Abstract
A nematode survey conducted in 2013 in Algeria, revealed that potato cyst nematodes (PCN) and cereal cyst nematodes (CCN) are widely distributed in several potato and cereal growing regions of the country. Sixteen PCN populations from five localities and five CCN populations from four of these localities were collected and characterized at the morphological and molecular levels. The PCN populations were identified as Globodera rostochiensis and G. pallida occurring separately or in mixed populations. Two species of CCN were detected. Heterodera avenae was found in four localities, whereas H. hordecalis only in one locality in association with H. avenae. The morphological and morphometric identification of PCN and CCN was confirmed by diagnostic ITS-RFLP profiles and sequencing. Phylogenetic analysis of the ITS, D2-D3 expansion domains of the 28S rRNA gene and 18S rRNA gene was made for PCN and CCN populations. Globodera pallida and G. rostochiensis from Algeria show great similarity with European and South American populations. Because of the high divergence among Algerian populations of G. pallida and G. rostochiensis it can be assumed that they were multi-introduced in Algeria. The most divergent population of G. pallida, that formed a well-separated group with some populations from Chile and Peru, suggests a later or independent introduction of this population into Algeria. Heterodera avenae and H. hordecalis formed a well-supported cluster with the corresponding populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyst nematodes, Heterodera spp. and Globodera spp. are important obligate parasites and an economically relevant group of harmful plant parasitic nematodes which are found world-wide (Subbotin et al. 2010a, b). In particular, the potato cyst nematodes (PCN) G. pallida Stone, 1973 and G. rostochiensis (Wollenweber 1923) Skarbilovich, 1959 are a serious threat for the potato industry in many countries (Scurrah et al. 2005) and subject to strict quarantine regulations (Hafez et al. 2008). They are mainly associated with potato production essentially in temperate areas (Mugnièry 1984), but they occur also in the Mediterranean countries were host plants are grown from mid-autumn to spring. The origin of the two species is in the Andean mountains of South America from where they were spread all over the world, possibly together with potato tubers (Plantard et al. 2008). In North Africa, PCN have been reported in Algeria, Egypt, Libya, Tunisia and Morocco (Schlùter 1976; Mai 1977; Hlaoua et al. 2008). In Algeria, PCN have been recorded for the first time in 1953 following the introduction of potato seeds of British origin at the end of the Second World War (Frezal 1954). By 1961, the infested area had increased greatly in 33 townships around Algiers (Scotto La Massese 1961). Thereafter, the plant protection service reported these nematodes in several potato producing regions, the most important of which are Ain Defla, Tipaza, Chlef, Mascara and Sétif.
Cereal cyst nematodes (CCN) are considered as major pest of cereals throughout the world (McDonald and Nicol 2005). The main species parasitizing cultivated cereals are Heterodera avenae Wollenweber, 1924, H. filipjevi Madzhidov, 1981, H. hordecalis Andersson, 1975 and H. latipons Franklin, 1969. Heterodera avenae is the most damaging pest that limits cereals production in the major cereal-producing regions of the world including North Africa (Rammah 1994; McDonald and Nicol 2005; Namouchi-Kachouri and B’Chir 2005; Mokrini et al. 2009). In Algeria, H. avenae was first reported by Scotto La Massese in 1962 and by Lamberti et al. (1975). More recently, this species was recorded in different cereal-producing regions of Algeria (Mokabli et al. 2001; Mokabli 2002; Haddadi et al. 2013).
Heterodera filipjevi occurs in Mediterranean and European countries (Subbotin et al. 2003; Holgado et al. 2004). Heterodera latipons appears as a typical nematode of the Mediterranean region but occurs also in several European countries and elsewhere (Subbotin et al. 2010b). Mokabli (2002) reported this nematode also in Algeria. Heterodera hordecalis is spread less than H. avenae (Subbotin et al. 2010b) and has never been found in Algeria.
In Algeria, precise information on the species of nematodes affecting potatoes and cereals, their relative distribution and population densities, their association with different crops and potential damage in different environmental conditions is needed. This information is instrumental to suggest appropriate integrated management of these nematodes.
The PCN and CCN species can be distinguished by morphological characters, but sometimes, these characters overlap in different populations of these species (Baldwin and Mundo-Ocampo 1991; Subbotin et al. 2000; Manduric et al. 2004; Subbotin et al. 2010a, b). Thus, the morphological identification is not always reliable and, therefore, it must be validated by using molecular approaches (Ibrahim et al. 2001; Abrantes et al. 2004; Subbotin et al. 2011).
