Abstract
Organochlorine pesticides (OCPs) pose a considerable threat to human and environmental health. Despite most OCPs have been banned, they are still reported to be used in developing countries, including Pakistan. We aimed to identify the distribution, origin, mobility, and potential risks from OCPs in three major environmental compartments, i.e., air, water, and soil, across Azad Jammu and Kashmir valley, Pakistan. The sums of OCPs ranged between 66 and 530 pg/g in soil, 5 and 13 pg/L in surface water, and 14 and 191 pg/m3 in air, respectively. The highest sum of OCPs was observed in the downstream zone of a river that was predominantly influenced by peri-urban and urban areas. The OCP isomers ratios (α-HCH/γ-HCH and o,p′-DDT/p,p′-DDT) indicate use of lindane and technical DDTs mixture as a source of HCH and DDT in the riverine environment. Similarly, the ratios of DDE and DDD/the sum of DDTs, α-endosulfan/β-endosulfan, and cis-chlordane/trans-chlordane indicate recent use of DDTs, endosulfan, and chlordane in the region. The air-water exchange fugacity ratios indicate net volatilization (fw/fa > 1) of α-endosulfan and trans-chlordane, and net deposition (fw/fa < 1) of β-endosulfan, α-HCH, γ-HCH p,p′-DDD, p,p′-DDE, and p,p′-DDT. Based on the risk quotient (RQ) method, we consider the acute ecological risks for fish associated with the levels of OCPs as negligible. However, more studies are recommended to evaluate the chronic ecological risks to other riverine-associated aquatic and terrestrial species as well as human health risks to the POPs exposure through food chain transfer in forthcoming years.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, contamination with organochlorine pesticides (OCPs) has been highlighted as a key global concern due to their bio-accumulative, toxic, persistent, and long-range transport nature within the environment (Parween et al. 2014; Ali et al. 2016). OCPs can have severe ecological and human health impacts, including oncological, reproductive, endocrine, neurological, and immunological effects (Sharma et al. 2009; Kalyoncu et al. 2009; Letcher et al. 2010). Owing to these characteristics, the production and usage of OCPs has been banned through a global initiative, i.e., Stockholm Convention on Persistent Organic Pollutants (UNEP 2001). However, they are still used in developing countries, particularly in the South Asian Region, which can be attributed to public acceptance, their effectiveness, low costs, and inappropriate regulatory frameworks (Eqani et al. 2012a; Bergkvist et al. 2012; Chakraborty et al. 2015; Baqar et al. 2018a).
The riverine environment in developing countries, particularly Pakistan, is subject to OCP contamination given that flowing water bodies integrate the pollution from the whole catchment (Jamil et al. 2015; Baqar et al. 2018a, b). Within the aquatic environment, OCPs are ultimately deposited in sediments due to their high hydrophobicity, i.e., affinity toward the organic matter of sediment particulates (Kucuksezgin and Tolga Gonul 2012; Salem et al. 2013). Such sediments can form alluvial soils and play a significant role as key global sink for these organochlorine compounds (Chakraborty et al. 2015). However, the OCPs in soil can re-volatilize into the atmosphere thereby acting as secondary source of OCPs in the environment (Cabrerizo et al. 2011).
Besides the fact that Pakistan is a party to the Stockholm Convention (2001), ongoing use of OCPs has been reported from Pakistan (Eqani et al. 2012a; Baqar et al. 2018a). Moreover, the substantial stock of the banned OCPs in obsolete pesticide stores in various parts of the country is a vital source of OCP contamination (Ahad et al. 2010). Several studies have reported OCP contamination from various riverine sections of the country (Eqani et al. 2011, 2012b; Mahmood et al. 2014; Syed et al. 2013, 2014; Sultana et al. 2014; Ali et al. 2016; Baqar et al. 2018a). However, to date, no study has focused on the occurrence of OCPs along the riverine environmental compartments from Azad Jammu and Kashmir (AJK). The AJK holds 31.5 metric tons stock of the banned obsolete OCPs (Ali et al. 2018a) that acts as a potential source of OCPs environmental contamination in the region. Furthermore, long-range atmospheric transportation (LRAT) via air masses could be another potential source of OCPs in AJK’s environment, receiving OCPs contaminants from warmer areas (Zhang et al. 2002). Recently, Ali et al. (2018a, b) have identified prevailing westerlies from Central Asia, Middle East, and Europe and air masses from agricultural farms and obsolete OCP stores from adjacent Punjab areas as principal sources of OCPs in the Lesser Himalayas from AJK. So, the riverine ecosystem of the AJK, which is host to most enriched aquatic biodiversity in the region and a lifeline for local economic growth, is vital to be evaluated for OCP contamination.
The passive sampling techniques were employed with the aim to evaluate the spatial distribution, air-water exchange, source apportionment, and ecological risks of OCPs in air, water, and soil matrices across the four major rivers, i.e., River Jhelum, River Neelum, River Poonch, and River Kunhar from AJK, Pakistan. To the best of our knowledge, the present study is the first employing passive sampling for OCPs monitoring from Pakistan, providing baseline data across the aforementioned riverine environment that can contribute substantially to future freshwater ecological studies.
Materials and methods
Study area and sampling strategy
The state of Azad Jammu and Kashmir (AJK) lies between 73°–75° E and 33°–36° N, with an area of 5134 sq mi (13,297 km2). The northern districts (Neelum, Muzaffarabad, Hattian, Bagh, Haveli, Poonch, and Sudhnoti) of the AJK encompass the Lesser Himalayan range; so are generally mountainous with moist temperate climatic conditions, while the southern districts (Kotli, Mirpur, and Bhimber) are relatively plain with dry subtropical climate. Traditional subsidence agricultural activities predominate along the alluvial plains in the region, with maize and wheat acted as foremost crops (Hafeez and Ahmed 2014). In this fragile environment, however, the ecological resources upon which people are dependent for their livelihood vary greatly. The main rivers of AJK are River Jhelum, River Neelum, River Poonch, and River Kunhar; of which, the first three are transboundary in nature, originating from Indian held Jammu and Kashmir.
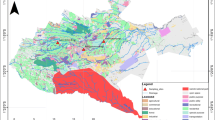
The present study covers all four riverine systems of AJK, including the River Jhelum, River Neelum, River Poonch, and River Kunhar. In total, 11 sampling sites were selected along the rivers, which were separated into 3 zones: (a) zone 1 (upstream zone) with three sampling sites, i.e., Kel, Sharda, and Authmuqam; (b) zone 2 (midstream zone) with five sampling sites, i.e., Chakothi, Hattian Bala, Sund Gran, Damashi, and Kohala; and (c) zone 3 (downstream zone) with three sampling sites, i.e., Dhan Gali, Raj Dhani, and Mangla (Fig. 1, see Table S1 for details on sampling sites). Zone 1 was defined as purely rural and agricultural area; zone 2 consists of peri-urban area, whereas zone 3 was located downstream of River Jhelum and River Poonch, which drain peri-urban and urban areas of the Mirpur and Kotli districts. Water, air, and alluvial soil samples were collected from eleven sampling sites during May–June, 2014.
