Abstract
Terpenes are secondary metabolites produced in different biological pathways as defense mechanisms against, among others, nematodes, insects and herbivores. Root-knot nematodes (RKN; Meloidogyne spp.) are one of the most serious pests of economically importance for vegetable production in Greece. The aim of this study was to investigate the nematicidal activity of geraniol on different life stages at 35–1000 ppm doses against the root-knot nematode Meloidogyne javanica. To our knowledge, this study is the first to report the effect of geraniol on egg differentiation and also its sub-lethal doses effect. Experiments testing the contact action of geraniol resulted in about 100% mortality of J2 s, whereas vapor showed only about 10%. Particularly, geraniol paralyzed 100% of second-stage juveniles (J2 s) and inhibited egg hatching in about 70%, at a dose of 500 ppm. In pot experiment, the use of geraniol at sub-lethal doses reduced female numbers in tomato roots. To the contrary, no nematostatic effects were observed in paralysis bioassays. The present study strongly demonstrates geraniol’s toxic effect against root-knot nematode Meloidogyne javanica.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Root-knot nematodes (RKN; Meloidogyne spp.) are one of the most widespread and economically important nematode genus (Huang et al. 2006) in vegetable crop production throughout the world. They have a very broad host range parasitizing about 2000 plant species of annual and perennial crops, which can cause major yield losses equivalent to billions worldwide (Chitwood 2003; Bleve-Zacheo et al. 2007). In plastic houses in Greece, tomato, cucumber and pepper are three of the most seriously affected crops in all growing regions. Meloidogyne spp. get also associated with other soil borne pathogens that result in complex diseases, which are more devastating and cause significant crop losses (Back et al. 2002).
In past years, nematicides have been widely used to suppress RKN population densities in vegetable production. However some nematicides are been banned or restricted due to their environmental impact. An example is the methyl bromide, a common fumigant that has been banned since 2005 (Martin 2003). For the ones still in use, their repetitive application in each cropping season reduced their persistence and efficacy while due to enhanced degradation (Karpouzas et al. 2004; Papadopoulou et al. 2016).
In face of the recent EU environmental restrictions, it is necessary to develop ecologically safer control techniques based upon pesticides of natural origin (Faria et al. 2016). Currently, scientists have been seeking alternatives to conventional nematicides, which are usually safer, eco-friendly and minimize the residual effects (Giannakou 2011). Plants are a significant source of phytochemicals, secondary metabolites playing an important role in plant defense against herbivores, pathogens and other interspecies defenses (Ntalli and Menkissoglu-Spiroudi 2011; Ntalli and Caboni 2012). Secondary metabolites are organic compounds which consist of three main groups: terpenenoids, alkaloids and phenolics (Kabera et al. 2014). Terpenes, known for their wide bioactive spectrum, play a significant role by providing the plant with natural protection from bacteria, fungus, insects, nematodes and other environmental stresses (Lee et al. 2002; D'Addabbo et al. 2014; Yu et al. 2015; Nasiou and Giannakou 2017). Among these, geraniol has been demonstrated to possess interesting antimicrobial (Melo et al. 2015), antioxidant (Aytac et al. 2016), antifungal (López-Meneses et al. 2017), insecticidal (Ali et al. 2013) and nematicidal (Ibrahim et al. 2006; Ntalli et al. 2010) properties.
Geraniol is an alcohol monoterpene with two double bonds (Fig. 1), isomeric with linalool, found in the essential oil of more than 160 species of herbs (especially the Cymbopogon genus) (La Rocca et al. 2017). Ntalli et al. (2011) stated that mixtures of terpenes have synergistic impact on Meloidogyne incognita which cause paralysis, and those pairs are trans-anethole/geraniol, trans-anethole/eugenol, carvacrol/eugenol, and geraniol/carvacrol. Echeverrigaray et al. (2010) reported that geraniol reduced effectively the number of galls at concentration of 100 mg/kg, and completely inhibited gall development at 250 mg/kg.
Specifically, this study aimed to assess: (1) the nematicidal and nematostatic activity of geraniol on second stage juveniles (J2 s) of M. javanica, (2) the differentiation of geraniol inhibition on undifferentiated eggs, (3) the hatch inhibition activity of geraniol in egg masses, (4) the effect of contact and vapor activity of geraniol in nematode infested soil, and (5) the sub-lethal doses of geraniol on nematodes infecting tomato plants.