Different molecular markers are used for the identification of Globodera and Heterodera species damaging potato and cereals (Abrantes et al. 2004; Thiéry and Mugniéry 1996; Subbotin et al. 2000; Grenier et al. 2001; Skantar et al. 2007; Madani et al. 2008; Quader et al. 2008; Abidou et al. 2005; Subbotin et al. 2011).
In Algeria, there is also little information on the molecular characteristics of these cyst nematodes. Therefore, the aims of this study were: (i) to investigate the occurrence and distribution of PCN and CCN species in Algeria; (ii) to provide an accurate identification of PCN and CCN by using morphological traits and molecular markers (ITS, 28 S and 18 S rDNA gene sequences); (iii) to investigate intraspecific variation between populations of G. rostochiensis and G. pallida and between Heterodera avenae group species; and (iv) to explore the phylogenetic relationships between potato and cereal cyst nematode populations collected in the Ain Defla region, in Algeria, and those occurring in other countries.
Material and methods
Area surveyed
The survey was conducted in the Ain Defla region (Algeria) in localities where potato was rotated with cereals. The Ain Defla region is located in the Midwest of Algeria and is characterized by a semi-arid Mediterranean climate; fine textured soils are predominant (80 %) and more than 45 % of them are represented by clay soils. In Ain Defla, the arable land is around 235,611 ha representing 51.85 % of the total area, with 731 million of kg of potatoes (DSA, Ain Defla 2013); the region ranks second in Algeria in term of potato production.
The Ain Defla region ranked twelth in Algeria in terms of cereal production in 2013 with 187 million of kg.
Nematode sampling and extraction procedures
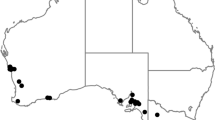
Soil samples were collected with a shovel or sampling tube from potato and cereal fields located in different sites in northern-central provinces of the Ain Defla region (Fig. 1). Cyst nematodes were extracted from soil samples by Fenwick’s apparatus. Specimens were preserved in permanent slides for morphological analysis or heat-killed and kept dry in an Eppendorf tube.
Morphological analyses
Identification of PCN and CCN populations was based on slides of permanent mounts of the perineal and vulval cone regions of cysts and of the measurements of main diagnostic characters of second stage juveniles (J2) according to Hooper (1970) and Wouts and Sturhan (1995). Results of morphological analysis of the cysts were compared with those of the J2 contained in the same cysts. Morphometric characters used were selected according to Golden (1986). Observations were made from live and mounted specimens using a Leitz Diaplan optical microscope equipped with differential interference contrast optics and Leica® DFC 425 camera. Measurements of specimens were made using LAS (Leica Application Suite) Version 3.6.0 software.
Statistical analysis was performed using the STATISTICA program (version 6.1, 2010). Analysis of variance (ANOVA) was applied to assess significant variation in morphological characters among the different populations of CCN and PCN. Two morphometric characters for cysts (Granex’s ratio and number of cuticular ridges between vulva and anus) and one for J2 (stylet length) of PCN were subjected to hierarchical classification and principal component analysis. These three characters were selected for their important diagnostic relevance for the identification of PCN species (OEPP/OEPP 2009, 2013). The morphological characters of CCN vulval cones listed in Table 4 (except presence of bullae and dimensions of the underbridge) were subjected to the same analyses.
Molecular analyses
DNA extraction, PCR amplification and sequencing
Individual cysts were crushed with a sterile micro-spatula under a stereo-microscope and the second stage juveniles were collected. Genomic DNA was extracted from fifteen individual nematodes as described by De Luca et al. (2004). The crude DNA isolated from each individual nematode was directly amplified. The ITS1–5.8S-ITS2 regions were amplified using the forward primer TW81 (5′-GTTTCCGTAGGTGAACCTGC-3′) and the reverse primer AB28 (5′-ATATGCTTAAGTTCAGCGGGT −3′) (Joyce et al. 1994); the 18S rDNA was amplified using the 18SnF (5′-TGGATAACTGTGGTAATTCTAGAGC-3′) and 18SnR (5′-TTACGACTTTTGCCCGGTTC-3′); the D2A-D3B expansion segments of 28S rRNA gene using the primers D2A (5′-ACAAGTACCGTGGGGAAAGTTG-3′) and the D3B (5′-TCGGAAGGAACCAGCTACTA-3′) (Nunn 1992). PCR cycling conditions used for all amplification reactions were: an initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 50 s, annealing at 55 °C for 50 s and extension at 72 °C for 1 min and a final step at 72 °C for 7 min. The size of the amplification products was determined by comparison with the molecular weight marker ladder 100 (Fermentas, St. Leon-Rot, Germany) following electrophoresis of 10 μl on a 1 % agarose gel. PCR products of the ITS containing region, the 18S rRNA gene and the D2-D3 expansion domains from three individual nematodes were purified using the protocol suggested by the manufacturer (High Pure PCR elution kit, Roche, Germany). Purified DNA fragments were cloned (pGEM-T Easy Vector System II, Promega) and sent for sequencing, in both directions, at MWG-Eurofin in Germany.