Sample collection
Passive air sampling
Polyurethane foam–passive air samplers (PUF-PAS) were employed for air sampling. Each PUF-PAS consisted of polyurethane foam (specifications: thickness, 1.35 cm; diameter, 14 cm; density, 0.0213 g/cm3). The design and development of PUF-PAS followed Jaward et al. (2005). The PUF disks were pre-cleaned with dichloromethane (DCM) and acetone solution (1:1). Transportation blank PUF disks were retained sealed during the sampling trips. Field blank PUF disks were transported to the respective sampling site(s) and opened for 5 min. Subsequently, they were closed tightly by sealing the glass jar lid with paraffin (Syed et al. 2013). Each PUF-PAS was installed at the sampling site for two months, i.e., May–June, 2014. The PUFs were then recovered, sealed and stored at − 20 °C until analysis. Concentrations of studied OCP masses measured in PUF-PAS were adjusted as given in Eq. (1), based upon the monitoring period (days), final reported concentrations of OCPs (pg/PUF sampler) and previously reported sampling rates (i.e., 3.5 m3 of air per day for PUF-PAS) (Shoeib and Harner 2002; Jaward et al. 2005).
Surface soil sampling
In total, thirty-three surface soil samples were collected from the sampling sites at the depths of 0–20 cm. Each sample was collected as a composite of five sub-samples, in triplicate within 500 m radius from PUF-PAS placement points along the riverine banks using a hand trowel, followed by labeling and transportation to the laboratory, where samples were air-dried, sieved (using 2 mm sieve), and stored at − 20 °C until analysis.
Passive water sampling
Polyethylene (PE) passive samplers were used for the passive sampling of OCPs in river water from the study area. First, 2-cm wide, 60-cm long, and hundred microns thick polyethylene (PE) tubing was cut off from a roll and cleaned twice for 24 h with 250 mL n-hexane. Subsequently, the tubes were dried for 30 min in a nitrogen stream, twice welded to front and back, and stored in a container until use. Two performance reference compounds (PRCs) (deuterated TCmX (2,4,5,6-tetrachloro-m-xylene) and deuterated PCB-209 (decachlorobiphenyl)), 10 μL each, were spiked into the tubes, which are substances that based on the remaining mass after environmental exposure provide information on the field conditions and allow for consideration of the between-site variability in the conditions in the calculation of time weighted average concentrations. For this step, a polyethylene tube was put in a 1.5-L bottle containing 1 L pure water with 10 μL of the deuterated substances. The bottle was capped and shaken for 72 h in an overhead shaker at 15 rpm. The solution was then discarded and the tubes were dried with pulp, and frozen until use. For exposure in the rivers, the tubes were placed within folded gratings, which were packed with screws (Figure S2). The gratings were fixed to a steel hook in the water bodies and exposed for approximately 3 weeks. Field blanks were carried to the sites and exposed to air during the installment of the samplers to capture the background contamination. After 3 weeks, the gratings were retrieved and treated as described in Schäfer et al. (2010). Final dissolved fractions of OCPs were calculated by using the equilibrium/disequilibrium of performance reference compounds (PRCs) added to the PE-samplers. Assuming that uptake and elimination rates are equivalent, the freely dissolved (or gas-phase) concentrations of individual OCPs (C water) were then calculated by the Eq. (2).
where CPE is the OCP concentration in the PE (g/L), Rs is the sampling rate (L/day), t is the deployment period (days), mPE is the mass of the PE sheet (g), and KPE − water is the sampler-water partitioning coefficients. Sampling rates were calculated using the PRCs as given in Eq. (3):
where f is the fraction of PRC retained in the passive sampler after deployment. More details on the PRC method/sampling rate calculation of PEs have been described elsewhere (Khairy and Lohmann 2014).
Experimental section
Extraction and cleanup procedure
The soil (20 g for each sample) and PUF-PAS samples were Soxhlet-extracted for 24 h with the DCM. TCmX and PCB-209, each in 10 μL quantity, were added as surrogate standards in every sample prior to the extraction for calculating method recovery (Zhang et al. 2008). To avoid elemental sulfur interferences, activated copper granules were added in the collection flask. For water-PE samples, extraction details can be found elsewhere (Khairy and Lohmann 2014). Briefly, the PE tubes were cold-extracted twice in in ethyl acetate each for 24 h after spiking with 10 μL of surrogate standards (TCmX and PCB-209). The extracts were then concentrated through rotary evaporation, and solvent phase was exchanged to n-hexane, followed by extract cleanup through alumina-silica columns (an 8-mm glass column), packed from the bottom to top, with 3 cm neutral alumina (3% deactivated), 3 cm neutral silica gel (3% deactivated), 3 cm of 50% sulfuric acid-silica (3 cm), and 1 cm anhydrous sodium sulfate. Prior to use, the columns were eluted with 50 mL of DCM: n-hexane (1:1) solution (Li et al. 2007). Purified fraction of extracts was further subjected to a gentle ultrapure nitrogen stream to concentrate samples up to 0.2 mL, followed by the addition of dodecane (25 μL) as a solvent keeper. Later, 10 μL of internal standard (PCB-54) was added, prior to GC-MS analysis.
Chromatographic analysis
Nineteen OCP isomers, including hexachlorobenzene (HCB), alpha-hexachlorocyclohexane (α-HCH), beta-hexachlorocyclohexane (β-HCH), gamma-hexachlorocyclohexane (γ-HCH), delta-hexachlorocyclohexane (δ-HCH), epsilonhexachlorocyclohexane (ε-HCH), heptachlor, α-endosulfan, β-endosulfan, oxy-chlordane (OC), trans-chlordane (TC), cis-chlordane(CC), o,p′-dichlorodiphenyldichloroethane (o,p′-DDD), p,p′-dichlorodiphenyl dichloroethane (p,p′-DDD), o,p′-dichlorodiphenyldichloroethylene (o,p′-DDE), p,p′-dichlorodiphenyldichloroethylene (p,p′-DDE), o,p′-dichlorodiphenyltrichloroethane (o,p′-DDT), p,p′-dichlorodiphenyltrichloroethane (p,p′-DDT), and mirex, were detected at the Chinese Academy of Sciences, China, using Agilent 7000A Triple Quadrupole GC/MS, attached with gas chromatograph (Agilent 7890A) and auto-sampler (Agilent 7693). A CP-Sil 8 CB (Varian 50 m × 0.25 mm; film thickness, 0.25 μm) capillary column was used to detect OCP isomers. The injector working temperature was set at 250 °C. Initially, temperature of the oven was 150 °C for 3 min that later increased to 290 °C, at the rate of 4 °C/min, and was held for 10 min. The OCP isomers were then determined with three fragment ions in SIM (selective ion mode) with EIS (electron impact spectrometry). The MSD source temperature was 230 °C and quadrupole temperature was 150 °C.