Materials and methods
Nematodes
The population of M. javanica was collected from infested tomato greenhouses in Heraklion, Crete and then reared on tomato seedlings (Solanum lycopersicum L.) cv. Belladonna in a greenhouse in the Agricultural University of Athens, Greece (25 ± 2 °C, 16 h light and 8 h dark). The seedlings used for inoculations were 6 weeks old, at the four leaf stage. After 40 days, the inoculated plants were uprooted and the galled roots with mature egg masses were gently washed free of soil and cut into 2 cm small pieces. Eggs of M. javanica were extracted using 1% sodium hypochlorite solution (Hussey and Barker 1973). Second stage juveniles (J2 s) were obtained by placing sterilized eggs on a Baermann funnel at the ambient temperature (27 ± 1 °C). The J2 s were used in the experiments were less than 2 days old.
Juveniles motility bioassays
Nematicidal activity
All bioassays were performed in Cellstar® flat bottom 24-well plates. Solutions of geraniol were tested for J2 motility at the doses of 62.5, 125, 250, 500 and 1000 ppm. Geraniol (Merck; Germany), indicated by the chemical formula C10H18O, was dissolved in ethanol (1%) (Sigma-Aldrich; Italy) to overcome the solubility problems and then serially diluted in distilled water containing 0.3% Tween-20 to prepare test solutions of the above doses. Nematodes exposed at these concentrations of ethanol and Tween-20 were not affected, as preliminary tests and previous work indicate (Ntalli et al. 2010). As controls we used distilled water and a mixture of water with ethanol and Tween-20 at concentrations as the ones in the treatment wells. Approximately 40 J2 s were used per treatment well in plates which were exposed to geraniol solutions. The plates were covered with a lid to diminish terpene volatilization, wrapped with aluminum foil to obtain total darkness and incubated at 26 ± 1 °C. Juveniles in the wells were observed with the aid of an inverted microscope (Zeiss, Germany) at 100× magnification after 12, 24, 48, and 96 h and were ranked into two distinct categories: motile or paralyzed. For a window of 10 s we checked the juveniles for motility by probing them with a needle. Lack of movement was considered a strong indication of paralysis. Each treatment was replicated five times, and the experiment was conducted twice.
Nematostatic activity
Geraniol was dissolved in ethanol and diluted serially in distilled water containing Tween-20 resulting in solutions at the doses of 62.5, 125, 250, 500 and 1000 ppm. These test solutions were placed in 250-ml Erlenmeyer flasks into which newly hatched J2 s were immersed subsequently (approximately 1200 J2 s per flask in 100 ml). The high number of nematodes in each flask made the need of oxygen high. For that reason, each flask was accommodated with oxygen supply through a plastic tube connected to an air pump. Evaporation was avoided by covering the flasks with cotton plug and incubating at 26 ± 1 °C temperature settings in dark. A solution of water with ethanol and Tween-20 at the same concentrations as the ones in the treatment flasks as well as distilled water were used as control.
After 12 h, two solutions of 5 ml were removed from each flask and used independently. The first one was placed into wells (approximately 40 J2 s per well) where the J2 s were observed under an inverted microscope (100×) and ranked as motile or paralyzed. For the second one, we placed the J2 s into wells with approximately 30 J2 s per well, after getting rid of the geraniol by rinsing it with tap water and using a 38 μm sieve. Motile and paralyzed J2 s were counted under an inverted microscope (100×) after 12 h. After evaluation, all J2 s were maintained in wells covered with a lid to avoid evaporation. The same procedure was repeated after 24 and 48 h as described above. If any J2 regained motility, the effect was considered as nematostatic (non permanent paralysis). Each treatment was replicated five times, and the experiment was conducted twice.
Effect of geraniol
Egg differentiation
Following the procedure described by Hussey and Barker 1973, we used hypochlorite solution to extract Meloidogyne javanica eggs, from tomato roots (Solanum lycopersicum cv. Belladona) that were infected with the nematode. The suspension of eggs was sieved through 53 and 38 μm, rinsed thoroughly with tap water to become clearer and was collected into a 100 ml beaker. The suspension of eggs was quantified by the aid of an inverted microscope (100×), the number of eggs per ml was adjusted and used directly in the bioassays.