Phylogenetic analysis
A BLAST (Basic Local Alignment Search Tool) search at NCBI (National Center for Biotechnology Information) was performed in order to confirm their nematode origins and species (Altschul et al. 1997). The newly obtained sequences for ITS containing region, the partial 18S gene and the D2-D3 expansion domains of 28S gene were aligned using ClustalW with default parameters with the corresponding published gene sequences of Globodera and Heterodera species. Sequence alignments were manually edited using BioEdit in order to improve the multi-alignment (Hall 1999). Outgroup taxa for each dataset were chosen according to the results of previously published data (Skantar et al. 2007; De Luca et al. 2013). Phylogenetic trees, obtained for ITS dataset, the partial 18S gene and the D2-D3 expansion domains were performed with Neighbour-Joining (NJ), Minimum Evolution (ME), Maximum Likelihood (ML) and Maximum Parsimony (MP) methods using MEGA version 6 software (Tamura et al. 2013). No significant conflict in branching order and support level among methods was observed and, therefore, only the ML tree is shown for each marker. The phylograms were bootstrapped 1000 times to assess the degree of support for the phylogenetic branching indicated by the optimal tree for each method. The newly obtained sequences were submitted to GenBank with the following accession numbers and shown in Tables 1 and 2.
RFLP analysis
Ten μl of the PCR product obtained using ITS primers for G. rostochiensis and G. pallida were digested with the following restriction enzymes: Alu I (Roche) and Rsa I (Roche) (5 U of enzyme for each digestion) at 37 °C overnight. For Heterodera avenae and H. hordecalis the five restriction enzymes: Alu I, Hae III, Hinf I, Pst I and Rsa I were used. The digested DNA fragments were loaded onto 2.5 % agarose gel and visualized by GelRed (Biotium) fluorescent stain. All gel images were stored digitally.
Results
Nematode sampling
Sixteen populations of PCN were found, mainly occurring in five localities (Table 1, Fig. 1). Five populations of CCN were also found in the same localities (except in Ain Defla) (Table 2, Fig. 1).
Morphological analysis
Measurements of cysts and J2 s for PCN and CCN are reported in Tables 3 and 4.
PCN identification
Morphological and morphometric analysis of the sixteen PCN populations collected from five localities in Ain Defla, identified two nematode species: G. pallida and G. rostochiensis in single or mixed populations (Table 3). Six (ADG3, EAG2, RG2, RG3, MEG and ARG) out of these 16 populations, listed in Table 1, were characterized at the morphological and molecular levels.
Cyst perineal region
Morphometrics of cyst perineal region (Table 3) revealed clear differences between populations of G. rostochiensis and those of G. pallida. The populations from Rouina RG2 and RG3 showed the highest values of vulva-anus distance, number of cuticular ridges between vulva and anus and Granek’s ratio, fitting well within ranges of G. rostochiensis (Subbotin et al. 2010a), whereas populations from Ain Defla ADG3 and El Amra EAG2 were rather close to those of G. pallida (Subbotin et al. 2010a). Mean values of Granek’s ratio and number of cuticular ridges of the populations from Arib ARG and Mekhatria MEG were closer to G. pallida although some overlap occurred with the ranges of G. rostochiensis, indicating that these populations could be mixed. The greatest differences, and to some extent conflicting, were observed in the range of the cuticular ridges between anus and vulva within populations of G. pallida that were 9–16 (ADG3), 8–18 (EAG2), 8–25 (MEG) and 7–27 (ARG). For the MEG and ARG populations, rather large ranges of the Granek’s ratio were observed (2.0–5.5 and 1.3–6.0, respectively) that overlap that for G. rostochiensis. This observation would suggest that they were mixed populations. The MEG population of G pallida had a mean hyaline tail region length of 31.7 ± 2.9 μm (range 28.5–36.5 μm) longer than that of the other populations of G pallida reported in this study and in the literature (Subbotin et al. 2010a).
