Abstract
The root-knot nematode Meloidogyne incognita causes losses in tomato production, making new methods/products to control this parasite desirable. Garlic essential oil (GEO) is potentially useful for controlling M. incognita. Here, GEO was obtained by hydrodistillation, dissolved in water and used against M. incognita in vitro. At 63 μg mL−1, this oil was more active against M. incognita eggs and second-stage juveniles (J2) than the nematicide Carbofuran at 173 μg mL−1. The main components of the oil, according to gas chromatography-mass spectrometry analysis, are diallyl trisulphide (66.7%) and diallyl disulphide (21.3%), which presented lethal concentrations to 50% (LC50) J2 equal to 36.2 ± 1.7 and 134.4 ± 19.4 μg mL−1, respectively, while the LC50 for Carbofuran was 151.6 ± 2.1 μg mL−1. When the J2 submerged in a solution of the oil at 250 μg mL−1 were used to infest tomato plants, the number of galls and eggs of M. incognita in the roots were reduced to values statistically equal to those obtained when J2 were submerged in a solution of Carbofuran at 415 μg mL−1. The vapour of the oil was also as active in vitro against M. incognita eggs and J2 as the fumigant nematicide Dazomet. The infectivity and reproduction of M. incognita in tomato plants cultivated in substrate inoculated with eggs of the nematode and treated with 0.2 mL of oil per L of substrate were statistically equal to those observed when the oil was replaced by 0.25 g of Dazomet per L of substrate. These findings confirm the activity of GEO and its components against M. incognita, suggesting its potential as a new fumigant nematicide to control the nematode in tomato plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The tomato (Solanum lycopersicum L.) is one of the most cultivated vegetables in the world. Of the 170 million tonnes of tomatoes produced worldwide, about 4 million were produced in Brazil in 2014 (FAO 2016). These numbers would be higher if the problems caused by root-knot nematodes (Meloidogyne spp.) could be avoided (Jones et al. 2013). In addition to directly affecting the tomato plant, these parasites make the tomatoes more susceptible to other diseases (Zhou et al. 2016). The most destructive root-knot nematode species for tomato plants is Meloidogyne incognita (Kofoid & White) Chitwood, which also affects several other crops of global economic importance (Sikora and Fernandez 2005).
Currently, the application of chemical nematicides is commonly used by farmers to control parasitic nematodes on their plants. Although the nematicides show good overall efficacy, the concerns about their residues in the food chain, risks to human health and negative environmental effects have resulted in the ban of methyl bromide and other substances (Wesemael et al. 2011).
Plants are promising sources of substances to circumvent the above-mentioned problem, as some of their metabolites can be used directly as pesticides or as starting models for the synthesis of improved chemical structures (Giannakou 2011; Ntalli and Caboni 2012). Among these metabolites are the compounds found in essential oils, which are volatile secondary metabolites produced by plants. Many essential oils can be used as natural antimicrobials (Júnior et al. 2014), and some are reported to be active against nematodes (Isman 2000; Barbosa et al. 2010; Faria et al. 2013; Andrés et al. 2012; Nasiou and Giannakou 2018).
Garlic (Allium sativum L.) is an essential oil producing plant that has been used for culinary and medicinal purposes for ages (Martins et al. 2016). In part, this is due to the antioxidant, antibacterial and antifungal activities of the metabolites produced by this plant (Block 2010). Some of these metabolites have also been reported to be active against insects (Chaubey 2016; Zhao et al. 2013) and even the pine wood nematode Bursaphelenchus xylophilus (Steiner & Buhrer) Nickle (Park et al. 2005). The cold pressed oil (Cetintas and Yarba 2010) and the essential oil (Al-Shalaby 2009; El-Saedy et al. 2014) from garlic are also reported to be active against M. incognita. The essential oil of garlic is rich in organosulfur compounds such as ajoene, diallyl disulphide (DADS), diallyl trisulphide (DATS), allyl methyl trisulphide and diallyl sulphide (Block 2010; Corzo-Martinez and Villamiel 2007). Apparently, sulphur compounds are responsible for the nematicidal activity of garlic essential oil (Park et al. 2005).
In the work done with B. xylophilus (Park et al. 2005), only in vitro tests were performed by the authors to evaluate the activity of garlic essential oil and its components against this nematode. However, the action of garlic essential oil under in vivo conditions remains unknown. Regarding the work done with M. incognita (Cetintas and Yarba 2010), only second-stage juveniles (J2) of the nematode were assayed. However, the majority of the nematode population is in the form of eggs (Evans and Perry 2009). Furthermore, the oil studied by Cetintas and Yarba (2010) was obtained by cold pressing, which results in oils containing several non-volatile compounds (Ferhat et al. 2007). As the oil used by the authors was not analysed, those components active against M. incognita were not identified. For example, such active components could be fatty acids that, according to Zhang et al. (2012), can be active against nematodes. Alternatively, these effects could be caused by allicin, a non-volatile substance that inhibits the hatching of M. incognita J2 at 0.5 μL mL−1 (Gupta and Sharma 1993). In the work carried out by El-Saedy et al. (2014), the amount of garlic essential oil used in the experiments with tomato seedlings was approximately 75–150 mg per 100 mL of soil, while N-[Ethoxy-(3-methyl-4-methylsulfanylphenoxy)phosphoryl]propan-2-amine (active ingredient of Nemacur® 10G) was used at approximately 2,5 mg per 100 mL of soil. If we consider Carbofuran, which controls M. incognita in tomato plants when approximately 0.8 mg of this commercial nematicide is applied to 110 mL of soil (Oliveira et al. 2019), the amount of garlic essential oil used by El-Saedy et al. (2014) seems even greater, which leads us to question whether such oil only showed activity against the nematode because it was used in very high concentrations. Something similar is observed in the work by Al-Shalaby (2009).