Solutions of geraniol were tested on development of eggs at the doses of 62.5, 125, 250, 500 and 1000 ppm. We used Tween-20 in water to bring geraniol to the desired volume after we dissolved it in ethanol (as previously described). As controls, we used distilled water and water with ethanol plus Tween-20 at the same concentrations as those in the treatment wells. An average of 50 eggs were used in each well, exposed to geraniol solutions and incubated at 26 ± 1 °C. Note that the 90% of those were undifferentiated. To prevent evaporation, we covered both treated and control plates with a lid.
The number of eggs developed and J2 s emerged were counted every 7 days under an inverted microscope (100×) (Zeiss, Germany) (Tzortzakakis and Trudgill 2005). For monitoring egg development, eggs were observed on day 0 with the aid of an inverted microscope and were distinguished either as differentiated (fully developed juvenile) or undifferentiated (eggs containing only cells).
After three weeks the experiment was terminated since no further egg differentiation was observed in the control treatment. Each treatment was replicated four times, and the experiment was conducted twice.
Hatching of J2 s
Mature egg masses were handpicked using sterilized forceps from roots previously rinsed thoroughly and placed in small plastic extraction trays made by six cm Petri dishes. Solutions of geraniol (62.5, 125, 250, 500 and 1000 ppm), initially dissolved in ethanol and brought to volume using Tween-20 in water (as above), were added to each extracting tray to cover egg masses. The latter were maintained for seven days and then test solutions were removed by washing them with tap water and placed in extracting trays filled with clean water. The extracting trays were covered to avoid loss of water and placed in incubator at 26 ± 1 °C. Hatching J2 s were counted every seven days, they were discarded and the water was renewed with fresh one. We counted the number of J2 s emerging over five weeks and terminated the experiment when no more J2 s emergence was observed. Each treatment was replicated five times, and the experiment was conducted twice.
Contact and vapor action
We collected sandy soil from Gargalianoi village in Messinia, an area in southern Greece. To clean soil from debris we passed it through a 2-mm sieve and subsequently we sterilized it for 20 min in an autoclave at 100 °C. The soil was also oven dried at 50 °C for 24 h to determine the water holding capacity (MWHC).
Plastic pots (7 cm deep and 5 cm diameter) were inoculated with a nematode suspension of approximately 500 J2 s after they were first filled with 40 g of soil each. In half of them, we replaced the plastic bottom with a plastic mesh net (size 1 mm) and added plain water (vapor mortality bioassay). To the ones left with the plastic bottom, we added genariol solutions (contact mortality bioassay). Subsequently, we arranged the pots in pairs, where a pot with a mesh net was placed on top of the one with a plastic bottom (contact-vapor mortality bioassay). To prevent moisture loss and minimize light effect, the pots were sealed with parafilm and covered with aluminum foil. Migration of the nematodes between the two pots was avoided by not allowing the upper pot to touch the soil surface of the bottom pot. The moisture content of the soil never exceeded the 20% of MWHC.
The efficacy of geraniol against nematodes was tested in a number of concentrations. i.e. 62.5, 125, 250, 500 and 1000 ppm. Geraniol stock solution was prepared in ethanol and Tween-20 (0.6%) to overcome insolubility, whereas distilled water with Tween-20 (0.6%) was used for further dilutions according to the above method. Pots under control treatment contained only soil with certain moisture and J2 s. The above experimental procedure was performed at two levels of temperature (20 °C and 30 °C).
For both temperatures, the plastic pots remained in climate room for three days. For the experiment at the 30 °C, the plastic pots were placed on a metal tray with dimensions 60 cm × 40 cm × 8 cm (L × W × H). At the bottom of the metal tray a flexible silicone resistance was placed which was connected with an automatic thermostat. Throughout the experiment the tray was filled with wet sand and plastic pots were immersed in the sand. After three days, in both temperature levels, nematodes were extracted from the soil of each pot of each treatment by a modification of Cobb’s decanting and sieving methods (Flegg 1967), as suggested by Brown and Boag (1988). Juveniles were allowed to pass through a modified Baermann funnel at 26 ± 1 °C for two days and, after collection, they were observed with the aid of an inverted microscope (Zeiss, Germany) at 100× magnification. Each treatment was replicated four times, and the experiment was conducted twice. Before statistical analysis, all numbers were calculated according to the Abbot (1925) formula: Efficacy % = ((mortality in treatment - mortality in control)/(100-mortality in control))*100.