J2
There were clear differences between morphological characters of J2 belonging to different populations. Analysis of variance showed significant differences for J2 body length (0.024) and c’ ratio (p = 0.018), highly significant for body width of J2 (p = 0.000588), very high meaningful differences for stylet length, body width at anus, tail length, hyaline tail length and c ratio, but for the ratios a and c, the differences were not significant (p = 0.75; p = 0.057 respectively).
Main morphometrics (stylet length and stylet knobs shape) of J2, listed in Table 3 for the populations examined were in agreement with data obtained from cysts and corresponded with data for G. pallida and G. rostochiensis reported in literature (Baldwin and Mundo-Ocampo 1991; EPPO/OEPP 2009).
Two distinct clusters were displayed by the ascending hierarchical classification based on minimal jump method and using the three most important diagnostic characters of Globodera genus (Fig.2). The cluster I is formed by the populations of G. pallida collected in Ain Defla, El Amra, Arib and Mekhatria, the last two localities contained both species. The cluster II contained the two populations of G. rostochiensis from Rouina.
Dendrogram of hierarchical classification of six Algerian PCN populations based on three morphological characters of cysts and second stage juveniles (Cluster1: G. pallida from Ain Defla ADG3, G. pallida from El Amra EAG2, mixed populations from Mekhatria MEG and mixed populations from Arib ARG; Cluster 2: two populations of G. rostochiensis from Rouina RG2 and RG3)
The principal component analysis separated the two populations of G. rostochiensis from Rouina (RG2 and RG3) from those of G. pallida collected from Ain Defla (ADG3) and El Amra (EAG2) (Fig. 3). The mixed populations from Arib (ARG) and Mekhatria (MEG) grouped in the same cluster with G. pallida because of dominance of G. pallida.
CCN identification
Morphology of the cyst vulval cones and J2 of the CCN populations from Rouina, Mekhatria, Arib and El Amra, revealed the presence of H. avenae in all sites sampled (Table 2). However, mixed populations of H. avenae and H. hordecalis were detected at El Amra. The populations ARH, EAH (Ha), EAH (Hh), MEH and RH were further characterized at the morphological and molecular levels.
Cyst vulval cones
Morphology of vulval cones of populations from Rouina, Arib, El Amra and Mekhatria showed typical features of H. avenae such as heavy bullaes, short vulval slit and absence of underbridge. Measurements of fenestral lengths and semi fenestral widths determined from all populations were close to each other. Variations were observed in the width of the vulval bridge of Rouina, Arib and Mekhatria populations (mean values 8.4, 7.6 and 10.1 μm, respectively) (Table 4). Specimens of the mixed population from El Amra fitting the morphology of H. avenae had the smallest width of vulval bridge (5 μm). Other specimens in this mixed population showed much larger fenestra length, larger vulval bridge and the presence of a strong under-bridge enlarged in the middle portion indicating that they were belonging to the species H. hordecalis (Table 4).
The analysis of variance showed very highly significant differences for fenestra length, vulval bridge width and vulval slit length (p < 0.0005) and highly significant difference for semi fenestra width (P = 0.0037) between H. avenae populations and H. hordecalis (Fig. 4).
Dendrogram of hierarchical classification of five Algerian CCN populations based on morphological characters and morphometrics of vulval cones and second stage juveniles (Cluster 1: H. avenae from Rouina, H. avenae from Arib, H. avenae from El Amra (Ha), H. avenae from Mekhatria; Cluster 2: H. hordecalis from El Amra EAH (Hh))
CCN populations were distributed into two clusters by the ascending classification based on minimal jump using selected morphological characters of vulval cones (Fig. 4). Cluster I included H. avenae (EAH (Ha), RH, MEH and ARH) populations, whereas cluster II contained the single population of H. hordecalis EAH (Hh). Similar results were also obtained using the principal component analysis, which discriminated the H. avenae populations from that of H. hordecalis (Fig. 5). The morphological identification of the population of H. hordecalis was confirmed by the results of the molecular analyses reported in the following sections.
J2
Among populations identified as H. avenae (Table 2), J2 from Mekhatria (MEH) showed the smallest body length, width, and c ratio whereas RH and ARH J2 had very similar measurement ranges.
Specimens of J2 in the mixed population from El Amra (EAH) had the shortest stylet, tail, terminal hyaline portion and c’ ratio. These characters were fitting those reported for of H. hordecalis rather than H. avenae (Table 4).
