Abstract
Environmental geochemical studies are carried out to assess the groundwater quality and identification of hydrogeochemical process in hard rock terrain of Bhongir watershed nearer to the Greater Hyderabad. A total of thirty-eight groundwater samples were collected and analysed for important physicochemical parameters, anions and cations. The analytical data of alkalis (Na+ and K+) and alkaline earths (Ca2+ and Mg2+), reveal that high concentration of Na+ than others (Na > Ca > Mg > K) is probability due to the loss of Ca2+ and Mg2+ and gain of Na+ by the cation exchange process. Among the anions, bicarbonate is identified in majority of the samples in the following order HCO3 > Cl > SO4 which confirms that all carbonate minerals might have been dissolved and leached to the groundwater system. Most of the samples (81%) are exceeding the WHO allowable limits of electrical conductivity for drinking. The data sets further suggest that the water chemistry in the study area is not homogeneous and influenced by complex contamination sources and geochemical processes. Besides, highest concentration of nitrate (565.7 mg/L), sulphate (414 mg/L) and chloride (1444 mg/L) firmly suggests the impact of agricultural activities such as irrigation return flow, fertilizer application on water chemistry. The elevated concentrations of fluoride (i.e. maximum 4.1 mg/L) in most of the water samples (66%) reveal the origin and geochemical mechanisms, i.e. rock–water interaction is driving its enrichment. As majority of the parameters are above the permissible limit, the groundwater is not potable for drinking.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Introduction
Groundwater is the major sources of drinking water both in urban and in rural areas of India. It is also an important source of agriculture and industrial sector. The demand of water has increased over the year, and this had led to water scarcity in many parts of the world. Generally, the variation of major elements in the groundwater can be controlled by cation exchanges, dissolution and precipitation of minerals, evaporation and oxidation–reduction reactions. These complicated hydrogeochemical processes help to get an insight into the contributions of rock–water interaction (geochemical study) that influences groundwater quality. These geochemical processes are responsible for the seasonal and spatial variations in groundwater chemistry (Matthess 1982; Kumar et al. 2006). Groundwater chemically evolves by interacting with aquifer minerals or internal mixing among different groundwater along flow paths in the subsurface (Domenico 1972; Wallick and Toth 1976; Toth 1984; Schuh et al. 1997). This indicated that the increase in solute concentrations in the groundwater is caused by spatially variable recharge, governed by geochemical evolution controls. Further, the weathering of primary and secondary minerals is also contributing cations and silica in the system (Jacks 1973; Freeze and Cherry 1979; Bartarya 1993). Many discussed analyses, such as leaching and dissolution, mixing, cation exchange, oxidation–reduction, precipitation, hydrolysis, control the water quality during its movement from the recharge to discharge areas. As far as the quality of groundwater concern, many states in India have been identified as endemic to fluorosis due to abundance in naturally occurring fluoride-bearing minerals. One such example is at Bhongir Watershed of Nalgonda district, Telangana state, as the groundwater is major source of drinking and agricultural purposes.
The aim of this work is to find out the hydrogeochemical processes controlling groundwater quality in granite aquifer based on major ion chemistry and groundwater quality assessment in the proposed watershed. The hydrochemical processes and geochemical evolution are studied and investigated through the evaluation of 38 water points tapping granite aquifer.
Description of the Study Area
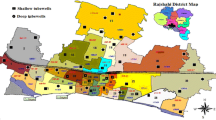
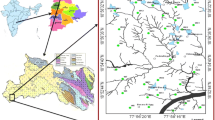
The study area has chosen on watershed scale in order to understand the hydrogeochemical behaviour of groundwater. Bhongir is a major town in the selected watershed, which is 48 km from the state capital Hyderabad. The watershed falls in the district of Nalgonda, Telangana, India. The study area covers 390 km2. The watershed experiences semi-arid-to-arid climate with temperature ranging from 28 to 44 °C. The average annual rainfall is 650 mm, of which 70% of rainfall contributes due to south-west monsoon (June to September). The location map of the study area (i.e. Bhongir Watershed) is shown in Fig. 1.
Geology of the Study Area
The study areas consist of major rock formation of medium- to coarse-grained granite. The granite can be divided into two major varieties based on colour: grey granite and pink granite. Both varieties occupied large part of the area, and it is not possible to draw a line of demarcation between two types, as they intermingle with no sharp point of contact. These granites are presumed to be the part of peninsular gneissic complex and to content basic enclaves of aplites, pegmatite, epidote and quartz vein. Dolerite dyke is frequently traversing the granite as well (GSI 1995).