Pot experiment
Effect of sub-lethal doses of geraniol on juvenile invasion
To evaluate the efficacy of the geraniol against the nematode invasion we used tomato seedlings, cv. Belladona, grown in plastic pots (10 cm deep and 6 cm diameter). All seedlings were 6 weeks old and at the four leaf stage. Four 250-ml Erlenmeyer flasks were prepared containing 100 ml of geraniol solutions at concentrations of 35, 70, 140 and 280 ppm, respectively. Fifteen thousand newly hatched J2 s were immersed in the flasks and incubated at 26 ± 1 °C. Geraniol was dissolved in ethanol (Sigma-Aldrich; Italy) and serially diluted in distilled water containing Tween-20 to produce test solutions of the above doses. Distilled water as well as a solution of water with ethanol and Tween-20 at concentrations equivalent to those in the treatment flasks, served as control. After 24 h, a 1/3 suspension from each flask was removed and washed gently with tap water to rinse out the excess of geraniol. Sieve of mesh size 38 μm was used for the transfer. Then, the nematodes were transferred to the wells of a 24-well plate and using an inverted microscope (Zeiss, Germany) they were categorized as motile or paralysed. The remaining J2 s in the flasks were maintained for another 48 and 96 h when the same procedure was repeated.
We infected the tomato plants of each pot, prepared as described earlier, with 300 motile J2 s M. javanica solution in flasks. The plants were uprooted and the stems removed after leaving the pots in a growth room at 26 ± 2 °C for 30 days. Roots were carefully washed free of soil and stained with acid fuchsin solution as described in Byrd et al. (1983). We prepared equal volumes of glycerol and distilled water and poured them in vials where the roots were put after rinsing. We counted the female nematodes in the whole root system of the plants using a stereoscopic microscope and choosing the12.5 × magnification setting. Each treatment was replicated five times, and the experiment was conducted once in a randomized block design.
Statistical analysis
Data were subjected to one-way analysis of variance (ANOVA) using the General Linear Model (GLM). Treatments means were compared using the LSD test. Statistical analysis in all cases was conducted using SAS statistical package (SAS University Edition). Whenever appropriate, two experiments were combined and analysed together if no variation was revealed between data.
Results
Juveniles motility bioassays
Nematicidal activity
The effect of geraniol on J2 s motility of M. javanica is presented in Table 1. The percentage of J2 s immobility increased by increasing the exposure period. Geraniol showed 100% paralysis at the dose of 500 and 1000 ppm after exposure of 12 and 24 h, respectively. Furthermore, at dose of 250 ppm about 30% of J2 s were paralyzed after 96 h exposure, while at doses 125 and 62.5 ppm no significant paralysis was recorded. Similar results were obtained in the second experiment (Table 1). Paralyzed juveniles were found to have a specific shape defined as either straight (I-shape), bent (banana-shape) and L-shape which can separate between paralyzed or motile nematodes.
Nematostatic activity
There was not observed a nematostatic effect in the number of paralyzed J2 s. The death of the nematodes was further confirmed by maintaining all nematodes in wells (Cellstar® 24-well plates) containing tap water for 12, 24, 48 and 96 h after evaluation. The percentage of paralyzed J2 s was similar to the previous experiment testing the nematicidal activity using bioassay (data not shown).
Effect of geraniol
Egg differentiation
The inhibitory effect of geraniol in different doses on eggs differentiation after exposure for 21 days is presented in Table 2. The lower percentage indicates higher efficiency in inhibiting the egg differentiation. All tested doses of geraniol caused some inhibition in egg differentiation. Compared to control treatment (88.9%), geraniol at doses of 250, 500 and 1000 ppm significantly inhibited eggs differentiation. Egg differentiation percentage was 69.5%, 49.2 and 38.9% respectively. Interestingly fewer undeveloped eggs were differentiated treated with 125 ppm geraniol solution resulting in about 24% more undeveloped compared to the control treatment (Table 2).
Hatching of J2 s
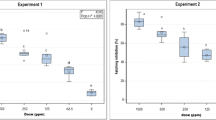
Geraniol demonstrated a significant egg hatch inhibition activity at different doses (Fig. 2) after exposure for 35 days in both experiments. Geraniol at doses of 500 and 1000 ppm exhibited the highest activity inhibiting in about 68 and 78% egg hatching, respectively, as compared to the control. Significantly fewer nematodes were hatched from egg masses treated with 125 and 250 ppm resulting in about 50 and 55% fewer nematodes counted respectively, compared to the control treatment. However, geraniol did not show significant inhibitory effect on hatching of M. javanica egg masses at dose of 62.5 ppm in both experiments (Fig. 2).