In addition, it is important to mention that in works with B. xylophilus (Park et al. 2005) and M. incognita (Cetintas and Yarba 2010) no nematicidal substance was used as a reference. It is therefore difficult to assess whether the products obtained from garlic are in fact potentially useful for the development of new nematicides. It is also important to consider the volatile properties of essential oils in general, as well as the propensity to oxidize the sulphur components produced by garlic (Yang et al. 2001). These characteristics likely render a short residence time of garlic essential oil in the soil in relation to non-fumigant nematicides. It is likely that after planting tomatoes in the field, several M. incognita individuals will not come across the essential oil applied to the soil, resulting in inefficient control of the nematode. Consequently, it makes more sense to use this oil as a fumigant nematicide to reduce the population of M. incognita in the soil prior to planting.
Therefore, in order to complete the knowledge about the efficiency of garlic essential oil in the control of plant parasitic nematodes, it is necessary to fill in some gaps, such as its effect on the nematode eggs, the effect of the oil gas phase, the real roles of the major components of the oil, and the comparison of different ways of applying the oil in the same paper. Considering these current issues, the present study was designed to: 1) evaluate the in vitro activity against J2 and eggs of M. incognita with aqueous solutions of garlic essential oil; 2) identify the components of garlic essential oil through gas chromatography and mass spectrometry analyses; 3) evaluate solutions of the main components of garlic essential oil through in vitro assays with M. incognita J2 and eggs; 4) evaluate the effect of aqueous solutions of garlic essential oil on the infectivity and reproduction of M. incognita on tomato plants; 5) investigate the in vitro activity against M. incognita J2 and eggs by vapour of garlic essential oil; and 6) evaluate the effect of vapour from garlic essential oil on the infectivity and reproduction of M. incognita on tomato plants.
Materials and methods
M. incognita inoculum
First, M. incognita was multiplied on tomato plants (Solanum lycopersicum L. ‘Santa Clara’) that were kept in a greenhouse for 50 days after inoculation with J2 previously obtained from other tomato plants in a greenhouse that had never undergone nematicide treatment. Nematode eggs were then extracted from the roots (according to Hussey and Barker 1973) and placed on an adapted Baermann funnel at 28 °C for hatching. The J2 that hatched during the first 24 h were discarded, while those hatched between 24 and 48 h were immediately used in the bioassays.
Garlic essential oil
Bulbs of garlic (Allium sativum L. ‘Lavínia’) in natura (1000 g) were purchased in the local market (Lavras - MG, Brazil), peeled, ground in a blender with distilled water and submitted to hydrodistillation for 60 min in a Clevenger apparatus type (Clevenger 1928). The obtained essential oil was separated from water and treated with anhydrous sodium sulphate to yield 7 mL of a pale yellow liquid that was stored in a freezer at −20 °C.
Chemicals
Diallyl disulphide (DADS, 80%), diallyl trisulphide (DATS, 98%) and Carbofuran (2,3-dihydro-2,2-dimethyl-1-benzofuran-7-yl N-methylcarbamate, 98%) were purchased from Sigma-Aldrich Co. (Milan, Italia), while Dazomet was purchased from BASF (Basamid®, 98%, Ludwigshafen, Germany). Although the nematicide Carbofuran has been withdrawn from the market in several countries due to its undesirable action on non-target organisms, its efficiency to control of plant parasitic nematodes is well known.
In vitro effect on mobility, mortality and hatching of M. incognita J2 by emulsions of garlic essential oil
Garlic essential oil was emulsified in an aqueous 0.01 g mL−1 Tween 80® solution to yield a final concentration of 10,000 μg mL−1. The resulting primary emulsion was then diluted with 0.01 g mL−1 Tween 80® to five different concentrations (2500, 1250, 625, 312 and 156 μg mL−1). Then, 100 μL of each resulting emulsion and 400 μL of an aqueous suspension of approximately 100 M. incognita J2 were placed into Eppendorf tubes (0.5 mL). The final concentrations of the oil were 500, 250, 125, 63 and 31 μg mL−1. Water, aqueous solutions of Tween 80® at 0.01 g mL−1 and Carbofuran at 865 μg mL−1 (final concentration: 173 μg mL−1) were used as controls. The experimental design was completely randomized, with six repetitions for each treatment. After 48 h at 28 °C, the Eppendorf tubes were agitated and opened so that 200 μL of their contents were transferred to 350 μL wells of a 96-well polypropylene plate. Mobile and immobile J2 were counted under a microscope. Then, one drop of freshly prepared 1.0 mol L−1 NaOH solution was added to each well (according to Chen and Dickson (2000) and adapted by Amaral et al. (2003)) and nematodes were counted again within 2 min of the addition. Immobile J2 after the NaOH addition were considered dead. This experiment was carried out in duplicate.
A procedure similar to the one used above was employed to evaluate the effect of garlic essential oil on J2 hatching, but using 240 M. incognita eggs per Eppendorf tube instead of 100 J2. Exposure of the eggs to the garlic essential oil emulsions and the controls lasted for seven days instead of the 48 h used in the previously described experiments with J2. After the exposure time, the evaluation consisted of counting intact eggs and hatched J2 (both alive and dead) under a microscope. This experiment was also carried out in duplicate.