Quality control and quality assurance
Quality control and assurance procedures were strictly followed for the entire analyses. Calibration standards were used daily for instrumental standardization and for every batch, mixtures of known concentrations of internal standards were injected into GC before the sample injection. HPLC grade chemicals were used during the experimentation, purchased from MERCK, Germany. Glassware was double-washed with deionized distilled water, followed by baking at 450 °C for 6 h. Agilent MSD Productivity Chemstation software was used for data acquisition and processing. Method detection limits (MDLs) were estimated as thrice the standard deviation of blank readings, and instrumental detection limits (IDLs) were assimilated when the signal-to-noise ratio was equal to 3. MDLs ranged from 0.02 to 0.1 pg/m3 for air, and 0.03 to 0.1 pg/g for water and soil samples, while the IDL range was determined as 0.01–0.09 ng per sample. Average surrogate recoveries in all samples ranged from 53 to 63% for TCmX (55–64%, 53–65%, and 61–68% in soil, air, and water samples, respectively), and from 77 to 81% for PCB-209 (77–79% in soil, 78–79% in air, and 78–81% in water samples). Calibration standards of 5, 10, 20, 50, 100, and 200 μg/L were used to quantify the calibration curves. All standards were purchased from Dr. Ehrenstorfer GmbH, Germany.
OCP isomer ratios assessment
In the past, the OCP isomer ratios have been acknowledged as a key tool in the environmental source apportionment of OCPs (Syed et al. 2014; Baqar et al. 2018a, b). The α-HCH/γ-HCH ratio was employed for the source identification of HCHs: distinction between lindane or technical HCHs mixture usage in the study area. The α-HCH/γ-HCH value less than 3 indicates the lindane formulation as a source of HCHs, while the α-HCH/γ-HCH ratio ranging between 3 and 7 is an indicator of technical HCHs (Sun et al. 2010). For DDTs, the o,p′-DDT/p,p′-DDT ratio was used to assess the source of DDT contamination, i.e., either technical DDTs mixture or dicofol. Typically, ratio values of 1.3 to 9.3 indicate dicofol, whereas ratios < 0.3 indicate technical mixture of DDT as source of environmental contamination (Da et al. 2013). Moreover, the ratio of DDT isomers and metabolites is considered to be vital to ascertain the potential ongoing DDT usage in a region. So, the (DDE + DDD)/ΣDDTs ratio was assessed for the study as ratio value less than 0.5 specifies the fresh input of DDTs (Li et al. 2012). Similarly, the DDE/DDD ratio was that relates to the aerobic or anaerobic decomposition of DDT in the region. A ratio value greater than 1 indicates degradation under aerobic conditions via oxidation-dehydrochlorination, and less than 1 indicates dominance of reductive-dechlorination under anaerobic conditions (Baqar et al. 2018a; Ma et al. 2016; Da et al. 2013). Whereas, the ratios of cis-chlordane/trans-chlordane and α-endosulfan/β-endosulfan are indicators for the usage history of technical mixtures of chlordane and endosulfan in an area, respectively. The α-endosulfan/β-endosulfan value > 1 and cis-chlordane/trans-chlordane < 1 specifies the newer or ongoing environmental addition of their technical mixtures in the region (Yu et al. 2014).
Air-water exchange
Air-water exchange is a two-way process—where the contaminants can be added to or from the atmosphere through volatilization and deposition, respectively. In this respect, the atmosphere serves as a potential source or sink of the OCPs to riverine environment at the air-water interface (Sabin et al. 2011). Therefore, the water-to-air fugacity ratio (fw/fa) method was employed to assess the air-water exchange of OCPs (Jantunen and Bidleman 2003, 2006; Dickhut et al. 2005; Cincinelli et al. 2009; Mai et al. 2016). The water-air fugacity values were computed as given in Eq. (4):
where, fw and fa are the fugacities in water and air, respectively (expressed in “Pa”). Cw and Ca are the concentrations of contaminants in water and air (both expressed in pg/m3); R is the ideal gas constant (8.314 Pa m3 mol-1 K-1); T is the air temperature (in K); and H is the Henry’s law constant (Pa m3 mol-1) at recorded surface water temperature at each site. The “H values” for α-HCH, γ-HCH, p,p′-DDT, p,p′-DDD, p,p′-DDE, α-endosulfan, β-endosulfan, cis-chlordane, and trans-chlordane at respective temperature were acquired from Cetin et al. (2006), provided as Table S2. According to literature, the water-air fugacity ratio fw/fa < 1 indicates net deposition from air to water; fw/fa > 1 expresses net volatilization from water to air; whereas = 1 represents equilibrium between both phases (Jantunen and Bidleman 2003; Cincinelli et al. 2009).
Ecological risk assessment
The ecological risk in the study area was assessed using the risk quotient (RQ) method (for examples of applications, see Palma et al. 2014; Xiong et al. 2015; Tang et al. 2018; Baqar et al., 2018a, b). The RQ benchmarks the concentration of contaminants in environment by their toxicity (Kouzayha et al. 2013) and was calculated using Eq. (5). We calculated the RQi for both mean and maximum environmental concentrations of a particular OCP, i.e., RQ meani and RQ maxi, respectively. The ecological risk was then computed by adding all the RQi values using the Eq. (6)
where, the MEC is the measured environmental concentration of an OCP isomer, and PNEC is the predicted no-effect concentration; which is computed as a ratio of a toxicologically relevant concentration (LC50 or EC50) to the assessment factor (f), i.e., f = 1000 for fish (Eq. (7)). The values for EC50 or LC50 to Labeo rohita for various OCPs were acquired from the PAN Pesticide Database (www.pesticideinfo.org) (Palma et al. 2014; Tang et al. 2018).
Moreover, the OCP concentrations in water were also compared with the CMC (criterion maximum concentration) and CCC (criterion continuous concentration) established in the National Recommended Aquatic Life Criteria Table by the United States Environmental Protection Agency (USEPA 2004). Likewise, the maximum allowable concentration (MAC) and annual average (AA) values established through Directive 2013/39/EU of the European Parliament and of the Council were also taken into consideration to assess the risk to the ecological integrities (European Council 2013).
Statistical analysis
The descriptive statistical analysis and univariate Pearson’s correlation matrix were assessed by using OriginPro 2018. The ArcGIS 10.5 (Esri) was employed to plot geographical graphs/maps.
Results and discussion
OCP profile analysis and comparative analysis
The descriptive statistics of OCPs in water, air, and soil compartments of the four major rivers across AJK are summarized in Table S3–S5. All analyzed OCP isomers/metabolites were detected in the environmental matrices from the region. The mean ƩOCP concentration ranged from 5 to 13 pg/L (mean ± SD, 9.3 ± 2.9 pg/L) in water, 14 to 191 pg/m3 (101.9 ± 65.1 pg/m3) in air, and 66 to 593 pg/g (231.3 ± 209.5 pg/g) in soil samples. The OCP concentrations were ordered as ƩDDTs > HCB > ƩChlordane > ƩEndosulfan > Heptachlor > ƩHCHs > Mirex; ƩDDTs > ƩChlordane > HCB > ƩEndosulfan > ƩHCHs > Mirex> Heptachlor; and ƩDDTs > ƩHCHs > ƩChlordane > HCB > ƩEndosulfan > Heptachlor in the water, soil, and air matrices, respectively.