Effect of geraniol on M. javanica hatch, after immersion of egg masses at the dose rates of 1000, 500, 250, 125, 62.5 and 0 ppm for 35 days. Values are means of five replicates and two experiments. Samples in boxplots contain a large amount of variation among the treatment means relative to the amount of variation within the treatment. F statistic is defined as: (variation among the treatment means) / (variation among individuals in the same treatment). F value is large indicating that the means are significantly different and is evidence against the null hypothesis that assumes equal means. F is zero only when all group means are the same
Contact and vapor action
Toxic effects on J2 s of geraniol were evaluated by the contact-vapor mortality bioassay (Figs. 3, and 4). It was observed that there was an increase in J2 mortality, as doses were increased from 62.5 to 1000 ppm. The number of J2 s was significantly low in contact bioassay at both levels of temperature. At doses of 500 and 1000 ppm geraniol indicated to have a strong contact mortality of J2 s (about 90 and 97% respectively) in both levels of temperature. On the other hand, lower effect of mortality was recorded at doses of 62.5 and 125 ppm at both levels of temperature, whilst an almost 60% decrease of J2 s in the soil observed in the contact bioassay at a dose of 250 ppm. On the contrary, no vapor toxicity against J2 s of M. javanica observed, regardless the level of temperature.
Effect of geraniol on contact and vapor mortality of M. javanica J2 s in the soil, at the dose rates of 1000, 500, 250, 125 and 62.5 ppm at 20–22 °C. Values are means of combined results from two experiments with 4 replicates each, since no significant differences were observed after ANOVA using the data of both experiments. Error bars represent the standard deviation of mean. Bars with the same letter indicate no significant differences according to LSD test; upper case letters refer to the contact bioassay, lower case letters to the vapor bioassay
Effect of geraniol on contact and vapor mortality of M. javanica J2 s in the soil, at the dose rates of 1000, 500, 250, 125 and 62.5 ppm at 30 °C. Values are means of combined data from two experiments with four replicates each, since no significant differences were observed after ANOVA using the data of both experiments. Error bars represent the standard deviation of mean. Bars with the same letter indicate no significant differences according to LSD test; upper case letters refer to the contact bioassay, lower case letters to the vapor bioassay
Pot experiment
Effect of sublethal doses of geraniol on juvenile invasion
The effect of sublethal doses activity of geraniol against M. javanica is shown in Fig. 5. Geraniol did not significantly decrease the number of females. In comparison with control treatment, it caused the lowest reduction of nematode population on roots in all doses after 24 h exposure (Fig. 5). However, the number of females decreased with increasing exposure time (48 and 96 h). Particularly, at doses of 140 and 280 ppm the number of females decreased to 59 and 56, whereas at control treatment there were 93 females per gram of root, after exposure of 48 h (Fig. 5). Also, there were no statistically significant differences on female number between lower doses of geraniol (35 and 70 ppm) and control treatment after exposure for 48 h. Geraniol at doses of 140 and 280 ppm showed also significant effect of sub-lethal doses activity against M. javanica after exposure of 96 h compared to control treatment (Fig. 5).
Numbers of females of M. javanica per gram of root after immersion in geraniol solutions at the dose rates of 0, 35, 70, 140 and 280 ppm for 24, 48 and 96 h. Error bars represent the standard deviation of mean (n = 5). Bars having the same pattern with the same letter indicate no significant differences according to LSD test (P < 0.001)
Discussion
Geraniol belongs to the category of acyclic alcohol monoterpenes and is found in many different plant essential oils. In this study, our experiments underlined geraniol’s high nematicidal activity against Meloidogyne javanica; a percentage of 99.5% of paralyzed J2 s at a dose of 500 ppm after exposure of 24 h suggested that geraniol has a potential efficacy from a control point of view. Such results agree with those reported by Ntalli et al. (2010) and Al-Banna et al. (2003) in which geraniol paralyzed a high percent of M. incognita J2 s.