GC-MS analysis of the essential oil of garlic
A gas chromatograph coupled to a mass spectrometer (model QP2010, Shimadzu, Japan), equipped with a RTX®-5MS capillary column (30 m × 0.25 mm ID × 0.25 μm film thickness; Restek), which used helium at 1.0 mL min−1 as the mobile phase was employed in this work. According to Adams (2007), the following conditions were adopted: 1) split/splitless injector temperature: 220 °C; 2) split ratio: 1:20; 3) initial column temperature: 60 °C; 4) elevation rate of the column temperature: 2 °C min−1 up to 200 °C and then 5 °C/min until final temperature; 5) final temperature of the column: 250 °C; 6) temperature of the interface between the gas chromatograph and the mass spectrometer: 220 °C; 7) ionization of each molecule in the spectrometer: electron impact at 70 eV; 8) range of mass/charge (m/z) analyses in the mass spectrometer: 45–400; 9) mass spectrum acquisition time: 0.5 s. The essential oil of garlic was dissolved in acetone to a concentration of 10 mg mL−1, and 1 μL of this solution was injected into the gas chromatograph. A solution of homologous linear alkanes, containing C9–C20 carbon atoms, was used as an external standard. All mass spectra were compared to those in the NIST 05 Mass Spectral Library (2005) and all peaks in the chromatogram with similarity index below 80% were considered unidentified. For each of the remaining peaks, the arithmetic index (AI) was calculated according to the following formulae: AI = {100Xz + 100[(RT – RTXz)/(RTXz + 1 - RTXz)]}, where Xz = number of carbon atoms of the linear alkane with retention time immediately below that of the substance to be identified in the chromatogram; RT = retention time (min) of the substance to be identified in the chromatogram; RTXz = retention time (min) of the linear alkane with number of carbon atoms equal to Xz; RTXz + 1 = retention time (min) of the linear alkane with number of carbon atoms equal to Xz + 1. Substances with calculated values of AI corresponding to an error ≥ 3% in relation to the AI described by Adams (2007) were considered not identified.
In vitro effect of garlic essential oil and its main components on mobility, mortality and hatching of M. incognita J2
The experiments were carried out twice as described before using the essential oil of garlic (final concentrations: 500, 250, 125, 63 and 31 μg mL−1), DATS (final concentrations: 335, 168, 84, 42 and 21 μg mL−1), DADS (final concentrations: 106, 53, 27, 14 and 7 μg mL−1) and DATS + DADS (final concentrations: 335 + 106, 168 + 53, 84 + 27, 42 + 14 and 21 + 7 μg mL−1). For the mobility and mortality assay four final concentrations were employed for Carbofuran: 200, 173, 150, 100, and 50 μg mL−1. For the hatching assay only one concentration was used for Carbofuran: 173 μg mL−1. Concentrations of DADS and DATS were proportional to their amounts in the oil (AMO): 0.213 AMO and 0.667 AMO for DADS and DATS, respectively.
Infectivity and reproduction of M. incognita in tomato plants after exposition to aqueous emulsion of garlic essential oil
Tomato seeds (Solanum lycopersicum L. ‘Santa Clara’) susceptible to M. incognita were sown on a commercial substrate (Tropstrato ®, Vida Verde Indústria e Comércio de Insumos Orgânicos Ltda., Mogi Mirim, São Paulo, Brazil) contained in 121 mL wells within a 72-well Styrofoam seedling tray. Thirty days later, an aqueous suspension (24 mL) containing approximately 3000 M. incognita J2 and aqueous emulsions (24 mL) of garlic essential oil (250, 125 and 63 μg mL−1) in 0.01 g mL−1 Tween 80® were combined. Water, Tween 80® (0.01 g mL−1) and Carbofuran (415 μg mL−1) were also combined with the aqueous J2 suspension to be used as controls. Aliquots (8 mL, approximately 500 J2) of each resulting suspension were added to the substrate of each plant through four equidistant holes (0.4 cm wide × 1.5 cm deep) around the stem. This experiment was arranged in a completely randomized design, with six repetitions for each treatment. The tray was kept in a dark room at 28 °C for 48 h and then transferred to a greenhouse, where it was maintained for 30 days. After this period, the roots were carefully removed, washed with water, dried on paper towels and weighed. After gall counting, eggs were extracted from the roots following the technique by Hussey and Barker (1973). Eggs retained on a 500 mesh (ASTM) sieve were suspended in 50 mL water and counted in a Peters chamber under a microscope. This experiment was carried out in duplicate.
In vitro effect of vapour from garlic essential oil on mobility, mortality and hatching of M. incognita J2
Adapting the method described by Barros et al. (2014), sand (30 g) was sterilized by autoclaving at 120 °C for 30 min and poured into Supelco™ SPME flasks (28 mm wide × 80 cm deep, Sigma-Aldrich, Bellefonte, PA, USA). Two Eppendorf tubes (0.5 mL) were partially immersed in the sand of each flask and 100 μL of garlic essential oil was poured into one of the tubes. The commercial fumigant nematicide Dazomet (80 mg) and water (100 μL) were used as positive and negative controls, respectively. The flask was immediately sealed with a screw cap, internally coated with silicone and kept at 28 °C for 72 h. Employing a syringe with a needle to punch the silicon septa of the Supelco™ SPME flask, 100 μL of an aqueous suspension (1000 J2 mL−1) was injected into each empty Eppendorf tube. This experiment was completely randomized, with six replicates for each treatment. After 48 h at 28 °C, the flasks were opened for the homogenization of the J2 suspension, from which aliquots (20 μL) were transferred to 350 μL wells of a 96-well polypropylene plate. These aliquots were diluted with 100 μL water before counting mobile and immobile J2 under a microscope. The J2 mortality was evaluated using the methodologies described by Chen and Dickson (2000) and Amaral et al. (2003). This experiment was carried out in triplicate.
The experiment aimed to evaluate the effect of vapour from garlic essential oil on hatching of M. incognita J2 followed a similar procedure as the previous experiment, but with a suspension containing M. incognita eggs (3000 eggs mL−1) instead of J2. The experiment time also changed, as number of intact eggs and hatched J2 (alive or dead) was evaluated seven days after injecting the aqueous egg suspension into each empty Eppendorf tube. This experiment was carried out in triplicate.