Molecular analyses
PCR-RFLP diagnostics of PCN and CCN populations
Amplification of the ITS containing region, the 18S rRNA gene and the D2-D3 expansion segments of the 28S gene of G. pallida and G. rostochiensis yielded single fragments of 1188 bp, 1628 bp and 700 bp, and 1190 bp, 1628 bp and 700 bp, respectively. Diagnostic PCR-ITS-RFLP profiles for G. pallida and G. rostochiensis from Algeria are given in Fig. 6. The two diagnostic enzymes produced RFLP patterns that allowed discriminating the two Globodera species from Algeria, even for mixed populations. The Alu I (lanes 1, 2, 5 and 7; fragment sizes 505 and 383 bp) digestion of Algerian G. pallida populations showed identical restriction profiles among geographical populations and were identical to those of G. pallida from Peru, UK (York) and USA (Idaho) (Skantar et al. 2007, 2011; Subbotin et al. 2011).
PCR-RFLP profiles for Globodera pallida and G. rostochiensis populations from Algeria digested by using the Alu I and Rsa I enzymes. M: 100 bp ladder DNA; lane 1: G. pallida from Ain Defla; lane 2: G. pallida from El Amra; lane 3: G . rostochiensis from Rouina 1; lane 4: G. rostochiensis from Rouina 2; lane 5: G. pallida from Arib; lane 6: G. rostochiensis from Arib; lane 7: G. pallida from Mekhatria
Rsa I digestion (lanes 1, 2, 5 and 7; fragment sizes 587 and 385 bp) produced a restriction profile for G. pallida populations from Ain Defla and El Amra (lanes 1and 2) identical to that of G. pallida populations from Europe, Asia, North and South America and Oceania, but populations from Arib and Mekhatria showed a polymorphism at Rsa I site resulting in missing restriction sites (lanes 5 and 7).
The Alu I (lanes 3, 4 and 6) and Rsa I (lanes 3, 4 and 6) profiles for Algerian G. rostochiensis populations were identical each other but different from those of G. rostochiensis published in the literature.
PCR amplicons of the the ITS region, the D2-D3 expansion segments of the 28S rDNA and the partial 18S rRNA gene of H. avenae and H. hordecalis produced single fragments of 1057 bp, 700 bp, and 1600 bp and 1142 bp, 700 bp and 1600 bp, respectively. Five restriction enzymes generated ITS-RFLP profiles for H. avenae and H. hordecalis allowing for differentiation of these species even when present as mixed populations (El Amra). Intraspecific polymorphism was not observed within H. avenae and H. hordecalis populations. However, Algerian H. avenae populations contain ITS belonging to the type B because it was restricted by Alu I and Rsa I enzymes (Fig. 7). Furthermore, the restriction profiles with Hae III and Hinf I enzymes of H. avenae, characterized in this study, are identical to those of H. avenae from Algeria reported by Rivoal et al. (2003). These profiles were also typical of H. avenae populations from India, Syria, Turkey, Italy, France and Oregon, but different from those obtained from Tunisian populations.
ITS-RFLP of H. hordecalis from Algeria showed identical profiles of H. hordecalis populations from Scotland and Italy (Subbotin et al. 1999; Madani et al. 2004) but different from those of a population of H. hordecalis from Iran by Tanha Maafi et al. (2003) (Fig. 8).
Phylogenetic analysis
Intraspecific variations among Algerian populations were as follows: G. pallida (six sequences) 7–22 nucleotides; G. rostochiensis (five sequences) 11–20 nucleotides. The most variable populations of G. pallida and G. rostochiensis were those from Mekhatria and Rouina RG3, respectively.
Phylogenetic relationships of G. pallida and G. rostochiensis from Algeria were inferred from analyses of the ITS, 18S and D2-D3 expansion domains sequences of the closest species of cyst nematodes by using ML method. Sequence alignment of the ITS regions included 64 sequences, 11 (six for G. pallida and five for G. rostochiensis from Algeria) of which were newly obtained in this study (Fig. 9). Globodera artemisiae and G. capensis were used as outgroups for the ITS dataset. The ML tree as inferred by the analyses of the ITS rRNA gene sequences shows the phylogenetic relationship of the PCN populations studied. All G. pallida and G. mexicana isolates clustered in a well-supported clade, which contained four groups (I-IV). All G. rostochiensis and G. tabacum isolates clustered also in a separated well-supported clade containing four groups (V-VIII). Globodera artemisiae and G. capensis formed a small clade at the bottom of the tree. The four groups in the G. pallida and G. mexicana clade included: I) G. pallida sequences from Algeria (Ain Defla, Arib and El Amra) that fitted into a highly supported cluster (92 % support) along with all sequences of populations from Australia, Canada, Europe, Peru, and USA (Idaho); II) G. mexicana group as sister species of group I; III) G. pallida sequences from Mekhatria (Algeria), Peru, and Chile; IV) G. pallida from Peru and South Africa isolates at basal position (86 % support) of all G. pallida isolates used in this analysis. The four groups in the G. rostochiensis and G. tabacum clade included: V) a population of G. pallida from Argentina confirming that this isolate could represent a hybrid or a naturally occurring variant closely related to G. rostochiensis; VI) G. tabacum closely related to G. rostochiensis (95 % support); VII) G. rostochiensis from Bolivia, which grouped as sister species of all G. rostochiensis (86 % support); VIII) G. rostochiensis isolates from Algeria, Europe and Canada.