The egg stage is the most resistant stage in the nematode’s life cycle, due to its three-layer shell; an outer vitelline, a middle chitinous and an inner lipid (Moens et al. 2009), providing a remarkable shield of protection and rendering it the most important barrier when it comes to breaching and enabling nematicidal activity. To the best of our knowledge, this work is the first one to report the effect of geraniol on egg differentiation in M. javanica. Geraniol showed at higher doses (1000 and 500 ppm) significant inhibition on egg differentiation for M. javanica eggs. Particularly, at doses of 500 and 1000 ppm the majority of isolated eggs remained undifferentiated despite the long incubating period and only 49,1% and 38,9% eggs were differentiated, respectively.
In more details, the increase of the exposure dose, in different geraniol solutions, resulted in a gradual decrease of juveniles hatched from egg masses. Geraniol showed noteworthy anti-hatching activity against M. javanica compared to the control treatment. A decrease of about 69% of juveniles hatched from egg masses was recorded after 35 days at 500 ppm dose. Echeverrigaray et al. (2010) evaluated that geraniol reduced hatching (>75%) at a concentration of 250 mg/l against M. incognita. Egg hatching experiments are more accurate than counting immobile juveniles in a particular nematode population (Oka et al. 2000). In soil, root knot nematode eggs are aggregated within egg masses. These are surrounded by a gelatinous matrix which serve as a protective barrier against soil-borne antagonists (Orion et al. 2001). Therefore, the egg hatch inhibition activity tested on egg masses is an indication of the terpene ability to penetrate the gelatinous matrix and act on nematode eggs.
In the present study, we tested the contact and fumigant effects of geraniol against M. javanica J2 s in nematode infested soil. Our experiments proved that geraniol by its contact activity exhibits strong nematicidal effect against J2 s, inducing about 59 and 96% at a dose of 250 and 1000 ppm compared to the control, at both levels of temperature. However, by examining the fumigant toxicity of geraniol, no significant differences in mortality were recorded compared to the control. In our previous work (Nasiou and Giannakou 2017), we studied the contact and fumigant activity of carvacrol against M. javanica J2 s, which has the same behaviour of biological action as geraniol.
It seems that geraniol has a sub-lethal dose-effect relationship when used to prevent nematode invasion in roots. Most of them remained motile, although there were some juveniles that were unable to locate and infect roots. Geraniol at doses of 140 and 280 ppm reduced the number of females per gram of root compared to the control, after exposure of 48 and 96 h (Fig. 5). These results suggest that the duration of exposure and the concentration of the terpene could disorientate nematodes during root location. However, no effect on the sub-lethal activity of geraniol at concentrations of 35 and 70 ppm was detected (Fig. 5). Walker and Melin (1996) reported that geraniol at 1500 mg oil/kg soil reduced the number of galls caused by M. arenaria.
In the literature, the mechanism of action of monoterpenes is unclear. Experiments focusing on identifying the behavior of terpenes and their effect on nematodes is of high importance and their results can be extensively leveraged for nematode control. They can provide useful information regarding the most appropriate formulation yielding the most desirable nematicidal activity. Work by Oka et al. (2000) has already suggested the use of terpenes as means to not only interrupt the nematode nervous system but also to change the permeability of the cell membrane.
In conclusion, our work showed that the use of geraniol can control M. javanica and prevent nematode population increase in autoclaved soil. Our results underlined the paralysis activity on J2 s together with the inhibition activity in egg differentiation and hatching as such the reduction of female numbers in tomato roots. Further experimental work is needed to determine the most efficient rate and dose of geraniol along with the economic aspects related to its use under field conditions.
References
Al-Banna, L., Darwish, R. M., & Aburjai, T. (2003). Effect of plant extracts and essential oils on root-knot nematode. Phytopatologia Meditarranea, 42(2), 123–128.
Ali, A., Murphy, C. C., Demirci, B., Wedge, D. E., Sampson, B. J., Khan, I. A., Baser, K. H. C., & Tabanca, N. (2013). Insecticidal and biting deterrent activity of rose-scented geranium (Pelargonium spp.) essential oils and individual compounds against Stephanitis pyrioides and Aedes aegypti. Pest Management Science, 69(12), 1385–1392.
Aytac, Z., Yildiz, Z. I., Kayaci-Senirmak, F., Keskin, N. O. S., Tekinay, T., & Uyar, T. (2016). Electrospinning of polymer-free cyclodextrin/geraniol-inclusion complex nanofibers: Enhanced shelf-life of geraniol with antibacterial and antioxidant properties. RSC Advances, 6(52), 46089–46099.