Effect of vapour from garlic essential oil on the infectivity and reproduction of M. incognita on tomato plants
M. incognita eggs (150,000) were added to 1 L of a commercial substrate (Tropstrato®, Vida Verde Indústria e Comércio de Insumos Orgânicos Ltda., Mogi Mirim, São Paulo, Brazil) contained in 2-L polyethylene terephthalate bottles. Essential oil of garlic was then splashed on the substrate surface to the following concentrations: 0.5, 0.2 and 0.1 mL per L of substrate. The commercial fumigant nematicide Dazomet at 0.25 g (L of the substrate)−1 and water (1.0 mL) were used as positive and negative controls, respectively. This experiment was carried out with six replicates for each treatment, under a completely randomized design. All bottles were cap closed and the resulting mixtures were shaken by hand for five minutes. After standing at 28 °C for 3 days, the bottles were opened. Five days later, the substrate inside them was poured into cells (121.2 mL) of a 72-cell Styrofoam seedling tray (six cells for treatment). Twenty-day old tomato plants (Solanum lycopersicum L. ‘Santa Clara’) were transferred to the tray (six plants per treatment), which was kept in a greenhouse for 30 days. After this period, roots were carefully washed, dried with a paper towel and weighed. After counting galls, M. incognita eggs were extracted (method described by Hussey and Barker 1973). The obtained eggs were counted in a Peters chamber under a microscope. This experiment was carried out in duplicate.
Data analysis
A combined analysis was performed for each assay, and the data presented are the combined results of the repeat experiments. The results (values from the in vitro tests were converted into percentage) were previously submitted to a normality test (Shapiro-Wilk’s test) and homoscedasticity test (Bartlett’s test), and based on those results, no transformation was necessary. The values were then submitted to analysis of variance (ANOVA; P = 0.05) and to separate treatment means, the Scott and Knott (1974) test was applied. The software SISVAR (Ferreira 2011) was used to carry out the statistical calculations. Percentage values of the in vitro assays were subjected to a nonlinear regression with the software Scidavis 0.2.4 (SciDAVis 2017). Lethal concentrations for 50% (CL50) and 90% (CL90) of the nematode were calculated from the values obtained for the in vitro mortality of the J2 through Logit analysis, which was performed with the drc 3.0–1 package (Ritz et al. 2015) of software R 3.6.2 (R Core Team 2017), using the graphical interface RStudio Desktop 1.1.463 (RStudio Team 2015).
Results and discussion
In vitro effect on mobility, mortality and hatching of M. incognita J2 by emulsions of garlic essential oil
Emulsions of garlic essential oil were very active against M. incognita, causing 100% J2 death and immobility at concentrations equal to or greater than 125 μg mL−1. At 62 μg mL−1, the oil caused the death of 82.3% of J2, while the nematicide Carbofuran at 173 μg mL−1 caused the death of only 63.0%. (Table 1). These results are qualitatively in line with those from studies with B. xylophilus (Park et al. 2005) and M. incognita (Cetintas and Yarba 2010). However, a quantitative comparison cannot be made with those results because those authors did not use a nematicide as control. At 125 μg mL−1, the oil reduced the J2 hatching to a value statistically equal to that obtained with Carbofuran at 173 μg mL−1 (Table 1).
GC-MS analysis of the essential oil of garlic
As the essential oil of garlic was active against M. incognita (Table 1), it underwent GC-MS analysis, which led to the identification of six compounds that represented 93.8% of the total composition. The most abundant were DATS (66.7%) and DADS (21.3%) (Table 2). Although this result is in agreement with previous reports (Martinez-Velazquez et al. 2011; Kocić-Tanackov et al. 2012; Zhao et al. 2013; Foe et al. 2016; El-Sayed et al. 2017), it is worth mentioning there is large variation described in the literature regarding the composition of garlic essential oil. For example, in a sample from Egypt, diallyl sulphide (21.5%), allyl methyl disulphide (3.3%), DADS (27.4%), allyl methyl trisulphide (8.0%), DATS (26.3%), allyl methyl tetrasulphide (3.4%) and diallyl tetrasulphide (9.93%), were identified (Nashwa 2015), while in a sample from Tunisia, DATS (30.38%) and DADS (49.1%) were primarily the identified substances (Chekki et al. 2014). In part, these differences may be due to factors such as climatic conditions, harvesting period, distillation technique and the cultivar of garlic (Lahlou 2004; Block 2010).
In vitro effect of the main components of the essential oil of garlic on mobility, mortality and hatching of M. incognita J2
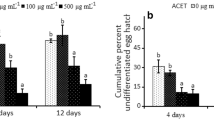
The number of J2 immobilized and killed by the combination of DADS and DATS was larger than the sum of the values obtained for these substances separately (Fig. 1). For example, DADS (7 μg mL−1), DATS (21 μg mL−1) and DADS+DATS (7 + 21 = 28 μg mL−1) caused 5.2, 44.4 and 100% mortality of J2, respectively. This result suggests a synergistic effect between DADS and DATS, which is logical when considering the behaviour of some other samples of natural origin. For example, Jiang et al. (2009) observed the synergistic effect of some components of the essential oils of Litsea pungens Hemsley and Litsea cubeba (Lour.) Persoon. Likewise, Miresmailli et al. (2006) reported a synergistic effect between inactive and active components of the essential oil of Rosmarinus officinalis L. Although there is no report of such behaviour in assays carried out with nematodes and garlic oil components, the synergistic effect of these components has already been observed (Amagase et al. 2001). For example, the combination of allyl alcohol with DATS and DADS increases the antifungal activity against Candida utilis (Henneberg) Lodder & Kreger-van Rij (Chung et al. 2007).