Phylogenetic tree of ITS containing region describing the evolutionary relationships among different geographical populations of PCN (Globodera pallida and G. rostochiensis) using Maximum Likelihood (ML) method. Branch lengths are proportional to the distances as derived from the distance matrix obtained using the GTR method with the invariant site plus gamma options. Numbers at nodes indicate bootstrap values
Sequence alignments of the 18S gene included 31 sequences, seven of which were obtained in this study. The phylogenetic tree of the 18S rRNA gene revealed no clear phylogenetic relationships among the different isolates of G. pallida and G. rostochiensis (unpublished data).
The phylogenetic tree inferred by the analysis of the D2-D3 of the 28S rRNA gene sequences of CCN and a selected number of PCN populations revealed that all sequences of PCN populations clustered in a clade separated from that of the CCN populations (Fig. 11). The G. pallida populations from Algeria grouped with the corresponding sequences of G. pallida from distant geographical areas with high support, and resulted to be closely related to those of G. rostochiensis group (Fig. 11).
Phylogenetic relationships of H. avenae and H. hordecalis from Algeria were inferred from analyses of the ITS rRNA, the 18S and the D2-D3 of the 28S rRNA gene sequences of the closest species of cyst nematodes by using the ML method. Cryphodera brinkmani was used as outgroup.
Forty-five ITS sequences of the CCN populations were aligned. These populations belonged to the H. avenae group and included those (three of H. avenae, two of H. hordecalis and one Heterodera sp. isolate 57) identified in this study. The phylogenetic tree, inferred by the analysis of these ITS rRNA gene sequences contained a major clade with all the CCN populations studied and a small clade with the outgroup Cryphodera brinkmani (Fig. 10). The major clade was clearly divided into four groups. Group I included all H. avenae populations along with those from Algeria, H. filipjevi, H. iri, and H. mani. Group II included all sequences of H. hordecalis along with those from Algeria. Group III included all H. latipons populations. Group IV included, at the basal position of the major clade, the sequence of an unidentified Heterodera sp. isolate 57 from Algeria. In the 18S phylogenetic tree, 25 sequences of CCN, belonging to the H. avenae group, were aligned with C. brinkmani used as outgroup. Two subgroups were observed: subgroup I included all H. avenae populations, H. filipjevi, H. mani and H. hordecalis determined in this study. Subgroup II included all Heterodera sequences from the database including one sequence of H. hordecalis and Heterodera sp. from Algeria (isolate 57) confirming that this isolate does not belong to the H. avenae group (data not shown).
Phylogenetic tree of ITS containing region describing the evolutionary relationships among different geographical populations of CCN belonging to the Heterodera avenae group using Maximum Likelihood (ML) method. Branch lengths are proportional to the distances as derived from the distance matrix obtained using the GTR method with the invariant site plus gamma options. Numbers at nodes indicate bootstrap values
Forty-four sequences of the D2-D3 of 28S rRNA gene obtained from CCN and selected PCN populations were aligned. Twelve of these sequences (six identified as H. avenae, two as H. hordecalis and four as G. pallida) were newly obtained in this study. The D2-D3 phylogenetic tree showed a separation of CCN clade from that containing the PCN populations (Fig. 11). The populations of Heterodera avenae group (CCN) clustered in different groups, which fitted those reported in the literature (Fu et al. 2011; De Luca et al. 2013). In particular, H. avenae and H. hordecalis from Algeria clustered with all Asian, African and European H. avenae group populations (Fig. 11).