The dominance of ƩDDTs over ƩHCHs and other OCPs may be attributed to their relatively lower vapor pressure and degradability than HCHs (Syed and Malik 2011; Ulusoy et al. 2016). Moreover, DDT is still used in the region owing to its widespread public acceptability and inappropriate regulation (Eqani et al. 2011; Baqar et al. 2018a). Previous studies from the region also reported the predominance of DDTs over other OCPs (Baqar et al. 2018a, b; Syed and Malik 2011; Sultana et al. 2014; Chakraborty et al. 2016; Ali et al. 2018a, b). Similarly, the higher environmental evaporation and hydrolysis rate of heptachlor is proportionate to its relative low levels observed from the study area (Milun et al. 2016). Univariate Pearson’s correlation coefficient test was applied among OCPs classes (Table S6), where a strong positive correlation was observed in atmospheric samples for HCBs with heptachlors (r = 0.97), ƩEndosulfan (r = 0.69), ƩChlordane (r = 0.74), and mirex (r = 0.78); for heptachlor with ƩEndosulfan (r = 0.71), ƩChlordane (r = 0.62), and mirex (r = 0.69); and for ƩChlordane with mirex (r = 0.90). Similarly, in water and soil samples, strong positive correlation was determined for heptachlor with ƩHCHs (r = 0.66) and mirex (r = 0.72), respectively. Thus, the strong positive correlation among OCPs in various environmental matrices indicates common sources of contamination for these OCP compound in the study area.
The OCP concentrations in soil samples of the River Jhelum, River Neelum, River Poonch, and River Kunhar were lower or slightly comparable to those from other high-altitude areas, e.g., those reported from Himalayan studies from Nepal and India, Mountains of Tajikistan, and Ruoergai High Plateau, China. For instance, the ΣDDTs concentrations were comparable to those from Southern Himalayas, Nepal (67.3–1551.6 pg/g) (Gong et al. 2014), and the HCB concentration to levels from Southern Himalayas, Nepal (8.9–18.7 pg/g) and Eastern Himalayas, India (ND-270 pg/g) (Gong et al. 2014; Devi et al. 2015). Whereas, the ΣOCPs levels were found to be much lower than those from Ruoergai High Plateau, China (970–17,600 pg/g) (Gai et al. 2014), mountainous regions of Tajikistan (52830–247,980 pg/g) (Zhao et al. 2013), and Eastern Himalayas, India (280–2,144,000 pg/g) (Devi et al. 2015). However, the comparison in context to OCPs’ atmospheric and water concentrations from this study revealed much lower contamination levels in riverine environment than those reported in consulted studies from other developing countries (Table 1).
The nationwide comparison of OCP levels in soil revealed that the OCP levels along the River Jhelum, River Neelum, River Poonch, and River Kunhar were orders of magnitude lower than those reported from all other regions of Pakistan (Table 2). Similarly, the OCP levels in surface water were considerably lower than those reported from other rivers in the country (Table 2). However, the atmospheric OCP levels in the present study (14–192 pg/m3) are comparable to those reported from other regions such as Chashma Barrage (150.4 pg/m3), Taunsa Barrage (159.0 pg/m3), Rahim Yar Khan (123.0 pg/m3), and Lesser Himalayan Region, AJK (Sultana et al. 2014; Ali et al. 2018a). The considerably lower levels of OCPs determined in water and soil from the study area than all previous studies from Pakistan may be explained by the fact that those studies were conducted in low-lying, plain, and intensively cultivated areas of Pakistan, where broad usage of banned OCPs has been reported for the agriculture sector (Syed and Malik 2011; Eqani et al. 2012a; Zehra et al. 2015; Baqar et al. 2018a).
OCP composition and source apportionment
The compositional profiles of the DDTs, HCHs, endosulfan, and chlordane in water, soil, and air are provided in Table 3. Whereas, the OCP source apportionments calculated through OCPs isomer ratios are provided in Table 4.
HCHs
Among the HCHs, γ-HCH was the most dominant isomer in water (30%) and air (84.8%); while in soil matrix β-HCH (43.9%) was the most predominant HCH isomer. The dominance of β-HCH in soil matrix was associated with its lower vapor pressure, water solubility, and resistance to microbial decomposition (Xiao et al. 2004; Salvadó et al. 2013). The current findings are consistent with those previously reported from Neelum-Jhelum Riverine Catchment System, AJK (Ali et al. 2018a). The α-HCH is the principal constituent of technical HCHs mixture (60–70%) and γ-HCH is of lindane (> 99%) (Barakat et al. 2013; Yu et al. 2014). The mean calculated ratio of α-HCH/ γ-HCH was less than 3 in all environmental matrices (Table 4), indicating usage of lindane in the region as a source of HCHs (Sultana et al. 2014). Consistently, lindane use was reported from AJK (Ali et al. 2018a, b) and adjacent areas from Pakistan (Baqar et al. 2018a, b; Syed et al. 2014; Mahmood et al. 2014a). The lindane has wide-scale agricultural application in the region that primarily attributed to the application of lindane after the ban of technical HCHs mixture (Vijgen et al. 2006) and limited use in medicinal purpose as scabicide (Daud et al. 2010).
DDTs
The p,p′-DDT dominated the DDT isomers in all environmental matrices; water, 45.5%; air, 35.9%; and soil, 47.2% contribution to the total DDTs. The composition of DDTs was comparable to the adjacent study area (Ali et al. 2018a). Among the DDT metabolites, the dominance of o,p′-DDE was observed in water and air with 46.2% and 59.5% of the ƩDDTs contribution, respectively (Table 3).
The technical mixture of DDTs is typically composed of 75% p,p′-DDT, 15% o,p′-DDT, and 10% of DDT metabolites (Yu et al. 2014). Dicofol was commonly used in the region after the ban of DDT’s technical mixture and contains a relative higher content of o,p′-DDT (Mahmood et al. 2014a). The calculated o,p′-DDT/p,p′-DDT ratio ranged from 0.01 to 0.1 in the study area (Table 4), suggesting the technical mixture of DDTs as a source of DDT contamination throughout the study area. Whereas, the (DDE + DDD)/ΣDDTs ratio in the present study ranged from 0.2–0.8 (Table 4), representing both historical and fresh input of DDTs in environment. Similarly, the DDE/DDD ratio values in the present study ranged from 2.9 to 48.4, indicating the anaerobic degradation (DDE/DDD > 1) of parent DDTs compounds.