In vitro effects of emulsions of garlic essential oil and its main components, diallyl disulphide (DADS; at 0.213 x concentration of the oil) and diallyl trisulphide (DATS; at 0.667 x concentration of the oil), on mobility (upper graph), mortality (middle graph) and hatching (bottom graph) of Meloidogyne incognita second-stage juveniles (J2). In the DATS + DADS treatment: concentration of DATS = 3.14 x concentration of DADS. Error bars correspond to standard deviations. Results for each parameter are the mean values of two experiments with six repetitions each. Carbofuran (control) at 173 μg mL−1 increased immobile and dead J2 to 73% and 60%, respectively, and reduced the percentage of hatched J2 to 11.6%. In all nonlinear regressions: P < 0.05
When isolated, both DADS and DATS could not yield values of immobile or dead J2 greater than those observed for the essential oil at proportional concentrations (0.213 and 0.667 times de concentration of the oil, respectively; Fig. 1). However, when combined, these substances were more active than the essential oil. For example, a 56 μg mL−1 solution of DADS+DATS (14 + 42 μg mL−1, respectively) immobilised and killed 100% of J2. The essential oil at 62 μg mL−1 increased J2 immobility and death up to 85.5% and 82.3%, respectively. These results suggest that there is an interference in the action of DADS and DATS against M. incognita J2 by other components of the oil. The same conclusion can be drawn from the number of hatched J2. When tested alone, both DADS and DATS afforded percentage of hatched J2 that were statistically lower than those observed for the garlic essential oil at proportional concentrations (Fig. 1). For example, at 106 μg mL−1, DADS reduced J2 hatching to 0%, which is statistically lower than 5.8% of hatched J2 observed for the essential oil at 500 μg mL−1. These results also suggest that other components in the oil may interfere with the action of DADS and DATS against M. incognita.
Both DADS and DATS were more active against M. incongita eggs than the nematicide Carbofuran at 173 μg mL−1. For example, concentrations of 14 and 42 μg mL−1 for DADS and DATS, respectively, reduced J2 hatching to 5.8% and 1.3%, respectively, while the value observed for Carbofuran was 11.6% (Fig. 1). Consequently, these substances are promising for the development of new nematicides, especially considering that the majority of the population of M. incognita in the environment is in the form of eggs (Evans and Perry 2009).
While DADS seems to be as active against J2 as Carbofuran, DATS certainly is more active than this commercial nematicide. For example, at 84 μg mL−1, DATS killed 100% of J2, while at 173 μg mL−1, Carbofuran killed only 60% and at 106 μg mL−1, DADS killed 49.0% (Fig. 1). However, the combination of DADS and DATS is even more efficient than that commercial nematicide. For example, 28 μg mL−1 DADS+DATS (7 + 21 μg mL−1) killed 76.0% of J2.
The results described so far are in accordance with the calculated values of CL50 and CL90. For example, the values for the essential oil and for DATS are lower than those obtained for DADS and Carbofuran (Table 3). Similarly, the combination of DATS + DADS has much lower values than those observed for DATS, which makes evident the existence of a synergistic effect between these two substances. It is also worth noting that the LC50 value of the essential oil (33.9 μg mL−1) corresponds to 7.2 μg mL−1 of DADS and 22.6 μg mL−1, which results in 29.8 μg mL−1 for the combination DATS + DADS. This value is approximately 85% higher than the LC50 of DATS + DADS, which corroborates the existence of some component in the essential oil that interferes with the action of DATS + DADS on the nematode.
Our findings suggest that DADS and DATS account for the nematicidal activity observed for garlic essential oil, which is in accordance with the work by Park et al. (2005), who demonstrated the in vitro activity of these substances against the pine wood nematode, B. xylophilus. Another notable work is that of Anastasiadis et al. (2011), who demonstrated the nematostatic and nematicidal activity of DADS at 2 μL mL−1 against Meloidogyne javanica (Treub) Chitwood.
Infectivity and reproduction of M. incognita on tomato plants after exposition to aqueous emulsion of garlic essential oil
Garlic essential oil reduced the number of eggs per plant to 360 and 250 when used at 63 and 125 μg mL−1, respectively. Furthermore, at 250 μg mL−1 the reduction in number of eggs was statistically equal to that obtained for Carbofuran at 415 μg mL−1. Root mass was not affected by the oil, suggesting no phytotoxic effect within the studied concentration range (Fig. 2). This result seems to be in line with the work by Anastasiadis et al. (2011), who reported no phytotoxic activity of DADS against tomato plants.
Numbers of galls and eggs of Meloidogyne incognita per tomato plant inoculated with second-stage juveniles (J2) of the nematode and treated with emulsions of garlic essential oil at 250 (Oil-250), 125 (oil-125) and 63 (Oil-63) μg mL−1. Tween 80® (0.01 g mL−1), Carbofuran (415 μg mL−1) and water were used as controls. The mass of fresh root is also presented. Results are mean values of two experiments with six repetitions for each. Error bars correspond to standard deviations. Columns with the same letter in each bar plot do not statistically differ according to the Scott-Knott test (P ≤ 0.05)
This result is also in line with the previously described assay with garlic oil (Cetintas and Yarba 2010; El-Saedy et al. 2014; Al-Shalaby 2009). In the work by Cetintas and Yarba (2010) the oil obtained by cold pressing reduced the number of galls of M. incognita in tomato roots to approximately 13% of that observed for the untreated plants; no phytotoxic effect was observed by the authors. However, the number of eggs was reduced to approximately 67% by the cold pressing oil, while in the present work this parameter was reduced by garlic essential oil (250 μg mL−1) to approximately 7% of that observed for untreated plants (Fig. 2). Therefore, it is unclear whether the effect on the nematode by garlic oils obtained by cold pressing and by steam distillation are exactly the same.
In vitro effect of vapour from garlic essential oil on motility, mortality and hatching of M. incognita J2
Despite the excellent results obtained with emulsions of garlic essential oil (Fig. 2), it seemed important to consider the volatility of the oil and the sensitivities of its components to oxidizing agents (Yang et al. 2001), which can cause their persistence to be low in the soil. These characteristics are not appropriate for a non-volatile nematicide, but are excellent for a fumigant, which can reduce the nematode population in the field before planting. Therefore, the in vitro effect of vapours from garlic essential oil on M. incognita J2 was also studied, resulting in values that were statistically equivalent to those obtained for the commercial fumigant nematicide Dazomet (Fig. 3). This result is important, mainly because the vapour of the essential oil could affect nematode eggs, which corresponds to the major part of the nematode population under field conditions (Evans and Perry 2009).