The Srisailam formation: the youngest member of Cuddapa supergroup directly overlies the basement granite with a distinct unconformity. The meta-sediments of Srisailam formation include pebbly-gritty quartzite, shale, dolomite limestone, intercalated sequence of shale–quartzite and massive quartzite. The litho-units of this formation are dipping at angle ranging from 3° to 5° towards SE. The generalized stratigraphic sequence of the study area is shown in Table 1 (Natranjan and Murthy 1974).
Sample Collection
In order to study the hydrogeochemistry and quality of groundwater from the Bhongir Watershed, samples are collected during the month of June 2012 (pre-monsoon) only. Usually, this area is expected the monsoon in the month of June, but it delayed. The samples were collected according to the grid pattern (1.6 × 1.6 km) to spread the entire study area. Sample locations were recorded using GPS. Groundwater samples are collected from thirty-eight (38) bore wells in Bhongir Watershed of Nalgonda district in Telangana.
The water samples are collected in one-litre polypropylene (PP) bottles. Prior to the collection of samples, the bottles are rinse two times with water and completely filled to avoid air bubbling. The groundwater samples are transported to the laboratory on each day of the sample collection. Physicochemical parameters such as pH, EC and TDS values of groundwater are measured in situ in the field using portable metres.

Analytical Methods
The groundwater samples were analysed for various parameters according to the standard methods (APHA 2005). The pH, EC and TDS of water samples were measured using portable metres (Eutech Instruments, model: pHTester10, ECTester11+ and TDSTester11+, respectively). Bicarbonate and carbonate were analysed by classical volumetric method. Major cations, such as calcium, magnesium, sodium, potassium and anions, such as chloride, nitrate, sulphate, fluoride, were analysed by ion chromatography.
Results and Discussion
Hydrogeochemical Process
The groundwater hydrogeochemistry is primarily controlled by water–rock interactions as well as anthropogenic pollution. Thus, the following indices were evaluated to identify the hydrogeochemical process in hard rock terrain of the proposed watershed.
Physicochemical Parameters
Summary of analytical results of different parameters of groundwater samples is presented in Table 2. These include physicochemical parameters such pH, EC, TDS, total hardness as well as major anion and cation concentrations. The analytical results show that the pH value of the groundwater is in the range of 6.9–7.6, indicating that groundwater in the study area is in alkaline nature. However, its higher range accelerates the scale formations in water heating apparatus either in industrial or in domestic purposes. The EC values are varied in between 786 and 4840 µs/cm, while TDS values are in between 480 and 3040 mg/L, indicating that most of the samples are not suitable for drinking as these are above the desirable levels prescribed by WHO guideline values.
Major Ion Chemistry
The data of alkalis (Na+ and K+) and alkaline earths (Ca2+ and Mg2+) reveal high concentration of Na+ than others with the following order Na > Ca > Mg > K. In contrast, the increased Na+ concentration and decreased Ca2+ and Mg2+ concentration values can be explained by the probability of the loss of Ca2+ and Mg2+ and gain of Na+ by the cation exchange process (Shanmugam and Ambujam 2012).
Among the various anions (Table 2) determined in groundwater samples, HCO3 −, Cl−, SO4 2− are the major anions identified in the groundwater and their presence is in the following order HCO3 > Cl > SO4. This could be due to available carbonates in the rocks that might have been dissolved and added to the groundwater system during irrigation and rainfall infiltration, and groundwater movement increases the bicarbonate (Sunne et al. 2005). Further, an increased chloride concentration may be due to the process of the removal of other ions from the system, either by precipitation or by adsorption. The high content of chloride in water causes salinity-related problems.
Water Quality Assessment
The analytical results of groundwater samples (Table 3) were compared with the standard guideline values recommended by the World Health Organization (WHO 1993) for the drinking purpose. It included the most desirable limits and maximum allowable limits of various parameters. The following key parameters are considered and discussed in detail in order to assess the suitability of the groundwater in the study area for drinking purposes.
Physicochemical Parameters
The prescribed limit of pH value for drinking purpose is given by Bureau of Indian Standards (BIS 2012) and is in the range of 6.5–8.5. In the study area, most of the groundwater has pH value within the prescribed limits (Table 3) indicating its suitability for drinking.