Endosulfan
The technical mixture of endosulfan contains α-endosulfan and β-endosulfan in a relation of 70% to 30%, respectively (Sultana et al. 2014). After endosulfan application in the environment, α-endosulfan degrades more rapidly than β-endosulfan (Jiang et al. 2009). The mean α-endosulfan/β-endosulfan ratio in the present study was > 1 in all environmental matrices; except in the soil (Table 4). This suggests the ongoing use of endosulfan in the AJK region. The widespread use of endosulfan in cotton cultivation, the major economic crop of Pakistan was recently identified by Ahmad et al. (2018).
Chlordane
The technical chlordane mixture is typically composed of 8–13% of cis-chlordane and 8–15% of trans-chlordane, respectively (Ali et al. 2018b). Trans-chlordane concentrations decrease rapidly in the environment through volatilization (Martinez et al. 2012; Sultana et al. 2014). The cis-chlordane/trans-chlordane ratio in the current study was lower than 1 in all environmental compartments, indicating current addition of chlordane in the environment (Table 4). Although chlordane has never been registered in Pakistan, but its contamination is reported in the region that might attributed to its popular use as insecticides in the field agriculture and horticulture (Rashid 2011; Baqar et al. 2018a).
Spatial distribution pattern of OCPs
The ∑OCP contamination concentrations varied among the zones, sites, and compartments. The concentrations in air samples were more similar across sites and zones than soil and water samples. One of the soil and two of the water sampling sites exhibited the highest concentrations in zone 3; nevertheless, the other sites in this zone were below average or among the lowest, indicating a large among-site variability (given the low sample size, no formal statistical procedure was applied). The fact that the highest concentrations occurred in sites in zone 3 may be explained by agricultural runoff from upstream zones and wastewater from the adjacent urban areas (Figures S1).
The OCP class distribution patterns in water, air, and soil compartments showed that DDTs were the dominant OCPs in all three zones (Fig. 2), with variability in the contribution to the total concentrations among zones and compartments. The widespread contamination with DDTs in the study area is presumably due to its history of perceived efficiency in insect control in the region, leading to public acceptability and possible ongoing use as previously reported from the adjacent study areas (Eqani et al. 2012a; Mahmood et al. 2014a; Baqar et al. 2018a).
The prevailing westerlies from Central Asia, Middle East, and Europe are considered to be a vital source of OCP atmospheric and soil contamination in the AJK (Ali et al. 2018a, b). By contrast, the OCP contamination in water samples of the study area may be attributed to the release of pesticides from the glacier deposits. Similar findings were observed from adjacent countries, i.e., India (Indian Himalayas) and China (Tibetan Plateau), reporting Himalayan and Changwengluozha glacier melts as a key POP source in the Ganges floodplains and Tibetan Plateau, respectively (Sharma et al. 2015; Li et al. 2017). The riverine resources of the AJK receive a significant quantity of water from glacier melt within the catchment area. The glaciated area of the AJK stretches over 109 km2 that drains to Jhelum River and its tributaries, predominantly River Neelum, River Kunhar, and River Poonch (Ashraf et al. 2012). Considering that climate change may increase glacier melt in the future, it may become a more significant source of OCPs (Sohail et al. 2018).
Air-water exchange
The calculated water-to-air fugacity (fw/fa) ratio values revealed that α-endosulfan and trans-chlordane in the riverine environment of AJK undergo net volatilization. The fw/fa ratios for α-endosulfan and trans-chlordane ranged from 0.16 to 9 (mean, 2.6) and from 0.17 to 3.35 (mean, 1.25), respectively. In contrast, the fw/fa ratios of β-endosulfan (mean, 0.05), p,p′-DDT (mean, 0.02), p,p′-DDD (mean, 0.03), p,p′-DDE (mean, 0.23), α-HCH (mean, 0.01), and γ-HCH (mean, 0.03) indicated their net depositional air-water exchange as fw/fa value was < 1. However, cis-chlordane exhibited a close-to-equilibrium fugacity ratio (mean, 0.93). The volatilization flux of trans-chlordane in the present study was consistent with findings from Taihu Lake, China; Lake Ontario, Canada; and Great Lakes Basin, Canada and USA (Jantunen and Bidleman 2006; Qiu et al. 2008; Khairy et al. 2014). Similarly, the α-HCH, γ-HCH, and β-endosulfan also reported depositional flux of Izmir Bay, Turkey (Odabasi et al. 2008), Great Lakes Basin (Khairy et al. 2014), Western Antarctic Peninsula (Dickhut et al. 2005), and Ross Sea, Italy (Cincinelli et al. 2009).
Ecological risk assessment
The mean risk quotients (RQ meani) and maximum risk quotients (RQ maxi) are summarized in Table S7. Among the OCPs, α-endosulfan and Σendosulfan exerted highest levels of acute risk (based upon both RQ meani and RQ maxi) to fish, followed by heptachlor and HCB. The highest risk was posed by α-endosulfan (LC50, 1 μg/L for Labeo rohita) and Σendosulfan (LC50, 1.25 μg/L for Labeo rohita) which may be attributed to their relative lower toxicity values. The Labeo rohita was selected as a target fish species due to its common availability in the study area and wide usage in human food chain. The mean risk quotient (RQmean) and maximum risk quotient (RQmax) in water were evaluated to be 9.5E−04 and 2.2E−03, respectively (Table S7). Given that all risk values were below 0.1, the ecological risks in the rivers can be considered negligible (Palma et al. 2014)
The comparison of results with the CMC and CCC values established by USEPA revealed that all OCPs concentrations in the study area were far below limits (Table 5). Similarly, none of the OCP isomers exceeded the established MAC and AA values by the European Council (Table 5) that also suggested negligible ecological risk associated with the levels of OCPs in the riverine water system of the AJK.
Conclusion
The current study provides detailed baseline data of OCPs in the riverine environment of the AJK, Pakistan. Results revealed the DDT level dominance over other studied OCPs, which were comparable to other higher altitude studies from the Asian region. The OCP contamination levels were lower than those previously reported from studies conducted in extensively cultivated areas of Indus Basin. Notwithstanding, relatively high contamination was found for the peri-urban and urban environment in the AJK. The recent addition of banned technical DDTs, endosulfan, and chlordane (as indicated by isomer and/or metabolites ratios) along with the net deposition of most of the studied OCPs compounds in the study area is alarming, indicating potential serious human health and ecological health concerns through OCPs’ food chain transfer in the forthcoming years. Nevertheless, the ecological risk assessment indicated negligible acute ecological risks from OCPs to the riverine fish in the AJK region. Still, more studies, involving biomarkers are recommended to evaluate the human health risk, and chronic ecological risks to other riverine associated aquatic and terrestrial species through POPs exposure.