Effect of vapour from garlic essential oil (100 μL) on motility, mortality and hatching of Meloidogyne incognita second-stage juveniles (J2). Water and Dazomet (80 mg) were used as negative and positive controls, respectively. Error bars correspond to standard deviations. Results for each parameter are mean values of three experiments with six repetitions for each of them. Columns of the same colour with the same letter do not differ statistically according to the Scott-Knott test (P ≤ 0.05)
Effect of garlic essential oil vapour on the infectivity and reproduction of M. incognita in tomato plants
To further evaluate the potential of garlic essential oil as a fumigant nematicide, it was used in an in vivo assay with eggs of the nematode. At 0.2 mL (L of the substrate)−1, this oil reduced the numbers of galls and eggs of M. incognita to values that were statistically equivalent to those obtained with the commercial nematicide Dazomet at 0.25 g (L of the substrate)−1. In addition, the oil had the advantage of having no apparent phytotoxic effect, since root weights of treated plants were statistically the same as those of the water-treated plants, while root weights of plants treated with Dazomet were smaller (Fig. 4). These results corroborate the potential of the garlic essential oil to be used as a fumigant nematicide.
Number of galls and eggs of Meloidogyne incognita per tomato plant cultivated in substrate inoculated with eggs of the nematode and treated with garlic essential oil at 0.5, 0.2 and 0.1 mL (L of substrate)−1. Dazomet at 0.25 g (L of the substrate)−1 and water were used as controls. The mass of fresh root is also presented. Results are mean values of two experiments with six repetitions each. Error bars correspond to standard deviations. Columns with the same letter in each graphic do not differ statistically according to the Scott-Knott test (P ≤ 0.05)
To summarize, when dissolved in water, the essential oil obtained from garlic bulbs was more active in vitro against M. incognita J2 and eggs than the commercial nematicide Carbofuran. This property can be attributed to DADS and DATS, which reveal a synergistic effect when combined that makes such substances considerably more active than Carbofuran. Other substances in the oil appear to interfere with the activity of DADS and DATS, which, even at the same concentrations as those in the oil, are more active than the oil. When in solution, the oil reduced the infectivity and reproduction of M. incognita in tomato plants. The vapour of garlic essential oil was also active against M. incognita in vitro and reduced the infectivity and reproduction of the nematode. These reduced levels were statistically similar to those observed for the commercial fumigant nematicide Dazomet when the substrate inoculated with M. incognita eggs was treated with the oil and used to cultivate tomato plants. This result suggests that prior to planting tomatoes, garlic essential oil or its components can be used as a fumigant to reduce the nematode population.
References
Adams, R. P. (2007). Identification of essential oil components by gas chromatography/mass spectrometry. Illinois: Allured Publ. Corp. Carol Stream.
Al-Shalaby, M. E. M. (2009). The use of garlic extracts for biocontrol of Meloidogyne incognita infecting cucumber plants. International Journal of Nematology, 19(2), 208–214.
Amagase, H., Petesch, B. L., Matsuura, H., Kasuga, S., & Itakura, Y. (2001). Intake of garlic and its bioactive components. The Journal of Nutrition, 131(3), 955S–962S. https://doi.org/10.1093/jn/131.3.955S.
Amaral, D. R., Oliveira, F. E. R., & Oliveira, D. F. (2003). Purification of two substances from bulbs of onion with nematicidal activity against Meloidogyne exigua Goeldi. Nematology, 5(6), 859–864. https://doi.org/10.1163/156854103773040763.
Anastasiadis, I., Kimbaris, A. C., Kormpi, M., Polissiou, M. G., & Karanastasi, E. (2011). The effect of a garlic essential oil component and entomopathogenic nematodes on the suppression of Meloidogyne javanica on tomato. Hellenic Plant Protection Journal, 4(1), 21–24.
Andrés, M. F., González-Coloma, A., Sanz, J., Burillo, J., & Sainz, P. (2012). Nematicidal activity of essential oils: a review. Phytochemistry Reviews, 11(4), 371–390. https://doi.org/10.1007/s11101-012-9263-3.
Barbosa, P., Lima, A. S., Vieira, P., Dias, L. S., Tinoco, M. T., Barroso, J. G., Pedro, L. G., Figueiredo, A. C., & Mota, M. (2010). Nematicidal activity of essential oils and volatiles derived from Portuguese aromatic flora against the pinewood nematode, Bursaphelenchus xylophilus. Journal of Nematology, 42(1), 8–16 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3380513/.
Barros, A. F., Campos, V. P., Silva, J. C. P., López, L. E., Silva, A. P., Pozza, E. A., & Pedroso, L. A. (2014). Tempo de exposição de juvenis de segundo estádio a voláteis emitidos por macerados de nim e de mostarda e biofumigação contra Meloidogyne incognita. Nematropica, 44(2), 190–199.
Block, E. (2010). Garlic and other alliums: The Lore and the science (First ed.). Cambridge, UK: Royal Society of Chemistry.
Cetintas, R., & Yarba, M. M. (2010). Nematicidal effects on five plant essential oils on the southern root-knot nematode, Meloidogyne incognita race 2. Journal of Animal and Veterinary Advances, 9(2), 222–225. https://doi.org/10.3923/javaa.2010.222.225.
Chaubey, M. K. (2016). Fumigant and contact toxicity of Allium sativum (Alliaceae) essential oil against Sitophilus oryzae L. (Coleoptera: Dryophthoridae). Entomology and Applied Science Letters, 3(2), 43–48.