Phylogenetic tree of D2-D3 expansion domains describing the evolutionary relationships among different geographical CCN (Heterodera avenae group) and selected PCN (Globodera pallida and G. rostochiensis) populations using Maximum Likely (ML) method. Branch lengths are proportional to the distances as derived from the distance matrix obtained using the GTR method with the invariant site plus gamma options. Numbers at nodes indicate bootstrap values
Discussion
This survey demonstrates that the major potato growing areas in Algeria are commonly infested by G. pallida and G. rostochiensis. Fields in the same areas, previously cropped with cereals, were infested with H. avenae, in particular in El Amra area H. avenae was associated with H. hordecalis in mixed populations.
Our study confirmed that both PCN and CCN are present in regions with temperatures in the higher 20 °C in the high summer. Furthermore our data showed for the first time a mixed occurrence of G. pallida and G. rostochiensis in the same locality in Algeria, with the predominance of one species for each locality. Globodera pallida is dominant in Ain Defla and El Amra, whereas G. rostochiensis is dominant in the Rouina region. In Mekhatria and Arib regions both species were present as mixed populations. The occurrence of mixed populations of G. pallida and G. rostochiensis in the same field was already reported in South America (Evans et al. 1975), in New Zeland (Marshall 1993), in Northern Ireland (Turner 1996), in England and Wales (Ibrahim et al. 2001) and recently in Slovakia (Douda et al. 2014). This finding reinforces the hypothesis by Den Nijs (1992) of the cross-hybridization between these two nematode species that might result in a generation of new genotypes. The incidence of mixed populations of these two Globodera species complicates control measures because of the lack of resistant potato cultivars for G. pallida, in contrast for G. rostochiensis nearly all contemporary potato cultivars are resistant or tolerant to this species.
Perineal patterns of cysts and J2 stylet characteristics distinguished PCN species, but sometimes the diagnostic characters may overlap among various populations of the different species (Baldwin and Mundo-Ocampo 1991). To confirm the identification of PCN based on the morphology, molecular analyses were carried out to specifically identify mixed populations and to get precise information about polymorphism of G. pallida and G. rostochiensis populations from Algeria. ITS-RFLP analysis revealed species-specific profiles for G. pallida and G. rostochiensis from Algeria. The comparison of the ITS-RFLP profiles of G. pallida populations from Algeria by using Rsa I and Alu I enzymes with those obtained in other studies revealed the presence of additional bands (ITS haplotypes) that may result from a higher level of rRNA gene heterogeneity or from the presence of a natural hybrid between species occurring in the same area. In our study, G. pallida from Mekhatria and Arib localities showed identical restriction profiles by using Rsa I but different from those of G. pallida from Ain Defla and El Amra regions. Heterogeneity in ITS of Peruvian G. pallida populations was already reported by RFLP analysis and several restriction enzymes discriminate European and Peruvian populations (Grenier et al. 2001). Globodera rostochiensis populations from Algeria showed identical restriction profiles but differed from other populations of G. rostochiensis reported in literature (Subbotin et al. 2000). The heterogeneity found in G. pallida and G. rostochiensis from Algeria may be due to an incomplete process of homogenization among the different ribosomal repeats and thus the rDNA repeats in their genomes are present as a mixture of haplotyes with different sequences.
The phylogenetic relationships by using ITS sequences revealed that all European G. pallida isolates and those from Ain Defla, Arib and El Amra clustered in the subgroup I together with some Peruvian isolates (P4A, Cusco, Puno and Amantani). The G. pallida isolate from Mekhatria resulting the most divergent isolate of G. pallida from Algeria, grouped instead in the subgroup III together with isolates of G. pallida from different areas of Chile and Peru. As the two groups containing Algerian G. pallida isolates are well supported, each related to different South American populations (Peru and Chile), a multi-introduction origin of Algerian G. pallida from Europe and South America could be hypothesized. In the phylogenetic tree, the position of G. pallida isolates from Mekhatria, in the group III, and Arib, in the group I, is intriguing because although morphometric values are in agreement with those of G. pallida, some overlap occurs with G. rostochiensis. The morphometrics and morphology of the J2 of both populations fit well within the ranges known for G. pallida. The phylogenetic analysis revealed that Mekhatria and Arib populations could represent a naturally occurring variants that diverged from the European and some Peruvian G. pallida isolates or, in the case of Mekhatria, could represent a genetically intermediate population. Our data confirm that G. pallida may represent a species complex as already reported (Madani et al. 2010; Subbotin et al. 2011) and the possible existence of a third PCN species regarding some G. pallida populations from South America, characterized by higher sequence variability compared with other G. pallida populations, included in the subgroups III and IV.