References
Ahad K, Mohammad A, Khan H, Ahmad I, Hayat Y (2010) Monitoring results for organochlorine pesticides in soil and water from selected obsolete pesticide stores in Pakistan. Environ Monit Assess 166:191–199
Ahmad A, Shahid M, Khalid S, Zaffar H, Naqvi T, Pervez A, Bilal M, Ali MA, Abbas G, Nasim W (2018) Residues of endosulfan in cotton growing area of Vehari, Pakistan: an assessment of knowledge and awareness of pesticide use and health risks. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-018-3169-6
Alamdar A, Syed JH, Malik RN, Katsoyiannis A, Liud J, Li J, Zhang G, Jones KC (2014) Organochlorine pesticides in surface soils from obsolete pesticide dumping ground in Hyderabad City, Pakistan: contamination levels and their potential for air–soil exchange. Sci Total Environ 470–471:733–741
Ali U, Bajwa A, Chaudhry MJ, Mahmood A, Syed JH, Li J, Zhang G, Jones KC, Malik RN (2016) Significance of black carbon in the sediment–water partitioning of organochlorine pesticides (OCPs) in the Indus River, Pakistan. Ecotoxicology and Environmental Safety 126:177–185
Ali U, Sweetman AJ, Jones KC, Malik RN (2018a) Higher atmospheric levels and contribution of black carbon in soil-air partitioning of organochlorines in Lesser Himalaya. Chemosphere 191:787–798
Ali U, Riaz R, Sweetman AJ, Jones KC, Li J, Zhang C, Malik RN (2018b) Role of black carbon in soil distribution of organochlorines in Lesser Himalayan Region of Pakistan. Environ Pollut 236:971–982
Ashraf A, Naz R, Roohi R (2012) Monitoring and estimation of glacial resource of Azad Jammu and Kashmir using remote sensing and GIS techniques. Pakistan Journal of Meteorology 8:31–41
Baqar M, Sadef Y, Ahmad SR, Mahmood A, Li J, Zhang G (2018a) Organochlorine pesticides across the tributaries of River Ravi, Pakistan: human health risk assessment through dermal exposure, ecological risks, source fingerprints and spatio-temporal distribution. Sci Total Environ 618:291–305
Baqar M, Sadef Y, Mahmood A, Ahmad SR, Li J, Zhang G (2018b) Organochlorine contaminants in freshwater mussels; occurrence, bioaccumulation pattern, spatio-temporal distribution and human health risk assessment from the tributaries of River Ravi, Pakistan. Human and Ecological Risk Assessment: An International Journal 24:1268–1290
Barakat AO, Mohammed Khairy M, Aukaily I (2013) Persistent organochlorine pesticide and PCB residues in surface sediments of Lake Qarun, a protected area of Egypt. Chemosphere 90:2467–2476
Behfar A, Nazari Z, Rabiee MH, Raeesi G, Oveisi MR, Sadeghi N, Jannat B (2013) The organochlorine pesticides residue levels in Karun River water. Jundishapur Journal of Natural Pharmaceutical Products 8:41–46
Bergkvist C, Aune M, Nilsson I, Sandanger TM, Hamadani JD, Tofail F, Oyvind-Odland J, Kabir I, Vahter M (2012) Occurrence and levels of organochlorine compounds in human breast milk in Bangladesh. Chemosphere 88:784–790
Cabrerizo A, Dachs J, Jones K, Barceló D (2011) Soil-air exchange controls on background atmospheric concentrations of organochlorine pesticides. Atmos Chem Phys 11:12,799–12,811
Cetin B, Ozer S, Sofuoglu A, Odabasi M (2006) Determination of Henry’s law constants of organochlorine pesticides in deionized and saline water as a function of temperature. Atmos Environ 40:4538–4546
Chakraborty P, Zhang G, Li J, Sivakumar A, Jones KC (2015) Occurrence and sources of selected organochlorine pesticides in the soil of seven major Indian cities: assessment of air-soil exchange. Environ Pollut 204:74–80
Chakraborty P, Khuman SN, Selvaraj S, Sampath S, Devi NL, Bang JJ, Katsoyiannis A (2016) Polychlorinated biphenyls and organochlorine pesticides in River Brahmaputra from the outer Himalayan Range and River Hooghly emptying into the Bay of Bengal: occurrence, sources and ecotoxicological risk assessment. Environ Pollut 219:998–1006
Cincinelli A, Martellini T, Del Bubba M, Lepri L, Corsolini S, Borghesi N, King MD, Dickhut RM (2009) Organochlorine pesticide air-water exchange and bioconcentration in krill in the Ross Sea. Environ Pollut 157:2153–2158
Da C, Liu G, Tang Q, Liu J (2013) Distribution, sources, and ecological risks of organochlorine pesticides in surface sediments from the Yellow River Estuary, China. Environmental Science: Processes & Impacts 15:2288–2296
Daud Y, Daud-ur-Rehman, Farooq U (2010) Lindane toxicity in a 7 year old boy. Journal of Ayub Medical College Abbottabad 22(4):223
Devi NL, Yadav IC, Raha P, Shihua Q, Dan Y (2015) Spatial distribution, source apportionment and ecological risk assessment of residual organochlorine pesticides (OCPs) in the Himalayas. Environ Sci Pollut Res 22:20154–20166
Dickhut RM, Cincinelli A, Cochran M, Ducklow HW (2005) Atmospheric concentrations and air-water flux of organochlorine pesticides along the Western Antarctic Peninsula. Environ Sci Technol 39:465–470
Eqani SAMAS, Malik RN, Mohammad A (2011) The level and distribution of selected organochlorine pesticides in sediments from River Chenab, Pakistan. Environ Geochem Health 33:33–47
Eqani SAMAS, Malik RN, Alamdar A, Faheem H (2012a) Status of organochlorine contaminants in the different environmental compartments of Pakistan: a review on occurrence and levels. Bull Environ Contam Toxicol 88:303–310
Eqani SAMAS, Malik RN, Katsoyiannis A, Zhang G, Chakraborty P, Mohammad A, Jones KC (2012b) Distribution and risk assessment of organochlorine contaminants in surface water from River Chenab, Pakistan. J Environ Monit 14:1645–1654
European Council (2013) Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off J Eur Union L 226:1–17
Gai N, Pan J, Tang H, Chen S, Chen D, Zhu X, Lu G, Yang Y (2014) Organochlorine pesticides and polychlorinated biphenyls in surface soils from Ruoergai high altitude prairie, east edge of Qinghai-Tibet Plateau. Sci Total Environ 478:90–97
Gong P, Wang XP, Li SH, Yu WS, Li JL, Kattel DB, Wang WC, Devkota LP, Yao TD, Joswiak DR (2014) Atmospheric transport and accumulation of organochlorine compounds on the southern slopes of the Himalayas, Nepal. Environ Pollut 192:44–51
Hafeez, A. & Ahmed, K.K. (2014). Azad Jammu and Kashmir Agriculture Policy: a ten years perspective. Directorate General of Agriculture, Azad Government of the State of Jammu and Kashmir, Muzaffarabad.
He C, Jin J, Li G, Wang Y (2016) Exchange of organohalogen compounds between air and tree bark in the Yellow River region. Chemosphere 153:478–484
Hellar-Kihampa, H., De Wael, K., Lugwisha, E., Malarvannan, G., Covaci, A., & Van Grieken, R. (2013). Spatial monitoring of organohalogen compounds in surface water and sediments of a rural–urban river basin in Tanzania. Sci Total Environ,. 447, 186–197.