Chekki, R. Z., Snoussi, A., Hamrouni, I., & Bouzouita, N. (2014). Chemical composition, antibacterial and antioxidant activities of Tunisian garlic (Allium sativum) essential oil and ethanol extract. Mediterranean Journal of Chemistry, 3(4), 947–956. https://doi.org/10.13171/mjc.3.4.2014.09.07.11.
Chen, S. Y., & Dickson, D. W. (2000). A technique for determining live second-stage juveniles of Heterodera glycines. Journal of Nematology, 32(1), 117–121 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2620428/.
Chung, I., Kwon, S. K., Shim, S. -T., & Kyung, K. H. (2007). Synergistic antiyeast activity of garlic oil and allyl alcohol derived from alliin in garlic. Journal of Food Science, 72(9), M437–M440. https://doi.org/10.1111/j.1750-3841.2007.00545.x.
Clevenger, J. F. (1928). Apparatus for the determination of volatile oil. Journal of the American Pharmaceutical Association, 17(4), 346–349.
Corzo-Martinez, M., & Villamiel, M. (2007). Biological properties of onions and garlic. Trends in Food Science & Technology, 18(12), 609–625. https://doi.org/10.1016/j.tifs.2007.07.011.
El-Saedy, M. A. M., Mokbel, A. A., & Hammad, S. E. (2014). Efficacy of plant oils and garlic cultivation on controlling Meloidogyne incognita infected tomato plants. Pakistan Journal of Nematology, 32(1), 39–50.
El-Sayed, H. S., Chizzola, R., Ramadan, A. A., & Edris, A. E. (2017). Chemical composition and antimicrobial activity of garlic essential oils evaluated in organic solvent, emulsifying, and self-microemulsifying water based delivery systems. Food Chemistry, 221, 196–204. https://doi.org/10.1016/j.foodchem.2016.10.052.
Evans, A. A. F., & Perry, R.N. (2009). Survival mechanisms. In: R. N. Perry, ,M. Moens, & J. L. Starr (Eds). Root-knot nematodes (pp. 201–222). Wallingford: CABI Publishing.
FAO (2016). Crops. http://www.fao.org/faostat/en/#data/QC/visualize. Accessed 01 November 2018.
Faria, J. M. S., Barbosa, P., Bennett, R. N., Mota, M., & Figueiredo, A. C. (2013). Bioactivity against Bursaphelenchus xylophilus: Nematotoxics from essential oils, essential oils fractions and decoction waters. Phytochemistry, 94, 220–228. https://doi.org/10.1016/j.phytochem.2013.06.005.
Ferhat, M. A., Meklati, B. Y., & Chemat, F. (2007). Comparison of different isolation methods of essential oil from Citrus fruits: cold pressing, hydrodistillation and microwave ‘dry’ distillation. Flavour and Fragrance Journal, 22(6), 494–504. https://doi.org/10.1002/ffj.1829.
Ferreira, D. F. (2011). Sisvar: a computer statistical analysis system. Ciência e Agrotecnologia, 35(6), 1039–1042. https://doi.org/10.1590/S1413-70542011000600001.
Foe, F. M.-C. N., Tchinang, T. F. K., Nyegue, A. M., Abdou, J.-P., Yaya, A. J. G., Tchinda, A. T., Essame, J.-L. O., & Etoa, F.-X. (2016). Chemical composition, in vitro antioxidant and anti-inflammatory properties of essential oils of four dietary and medicinal plants from Cameroon. BMC Complementary and Alternative Medicine, 16, 117. https://doi.org/10.1186/s12906-016-1096-y.
Giannakou, I. O. (2011). Efficacy of a formulated product containing Quillaja saponaria plant extracts for the control of root-knot nematodes. European Journal of Plant Pathology, 130(4), 587–596. https://doi.org/10.1007/s10658-011-9780-8.
Gupta, R., & Sharma, N. K. (1993). A study of the nematicidal activity of allicin – an active principle in garlic, Allium sativum L., against root-knot nematode, Meloidogyne incognita (Kofoid and White, 1919) Chitwood, 1949. International Journal of Pest Management, 39(4), 390–392. https://doi.org/10.1080/09670879309371828.
Hussey, R. S., & Barker, K. R. (1973). A comparison of methods of collecting inocula for Meloidogyne spp. including a new technique. Plant Disease Reporter, 57(12), 1025–1028.
Isman, M. B. (2000). Plant essential oils for pest and disease management. Crop Protection, 19(8–10), 603–608. https://doi.org/10.1016/S0261-2194(00)00079-X.
Jiang, Z., Akhtar, Y., Bradbury, R., Zhang, X., & Isman, M. B. (2009). Comparative toxicity of essential oils of Litsea pungens and Litsea cubeba and blends of their major constituents against the cabbage looper, Trichoplusia ni. Journal of Agricultural and Food Chemistry, 57(11), 4833–4837. https://doi.org/10.1021/jf900274r.
Jones, J. T., Haegeman, A., Danchin, E. G. J., Gaur, H. S., Helder, J., Jones, M. G. K., Kikuchi, T., Manzanilla-López, R., Palomares-Rius, J. E., Wesemael, W. M. L., & Perry, R. N. (2013). Top 10 plant-parasitic nematodes in molecular plant pathology. Molecular Plant Pathology, 14(9), 946–961. https://doi.org/10.1111/mpp.12057.
Júnior, H. M. S., Campos, V. A. C., Alves, D. S., Cavalheiro, A. J., Souza, L. P., Botelho, D. M. S., Chalfoun, S. M., & Oliveira, D. F. (2014). Antifungal activity of flavonoids from Heteropterys byrsonimifolia and a commercial source against Aspergillus ochraceus: in silico interactions of these compounds with a protein kinase. Crop Protection, 62, 107–114. https://doi.org/10.1016/j.cropro.2014.04.012.
