere, 52, 1423–1430.
Zhou, R., Zhu, L., Yang, K., & Chen, Y. (2006). Distribution of organochlorine pesticides in surface water and sediments from Qiantang River, East China. Journal of Hazardous Materials, 137, 68–75.
Acknowledgments
The authors would like to thank the Department of Science and Technology (DST), University Grants Commission (UGC) for financial assistance. The service provided by UNU, Shimadzu Corporation, Japan for GC/MS analysis is highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumarasamy, P., Govindaraj, S., Vignesh, S. et al. Anthropogenic nexus on organochlorine pesticide pollution: a case study with Tamiraparani river basin, South India. Environ Monit Assess 184, 3861–3873 (2012). https://doi.org/10.1007/s10661-011-2229-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-011-2229-x