Back, M. A., Haydock, P. P. J., & Jenkinson, P. (2002). Disease complexes involving plant parasitic nematodes and soilborne pathogens. Plant Pathology, 51(6), 683–697.
Bleve-Zacheo, T., Mellilo, M. T., & Castagnone-Sereno, P. (2007). The contribution of biotechnology to root-knot nematode control in tomato plants. Pest Technology, 1(1), 1–16.
Brown, D. J. F., & Boag, B. (1988). An examination of methods used to extract virus-vector nematodes (Nematoda: Longidoridae and Trichodoridae) from soil samples. Nematologia Mediterranea, 16(1), 93–99.
Byrd, D.W., Krickpatrick, T, Barker, K.R. (1983). An improved technique for cleaning and staining plant tissue for detection of nematodes. Journal of Nematology, 15(1), 142–143.
Chitwood, D. J. (2003). Research on plant-parasitic nematode biology conducted by the United States Department of Agriculture-Agricultural Research Service. Pest Management Science, 59(6–7), 748–753.
D'Addabbo, T., Laquale, S., Lovelli, S., Candido, V., & Avato, P. (2014). Biocide plants as a sustainable tool for the control of pests and pathogens in vegetable cropping systems. Italian Journal of Agronomy, 9(4), 137–145.
Echeverrigaray, S., Zacaria, J., & Beltrão, R. (2010). Nematicidal activity of monoterpenoids against the root-knot nematode meloidogyne incognita. Phytopathology, 100(2), 199–203.
Faria, J. M. S., Sena, I., Ribeiro, B., Rodrigues, A. M., Maleita, C. M. N., Abrantes, I., Bennett, R. N., Mota, M., & Figueiredo, A. C. (2016). First report on Meloidogyne chitwoodi hatching inhibition activity of essential oils and essential oils fractions. Journal of Pest Science, 89(1), 207–217.
Flegg, J. J. M. (1967). Extraction of Xiphinema and Longidorus species from soil by a modification of Cobb’s decanting and sieving technique. Annals of Applied Biology, 60(3), 429–437.
Giannakou, I. O. (2011). Efficacy of a formulated product containing Quillaja Saponaria plant extracts for the control of root-knot nematodes. European Journal of Plant Pathology, 130(4), 587–596.
Huang, G., Allen, R., Davis, E. L., Baum, T. J., & Hussey, R. S. (2006). Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential rootknot nematode parasitism gene. Proceedings of the National Academy of Sciences of the United States of America, 103(39), 14302–14306.
Hussey, R. S., & Barker, K. R. (1973). A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant DiseaJse Reports, 57, 1025–1028.
Ibrahim, S. K., Traboulsi, A. F., & El-Haj, S. (2006). Effect of essential oils and plant extracts on hatching, migration and mortality of Meloidogyne incognita. Phytopathologia Mediterranea, 45(3), 238–246.
Kabera, J. N., Semana, E., Mussa, A. R., & He, X. (2014). Plant secondary metabolites: Biosynthesis, classification, Function and Pharmacological Properties. Journal of Pharmacy and Pharmacology, 2, 377–392.
Karpouzas, D. G., Hatziapostolou, P., Papadopoulou-Mourkidou, E., Giannakou, I. O., & Georgiadou, A. (2004). The enhanced biodegradation of fenamiphos in soils from previously treated sites and the effect of soil fumigants. Environmental Toxicology and Chemistry, 23(9), 2099–2107.
La Rocca, V., da Fonsêca, D. V., Silva-Alves, K. S., Ferreira-da-Silva, F. W., de Sousa, D. P., Santos, P. L., Quintans-Júnior, L. J., Leal-Cardoso, J. H., & de Almeida, R. N. (2017). Geraniol Induces Antinociceptive Effect in Mice Evaluated in Behavioural and Electrophysiological Models. Basic & Clinical Pharmacology & Toxicology, 120(1), 22–29.
Lee, B.-H., Lee, S.-E., Annis, P. C., Pratt, S. J., Park, B.-S., & Tumaalii, F. (2002). Fumigant Toxicity of Essential Oils and Monoterpenes Against the Red Flour Beetle, Tribolium castaneum Herbst. Journal of Asia-Pacific Entomology, 5(2), 237–240.