Kocić-Tanackov, S., Dimić, G., Lević, J., Tanackov, I., Tepić, T., Vujičić, B., & Gvozdanović-Varga, J. (2012). Effects of onion (Allium cepa L.) and garlic (Allium sativum L.) essential oils on the Aspergillus versicolor growth and sterigmatocystin production. Journal of Food Science, 77(5), 278–284. https://doi.org/10.1111/j.1750-3841.2012.02662.x.
Lahlou, M. (2004). Methods to study the phytochemistry and bioactivity of essential oils. Phytotherapy Research, 18(6), 435–448. https://doi.org/10.1002/ptr.1465.
Martinez-Velazquez, M., Rosario-Cruz, R., Castillo-Herrera, G., Flores-Fernandez, J. M., Alvarez, A. H., & Lugo-Cervantes, E. (2011). Acaricidal effect of essential oils from Lippia graveolens (Lamiales: Verbenaceae), Rosmarinus officinalis (Lamiales: Lamiaceae), and Allium sativum (Liliales: Liliaceae) against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Journal Medical Entomology, 48(4), 822–827. https://doi.org/10.1603/ME10140.
Martins, N., Petropoulos, S., & Ferreira, I. C. F. R. (2016). Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: a review. Food Chemistry, 211, 41–50. https://doi.org/10.1016/j.foodchem.2016.05.029.
Miresmailli, S., Bradbury, R., & Isman, M. B. (2006). Comparative toxicity of Rosmarinus officinalis L. essential oil and blends of its major constituents against Tetranychus urticae Koch (Acari: Tetranychidae) on two different host plants. Pest Management Science, 62(4), 366–371. https://doi.org/10.1002/ps.1157.
Nashwa, F. S. M. (2015). Extending shelf life of sunflower oil by aromatization with garlic and onion. Journal of Agroalimentary Processes and Technologies, 21(4), 358–364.
Nasiou, E., & Giannakou, I. O. (2018). Effect of geraniol, a plant-based alcohol monoterpene oil, against Meloidogyne javanica. European Journal of Plant Pathology, 152, 701–710. https://doi.org/10.1007/s10658-018-1512-x.
Ntalli, N. G., & Caboni, P. (2012). Botanical nematicides: a review. Journal of Agricultural and Food Chemistry, 60(40), 9929–9940. https://doi.org/10.1021/jf303107j.
Oliveira, D. F., Costa, V. A., Terra, W. C., Campos, V. P., Paula, P. M., & Martins, S. J. (2019). Impact of phenolic compounds on Meloidogyne incognita in vitro and in tomato plants. Experimental Parasitology, 199, 17–23.
Park, I. K., Park, J. Y., Kim, K. H., Choi, K. S., Choi, I. H., Kim, C. S., & Shin, S. C. (2005). Nematicidal activity of plant essential oils and components from garlic (Allium sativum) and cinnamon (Cinnamomum verum) oils against the pine wood nematode (Bursaphelenchus xylophilus). Nematology, 7(5), 767–774.
R Core Team. (2017). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org.
Ritz, C., Baty, F., Streibig, J. C., & Gerhard, D. (2015). Dose-response analysis using R. PLoS One, 10(12), e0146021.
RStudio Team. (2015). RStudio: Integrated development for R. RStudio, Inc., Boston, MA URL http://www.rstudio.com.
SciDAVis. (2017). http://scidavis.sourceforge.net/.
Scott, A. J., & Knott, M. A. (1974). A cluster analyses method for grouping means in the analyses of variance. Biometrics, 30(3), 502–512. https://doi.org/10.2307/2529204.
Sikora, R. A., & Fernandez, E. (2005). Nematode parasites of vegetables. In M. Luc, R. A. Sikora, & J. Bridge (Eds.), Plant parasitic nematodes in subtropical and tropical agriculture (Second ed., pp. 319–392). Wallingford, UK: CAB International.
Wesemael, W. M. L., Viaene, N., & Moens, M. (2011). Root-knot nematodes (Meloidogyne spp.) in Europe. Nematology, 13(1), 3–16. https://doi.org/10.1163/138855410X526831.
Yang, C. S., Chhabra, S. K., Hong, J.-Y., & Smith, T. J. (2001). Mechanisms of inhibition of chemical toxicity and carcinogenesis by diallyl sulfide (DAS) and related compounds from garlic. The Journal of Nutrition, 131(3), 1041S–1045S. https://doi.org/10.1093/jn/131.3.1041S.
Zhang, W.-P., Ruan, W.-B., Deng, Y.-Y., Gao, Y.-B., et al. (2012). Potential antagonistic effects of nine natural fatty acids against Meloidogyne incognita. Journal of Agricultural and Food Chemistry, 60(46), 11631–11637. https://doi.org/10.1021/jf3036885.
Zhao, N. N., Zhang, H., Zhang, X. C., Luan, X. B., Zhou, C., Liu, Q. Z., Shi, W. P., & Liu, Z. L. (2013). Evaluation of acute toxicity of essential oil of garlic (Allium sativum) and its selected major constituent compounds against overwintering Cacopsylla chinensis (Hemiptera: Psyllidae). Journal of Economic Entomology, 106(3), 1349–1354.
Zhou, L., Yuen, G., Wang, Y., Wei, L., & Ji, G. (2016). Evaluation of bacterial biological control agents for control of rootknot nematode disease on tomato. Crop Protection, 84, 8–13. https://doi.org/10.1016/j.cropro.2015.12.009.
Acknowledgements
The authors gratefully acknowledge financial support and fellowships from: Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
The authors declare this is not applicable for this paper.
Informed consent
The authors declare that this is not applicable for this paper.
Rights and permissions
About this article
Cite this article
Jardim, I.N., Oliveira, D.F., Campos, V.P. et al. Garlic essential oil reduces the population of Meloidogyne incognita in tomato plants. Eur J Plant Pathol 157, 197–209 (2020). https://doi.org/10.1007/s10658-020-02000-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-020-02000-1