Classifications of electrical conductivity of groundwater in study area are given in Table 4. It is found that 18% of the samples are within the permissible limits and 55% of samples fall in not permissible limit, but they are marginally poor in quality and only 26% of the sample locations are classified as hazardous. However, according to the WHO standards, 81% of the samples are exceeding the allowable limits for drinking (Table 3).
It is essential to classify the groundwater depending upon their TDS values obtained in the study area as reported in the literature (Davis and Dewiest 1966; Freeze and Cherry 1979) to understand their suitability for drinking purposes. The data are classified according to Davis and DeWiest (1966) and Freeze and Cherry (1979) and presented in Tables 5 and 6, respectively. Further, the contour map of TDS in groundwater is shown in Fig. 2. The data represent that only 2% of the samples is below 500 mg/L of TDS (Davis and DeWiest 1966) which can be used for drinking without any risk (Table 5). On the other hand, results demonstrated that the groundwater of the area 16% is only the freshwater type and remaining (84%) samples represent brackish water in accordance with Freeze and Cherry classification (Table 6). The spatial distribution of TDS in the watershed is shown in Fig. 2 exhibiting in the range of 480–3039 mg/L.
Total hardness of groundwater samples in the present study exhibited minimum content of 100 mg/L to a maximum of 1235 mg/L with the average of 586 mg/L. Please note that the maximum allowable limit of TH for drinking purpose is 500 mg/L and the most desirable limit is 100 mg/L as per the WHO standard (Table 3).
The spatial distribution of hardness (Fig. 3) shows that higher concentrations were observed in the south-western part of the study area. According to the Sawyer and McMacartly classification (Sawyer and McCarty 1967), 95% of the groundwater samples in the study area fall in the very hard category (Table 7) and rest of 5% of the samples are in moderately hard to hard category. Therefore, the groundwater in the study area is not suitable for drinking.
Cations
The analytical data on cations demonstrated that 32% of samples with Na+ and each of K+ and Ca2+ concentration (11% each) are exceeding the WHO limit, whereas Mg2+ concentrations are within permissible limits. This may be due to the ion exchange process occurring in the aquifer. The spatial distribution of sodium, potassium, calcium and magnesium in groundwater samples of the study area is shown in Figs. 4, 5, 6 and 7.
Anions
Anions such as bicarbonate 97% and nitrate 52% of the sample locations exceed the standards. The spatial distribution of bicarbonate in groundwater is shown in Fig. 8. The permissible limit for bicarbonate (i.e. 300 mg/L) exceeded in majority of the samples which confirm that all carbonate minerals are dissolved in the groundwater. This is further evident that no carbonate is detected in all the groundwater samples investigated.
Further, distribution of nitrate in groundwater samples of the study area is shown in Fig. 9. The groundwater samples exceed the WHO permissible limit of 45 mg/L nitrate, and it varies between below detection limit to 565.69 mg/L with an average of 110.63 mg/L. Nitrate content in groundwater is because of the excess use of fertilizer on to the agricultural land. Further, this may be evident from the reported literature (Jalali 2005).
Fluoride concentration varies between 0.87 and 4.12 mg/L with average 1.91 mg/L. The spatial distribution of the fluoride in the study area is shown in Fig. 10. On the other hand, high concentration of fluoride in the study area is observed in 66% of the sampling locations. The average concentration observed (1.91 mg/L) in the study area is also above the WHO permissible limit of 1.5 mg/L which further indicates that the people in the area are severely affected with dental fluorosis (Fig. 11).
Natural waters contain chloride and sulphate ions, and their concentrations vary considerably according to the mineral content of the earth in any given area. Low-to-moderate concentrations of both chloride and sulphate ions add palatability to water. In fact, they are desirable for this reason. Chlorides give salty taste to water. Excessive concentrations of either, of course, can make water unpleasant to drink. The high concentration of sulphate in drinking water is toxic and cause laxative effective on human beings.
The present investigations showed that chloride content varying between 54.08 and 1444 mg/L (average = 501.56 mg/L) with 30% of the total samples exceeded the permissible limit. Sulphate is found to be within the permissible limit of 400 mg/L as per WHO standards except 5% of the samples. The spatial distribution of chloride and sulphate is shown in Figs. 12 and 13.