Iram S, Ahmad I, Ahad K, Muhammad A, Anjum S (2009) Analysis of pesticide residues of Rawal and Simly lakes. Pak J Bot 41:1981–1987
Isogai N, Hogarh JN, Seike N, Kobara Y, Oyediran F, Wirmvem MJ, Ayonghe SN, Fobil J, Masunaga S (2016) Atmospheric monitoring of organochlorine pesticides across some West African countries. Environ Sci Pollut Res https://doi.org/10.1007/s11356-016-7284-y
Jamil N, Baqar M, Shaikh IA, Javaid I, Shahid A, Khalid R, Ahsan N, Qadir A, Arslan M (2015) Assessment of mercury contamination in water and soil surrounding a chlor-alkali plant: a case study. J Chem Soc Pak 37:173–178
Jantunen LM, Bidleman TF (2003) Air-water gas exchange of toxaphene in Lake Superior. Environ Toxicol Chem 22:1229–1237
Jantunen LM, Bidleman TF (2006) Henry’s law constants for hexachlorobenzene, p,p′-DDE and components of technical chlordane and estimates of gas exchange for Lake Ontario. Chemosphere 62:1689–1696
Jaward TM, Zhang G, Nam JJ, Sweetman AJ, Obbard JP, Kobara Y, Jones KC (2005) Passive air sampling of polychlorinated biphenyls, organochlorine compounds, and polybrominated diphenyl ethers across Asia. Environ Sci Technol 39:8638–8645
Jiang YF, Wang XT, Jia Y, Wang F, Wu MH, Sheng GY, Fu JM (2009) Occurrence, distribution and possible sources of organochlorine pesticides in agricultural soil of Shanghai, China. J Hazard Mater 170:989–997
Kafilzadeh F (2015) Assessment of organochlorine pesticide residues in water, sediments and fish from Lake Tashk, Iran. Achievements in the Life Sciences 9:107–111
Khairy MA, Lohmann, R (2014) Field calibration of low density polyethylene passive samplers for gaseous POPs. Environmental Science: Processes & Impacts 16:414-421
Kalyoncu L, Agca I, Aktumsek A (2009) Some organochlorine pesticide residues in fish species in Konya, Turkey. Chemosphere 74:885–889
Khairy M, Muir D, Teixeira C, Lohmann R (2014) Spatial trends, sources, and air-water exchange of organochlorine pesticides in the Great Lakes basin using low density polyethylene passive samplers. Environ Sci Technol 48:9315–9324
Kouzayha A, Al Ashi A, Al Akoum R, Al Iskandarani M, Budzinski H, Jaber F (2013) Occurrence of pesticide residues in Lebanon's water resources. Bull Environ Contam Toxicol 91:503–509
Kucuksezgin F, Tolga Gonul L (2012) Distribution and ecological risk of organochlorine pesticides and polychlorinated biphenyls in surficial sediments from the Eastern Aegean. Mar Pollut Bull 64:2549–2555
Kumarasamy P, Govindaraj S, Vignesh S, Babu Rajendran R, James AR (2012) Anthropogenic nexus on organochlorine pesticide pollution: a case study with Tamiraparani river basin, South India. Environ Monit Assess 184:3861–3873
Letcher RJ, Bustnes JO, Dietz R, Jenssen BM, Jørgensen EH, Sonne C, Verreault J, Vijayan MM, Gabrielsen GW (2010) Exposure and effects assessments of persistent organohalogen contaminants in arctic wildlife and fish. Sci Total Environ 408:2995–3040
Li J, Zhang G, Guo L, Xu W, Li X, Lee CS, Ding A, Wang T (2007) Organochlorine pesticides in the atmosphere of Guangzhou and Hong Kong: regional sources and long-range atmospheric transport. Atmos Environ 41:3889–3903
Li W, Yang H, Gao Q, Pan H (2012) Residues of organochlorine pesticides in water and suspended particulate matter from Xiangshan Bay, East China Sea. Bull Environ Contam Toxicol 89:811–815
Li J, Yuan GL, Wu MZ, Sun Y, Han P, Wang GH (2017) Evidence for persistent organic pollutants released from melting glacier in the central Tibetan Plateau, China. Environ Pollut 220(Pt A):178–185
Lu H, Liu W (2016) Distribution characteristics of organochlorine pesticides in soil, water, and sediment from the Bahe River, China. Environ Forensic 17:80–86
Ma J, Pan LB, Yang XY, Liu XL, Tao SY, Zhao L, Qin XP, Sun ZJ, Hou H, Zhou YZ (2016) DDT, DDD, and DDE in soil of Xiangfen County, China: residues, sources, spatial distribution, and health risks. Chemosphere 163:578–583
Mahmood A, Malik RN, Li J, Zhang G (2014a) Levels, distribution pattern and ecological risk assessment of organochlorines pesticides (OCPs) in water and sediments from two tributaries of the Chenab River. Pakistan Ecotoxicology 23:1713–1721
Mahmood A, Malik RN, Li J, Zhang G (2014b) Human health risk assessment and dietary intake of organochlorine pesticides through air, soil and food crops (wheat and rice) along two tributaries of river Chenab, Pakistan. Food and Chemical Toxicology 71:17–25
Mai C, Theobald N, Hühnerfuss H, Lammel G (2016) Persistent organochlorine pesticides and polychlorinated biphenyls in air of the North Sea region and air-sea exchange. Environ Sci Pollut Res 23:23648–23661
Martinez A, Erdman NR, Rodenburg ZL, Eastling PM, Hornbuckle KC (2012) Spatial distribution of chlordanes and PCB congeners in soil in Cedar Rapids, Iowa, USA. Environ Pollut 161:222–228
Milun V, Grgas D, Dragičević TL (2016) Assessment of PCB and chlorinated pesticide accumulation in mussels at Kaštela Bay (Eastern Adriatic). Sci Total Environ 562:115–127
Odabasi M, Cetin B, Demircioglu E, Sofuoglu A (2008) Air–water exchange of polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) at a coastal site in Izmir Bay, Turkey. Mar Chem 109:115–129
Palma P, Köck-Schulmeyer M, Alvarenga P, Ledo L, Barbosa IR, López de Alda M, Barceló D (2014) Risk assessment of pesticides detected in surface water of the Alqueva reservoir (Guadiana basin, southern of Portugal). Sci Total Environ 488–489:208–219
Parween M, Ramanathan AL, Khillare PS, Raju NJ (2014) Persistence, variance and toxic levels of organochlorine pesticides in fluvial sediments and the role of black carbon in their retention. Environ Sci Pollut Res 21:6525–6546
Qiu X, Zhu T, Wang F, Hu J (2008) Air–water gas exchange of organochlorine pesticides in Taihu Lake, China. Environ Sci Technol 42:1928–1932
Rashid, A. (2011). Investigations on organochlorine pesticide residues in soils from cotton growing areas of Pakistan. Department of Plant Sciences, Quaid–i–Azam University Islamabad–Pakistan (Ph.D. Dissertation).