López-Meneses, A. K., Sánchez-Mariñez, R. I., Quintana-Obregón, E. A., Parra-Vergara, N. V., González-Aguilar, G. A., López-Saiz, C. M., & Cortez-Rocha, M. O. (2017). In vitro Antifungal Activity of Essential oils and Major Components against Fungi Plant Pathogens. Journal of Phytopathology, 165(4), 232–237.
Martin, F. N. (2003). Development of alternative strategies for management of soilborne pathogens currently controlled with methyl bromide. Annual Review of Phytopathology, 41, 325–350.
Melo, C. H. D., de Freitas, M. A., Figueiredo, L. G. C. N., Sabino, A. R., de Alencar, F. J. V. & Cosmo, A. J. (2015). In vitro antimicrobial activity of geraniol and cariophyllene against Staphylococcus aureus. Revista Cubana de Plantas Medicinales, 20(1), 98–105.
Moens, M., Perry, N. P., & Starr, L. J. (2009). Root-knot nematodes. Wallingford: CAB International.
Nasiou, E., & Giannakou, I. O. (2017). The potential use of carvacrol for the control of Meloidogyne javanica. European Journal of Plant Pathology, 149(2), 415–424.
Ntalli, N. G., & Caboni, P. (2012). Botanical nematicides in the mediterranean basin. Phytochemistry Reviews, 11(4), 351–359.
Ntalli, N.G. & Menkissoglu-Spiroudi, U. (2011). Pesticides of botanical origin: A promising tool in plant protection. In M. Stoytcheva (Ed.), Pesticides-formulations, effects, fate. Ch. 1, (pp. 3-24). InTech..
Ntalli, N. G., Ferrari, F., Giannakou, I., & Menkissoglu-Spiroudi, U. (2010). Phytochemistry and nematicidal activity of the essential oils from 8 greek lamiaceae aromatic plants and 13 terpene components. Journal of Agricultural and Food Chemistry, 58(13), 7856–7863.
Ntalli, N. G., Ferrari, F., Giannakou, I., & Menkissoglu-Spiroudi, U. (2011). Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest Management Science, 67(3), 341–351.
Oka, Y., Nacar, S., Putievsky, E., Ravid, U., Yaniv, Z., & Spiegel, Y. (2000). Nematicidal activity of essential oils and their components against the root-knot nematode. Phytopathology, 90(7), 710–715.
Orion, D., Kritzman, G., Meyer, S. L., Erbe, E. F., & Chitwood, D. J. (2001). A role of the gelatinous matrix in the resistance of root-knot nematode (Meloidogyne spp.) eggs to microorganisms. Journal of Nematology, 33(4), 203–207.
Papadopoulou, E. S., Lagos, S., Spentza, F., Vidiadakis, E., Karas, P. A., Klitsinaris, T., & Karpouzas, D. G. (2016). The dissipation of fipronil, chlorpyrifos, fosthiazate and ethoprophos in soils from potato monoculture areas: First evidence for the enhanced biodegradation of fosthiazate. Pest Management Science, 72(5), 1040–1050.
Tzortzakakis, E. A., & Trudgill, D. L. (2005). A comparative study of the thermal time requirements for embryogenesis in Meloidogyne javanica and M. incognita. Nematology, 7(2), 313–315.
Walker, J. T., & Melin, J. B. (1996). Mentha piperita, Mentha spicata and effects of their essential oils on Meloidogyne in soils. Journal of Nematology, 28(4S), 629–635.
Yu, D., Wang, J., Shao, X., Xu, F., & Wang, H. (2015). Antifungal modes of action of tea tree oil and its two characteristic components against Botrytis cinerea. Journal of Applied Microbiology, 119(5), 1253–1262.
Acknowledgements
Authors are thankful to Tzortzakakis Emmanuel for the nematode specie speciment.
Author information
Authors and Affiliations
Contributions
Nasiou Eleni is a PhD student, designed and conducted the exrperiments, analyzed data and wrote the manuscript. Ioannis Giannakou is Ms. Nasiou’s supervisor, supervised the experiments and corrected the first draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
There are no ethical issues related to this manuscript.
Rights and permissions
About this article
Cite this article
Nasiou, E., Giannakou, I.O. Effect of geraniol, a plant-based alcohol monoterpene oil, against Meloidogyne javanica. Eur J Plant Pathol 152, 701–710 (2018). https://doi.org/10.1007/s10658-018-1512-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-018-1512-x