Conclusions
The study of major ion concentration of the groundwater of the Bhongir watershed showed high concentration of Na+ than the others cations (Na>Ca>Mg> K) is probably due to the loss of Ca2+ and Mg2+ and gain of Na+ by the cation exchange process. Among the anions, bicarbonate is identified in majority of the samples in the following order HCO3 > Cl > SO4 which confirms that all carbonate minerals might have been dissolved and added to the groundwater system. Nitrate and fluoride levels are higher than the permissible levels in most of the samples around 53 and 66% of samples, respectively. This indicates that the nitrate may be due to anthropogenic influence, whereas fluoride is due to geogenic sources.
It is evident from the higher values of physicochemical results, especially hardness, alkalinity and bicarbonates, that most of the water samples analysed in this study are contaminated due to geogenic as well as anthropogenic activities. The groundwater quality is poor in the study area due to various contaminants at higher concentration than permissible; thus, it is unfit for drinking purposes. The present investigations recommended stringent monitoring and control/mitigation measures in the regions where low groundwater quality is essential to ensure sustainable safe use of the resource.
References
APHA, American Public Health Association (2005) Standard methods for the examination of water and wastewater, 21st edn. APHA, Washington D.C
Bartarya SK (1993) Hydrochemistry and rock weathering in a subtropical lesser Himalayan river basin in Kumaun, India. J. Hydrol. 146:149–174
BIS (2012) Indian Standard Specification for drinking water, IS: 10500, New Delhi
Domenico PA (1972) Concepts and models in groundwater hydrology. McGraw-Hill, New York, U.S.A
Davis SN, Dewiest RJ (1966) Hydrogeology. Wiley, New York
Freeze RA, Cherry JA (1979) Groundwater. Prentice-Hall, Englewood Cliffs, p 604
GSI (1995) Geological survey of Indian geology and minerals map, Nalgonda district A.P
Jacks G (1973) Chemistry of groundwater in a district in Southern India. J Hydrol 18:185–200
Jalali M (2005) Nitrates leaching from agricultural land in Hamadan, western Iran. Agric Ecosyst Environ 110:210–218
Kumar M, Ramanathan AL, Rao MS, Kumar B (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. J Environ Geol. 50:1025–1039
Matthess G (1982) The properties of groundwater. Wiley, New York, p 498
Natranjan V, Murthy SRN (1974) Fluorite bearing granite of Sivannagudem area, Nalgonda district A.P. Indian Academy of Geological Science. Proceedings, symposium on fluorosis, Hyderabad, pp 49–33
Sawyer CN, McCarty PL (1967) Chemistry for sanitary engineers, 2nd edn. McGraw-Hill, New York, p 518
Schuh WM, Klinekebiel DL, Gardner JC, Meyar RF (1997) Tracer and nitrate movements to groundwater in the Norruem Great Plains. J Environ Qual 26:1335–1347
Shanmugam P, Ambujam NK (2012) A hydrochemical and geological investigation on the Mambakkam mini watershed, Kancheepuram District, Tamil Nadu. Environ Monit Assess 184(5):3293–3306
Sunne VX, Villa S, Carrera J (2005) Introductory review of specific factors influencing urban groundwater—an emerging branch of hydrogeology, with reference to Barcelona, Spain. Hydrogeology J 13:522–533
Toth J (1984) The role of regional gravity flows in the chemical and thermal evolution of groundwater. In: Proceedings of 1st Canadian/American conference on hydrogeology, Banff, Alta, June 1984
Wallick EI, Toth J (1976) Methods of regional groundwater flow analysis with suggestions for the use of environmental isotope and hydrochemical data in groundwater hydrology. IAEA, Vienna, pp 37–64
WHO (1993) Guidelines for Drinking Water, vol 1: Recommendations, vol 1. World Health Organization, Geneva
Acknowledgements
The authors are thankful to Director, CSIR-National Geophysical Research Institute (NGRI), Hyderabad, for his kind permission and encouragement to publish this work. The authors are also grateful to CSIR-NGRI, for their financial support through MLP-6601-28 (KRM). Further, the authors are acknowledged the support from project staff and trainee students during the field and experimental work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Rama Mohan, K., Keshav Krishna, A. (2018). Assessment of Groundwater Quality and Identification of Hydrogeochemical Process in Hard Rock Terrain. In: Singh, V., Yadav, S., Yadava, R. (eds) Groundwater. Water Science and Technology Library, vol 76. Springer, Singapore. https://doi.org/10.1007/978-981-10-5789-2_12
Download citation
DOI: https://doi.org/10.1007/978-981-10-5789-2_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5788-5
Online ISBN: 978-981-10-5789-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)