Sabin LD, Maruya K, Lao W, Diehl D, Tsukada D, Stolzenbach KD, Schiff KC (2011) A pilot study of air-water exchange of organochlorine compounds at three coastal estuaries in southern California, SCCWRP Annual Report 2011. University of California, Los Angeles
Salem DMA, Khaled A, El Nemr A (2013) Assessment of pesticides and polychlorinated biphenyls (PCBs) in sediments of the Egyptian Mediterranean coast. Egypt J Aquat Res 39:141–152
Salvadó JA, Grimalt JO, López JF, de Madron XD, Pasqual C, Canals M (2013) Distribution of organochlorine compounds in superficial sediments from the Gulf of Lion, northwestern Mediterranean Sea. Prog Oceanogr 118:235–248
Schäfer RB, Hearn L, Kefford BJ, Mueller JF, Nugegoda D (2010) Using silicone passive samplers to detect polycyclic aromatic hydrocarbons from wildfires in streams and potential acute effects for invertebrate communities. Water Res 44:4590–4600
Sharma CM, Rosseland BO, Almvik M, Eklo OM (2009) Bioaccumulation of organochlorine pollutants in the fish community in Lake Arungen, Norway. Environ Pollut 157:2452–2458
Sharma BM, Nizzetto L, Bharat GK, Tayal S, Melymuk L, Sáňka O, Přibylová P, Audy O, Larssen T (2015) Melting Himalayan glaciers contaminated by legacy atmospheric depositions are important sources of PCBs and high-molecular-weight PAHs for the Ganges floodplain during dry periods. Environ Pollut 206:588–596
Sultana J, Syed JH, Mahmood A, Ali U, Rehman MYA, Malik RN, Li J, Zhang G (2014) Investigation of organochlorine pesticides from the Indus Basin, Pakistan: sources, air– soil exchange fluxes and risk assessment. Sci Total Environ 497:113–122
Sun JH, Feng JL, Liu Q, Li QL (2010) Distribution and sources of organochlorine pesticides (OCPs) in sediments from upper reach of Huaihe River, East China. J Hazard Mater 184:141–146
Syed JH, Malik RN (2011) Occurrence and source identification of organochlorine pesticides in the surrounding surface soils of the Ittehad Chemical Industries Kalashah Kaku Pakistan. Environ Earth Sci 62:1311–1321
Syed JH, Malik RN, Liu D, Xu Y, Wang Y, Li J, Zhang G, Jones KC (2013) Organochlorine pesticides in air and soil and estimated air–soil exchange in Punjab, Pakistan. Sci Total Environ 444:491–497
Syed JH, Malik RN, Li J, Chaemfa C, Zhang G, Jones KC (2014) Status, distribution and ecological risk of organochlorines (OCs) in the surface sediments from the Ravi River, Pakistan. Sci Total Environ 472:204–211
Tang J, An T, Li G, We C (2018) Spatial distributions, source apportionment and ecological risk of svocs in water and sediment from Xijiang River, Pearl River Delta. Environ Geochem Health 40(5):1853-1865
Ulusoy Ş, Özden Ö, Päpke O (2016) Distribution of OCPs and PCBs in Mussels (Mytilus galloprovincialis) from the Marmara Sea coastal sites. Bull Environ Contam Toxicol 97:191–197
UNEP (2001) Final act of the conference of plenipotentiaries on the Stockholm Convention on persistent organic pollutant. United Nations Environment Program, Geneva, Switzerland
US Environmental Protection Agency (USEPA) (2004). National Recommended Water Quality Criteria. Office of Science and Technology (4304T). https://www.epa.gov/sites/production/files/2015-06/documents/nrwqc- 2004.pdf (accessed 24.05.17).
Vijgen J, Yi LF, Forter M, Lal R, Weber R (2006) The legacy of lindane and technical HCH production. Organohalogen Compd 68:899–904
Wang D, Yang S, Wang G, Gao L, Wang Y, Jiang Q, Chen Y (2016) Residues and distributions of organochlorine pesticides in China’s Weihe River. Pol J Environ Stud 25:1285–1292
Wong F, Alegria HA, Bidleman TF (2010) Organochlorine pesticides in soils of Mexico and the potential for soil-air exchange. Environ Pollut 158:749–755
Xiong JK, An TC, Zhang CS, Li GY (2015) Pollution profiles and risk assessment of PBDEs and phenolic brominated flame retardants inwater environments within a typical electronic waste dismantling region. Environ Geochem Health 37:457–473
Xiao H, Li NQ, Wania F (2004) Compilation, evaluation, and selection of physical-chemical property data for α-, β-, and γ-hexachlorocyclohexane. J Chem Eng Data 49:173–185
Yu Y, Li Y, Shen Z, Yang Z, Mo L, Kong Y, Lou I (2014) Occurrence and possible sources of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) along the Chao River, China. Chemosphere 114:136–143
Zehra A, Eqani SAMAS, Katsoyiannis A, Schuster JK, Moeckel C, Jones KC, Malik RN (2015) Environmental monitoring of organo-halogenated contaminants (OHCs) in surface soils from Pakistan. Sci Total Environ 506–507:344–352
Zhang G, Parker A, House A, Mai B, Li X, Kang Y, Wang Z (2002) Sedimentary records of DDT and HCH in the Pearl River Delta, south China. Environ Sci Technol 36:3671–3677
Zhang G, Chakraborty P, Li J, Sampathkumar P, Balasubramanian T, Kathiresan K, Takahashi S, Subramanian A, Tanabe S, Jones KC (2008) Passive atmospheric sampling of organochlorine pesticides, polychlorinated biphenyls, and polybrominated diphenyl ethers in urban, rural, and wetland sites along the coastal length of India. Environ Sci Technol 42:8218–8223
Zhang L, Dong L, Yang W, Zhou L, Shi S, Zhang X, Niu S, Li L, Wu Z, Huang Y (2013) Passive air sampling of organochlorine pesticides and polychlorinated biphenyls in the Yangtze River Delta, China: concentrations, distributions, and cancer risk assessment. Environ Pollut 181:159–166
Zhao Z, Wang Y, Zhang L, Cai Y, Chen Y (2013) Organochlorine pesticide (OCP) residues in mountain soils from Tajikistan. Environmental Science: Processes and Impacts 15(3):608–616
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Ester Heath
Electronic supplementary material
ESM 1
(DOC 1055 kb)
Rights and permissions
About this article
Cite this article
Ullah, R., Asghar, R., Baqar, M. et al. Assessment of organochlorine pesticides in the Himalayan riverine ecosystems from Pakistan using passive sampling techniques. Environ Sci Pollut Res 26, 6023–6037 (2019). https://doi.org/10.1007/s11356-018-3987-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3987-6