In contrast to G. pallida, a lower variability was displayed by ITS sequence diversity among G. rostochiensis populations from Algeria and from all over the world. In the phylogenetic tree, all populations of G. rostochiensis from Europe, Canada and Algeria formed a supported cluster (with 93 % support) with four subgroups, even some relationships among G. rostochiensis sequences were unresolved. Globodera rostochiensis populations from Algeria contain a mixture of ITS haplotypes that clustered in different groups suggesting that the process of homogenization among repeats is still incomplete as shown by RFLP analysis. This finding also suggests that the presence of G. rostochiensis in Algeria is the result of multiple introductions of European isolates rather than of direct import of infected potatoes from Latin America. The phylogenetic tree of the 18S rRNA gene revealed that the origin of both species in Algeria was consistent with a bush-like radiation as no clear phylogenetic relationships was revealed between the different haplotypes of G. pallida and G. rostochiensis.
A previous survey of CCN conducted by Mokabli (2002) recorded the occurrence of H. avenae and H. latipons in other regions of Algeria such as Setif, Béjaia, Relizane, Dar El Beida and Mascara. Our survey reveals that H. avenae is more spread than previously reported and H. hordecalis is recorded for the first time in Algeria and at present only in El Amra region. The use of the enzyme Rsa I in the PCR-RFLP analyses clearly identified H. avenae from Algeria as type B also reported from Iran (Tanha Maafi et al. 2007), from Turkey (Abidou et al. 2005), from Italy (De Luca, unpublished data). Furthermore Alu I profile revealed heterogeneity of the ITS region among several specimens from different Algerian H. avenae populations as reported for Iranian H. avenae (Tanha Maafi et al. 2010). The Alu I, Pst I and Rsa I patterns of the Algerian H. hordecalis population were identical to those from the Northern Europe and Iran but different from those of the Italian population reported by Subbotin et al. (2000) and Tanha Maafi et al. (2003). Furthermore a high level of sequence divergence between those populations of H. hordecalis was already observed (Madani et al. 2004). Species identification of H. avenae and H. hordecalis based on combinations of Hinf I, Hae III and Rsa I enzymes and sequencing of the ITS, 18S gene and D2-D3 expansion domains was congruent with the morphological and morphometrical data of these species (Subbotin et al. 2000; Tanha Maafi et al. 2010) and resulted useful for species identification. The ML tree based on the ITS sequences revealed that both H. avenae and H. hordecalis from Algeria grouped with populations of the same species from different geographical areas. However, H. hordecalis from Algeria formed a well-supported group close to that of H. latipons confirming that these species constitute a species complex within the H. avenae group as suggested by Rivoal et al. (2003). The Heterodera sp. isolate 57 from Algeria grouped at basal position of the phylogenetic tree revealing that this is a different species related to the H. avenae group, suggesting further investigation for its correct identification.
The D2-D3 sequences were obtained for the first time for H. hordecalis. Phylogenetic relationships inferred using the D2-D3 expansion domains showed clades similar to those of the ITS tree. Heterodera hordecalis from Algeria formed a well-supported group closely related to H. avenae group sensu stricto, while H. latipons populations grouped together at basal position of the H. avenae group and H. hordecalis.
In conclusion, the correct and rapid identification of PCN and CCN is essential for their control as they can be present as mixtures. This study has elucidated the geographical distribution and the genetic variation of Heterodera and Globodera species in Algeria and has provided accurate molecular tools for the identification of PCN and CCN in the country. Furthermore, the occurrence of Heterodera and Globodera species in the same localities in Algeria is reported for the first time so far, suggesting that the cultural conditions, especially rotation type based on cereals-potato in these localities may be the cause of the presence of both nematode species as mixture in the same fields.
Cereal cyst nematodes, especially H. avenae, are very damaging in rather finely structured soils and mild climates. In Algeria these conditions occur in the regions bordering the Mediterranean sea and, therefore, as reported from Tunisia (Namouchi-Kachouri and B’Chir 2005), severe yield losses of cereals must be expected whenever the soil population densities of these nematodes exceed the tolerance of the host crop, estimated in 1.3 eggs/g soils. Potato cyst nematodes are well adapted to the same environmental conditions although they would tolerate also less finely structured soils. These nematodes are expected to severely affect potato when the soil population densities exceed 1.9 eggs/g soil, as reported in Italy (Greco et al. 1982). The control of cyst nematodes should be based mainly on the use of environment friendly methods, such as the use of appropriate crop rotations by intercropping a leguminous crop between cereal and potato and by adding resistant cultivars if available. This requires information on the soil population densities of different nematodes, precise identification to species level and identification of the race/pathotype. Haddadi et al. (2013) reported the reaction to some Algerian populations of H. avenae of several cereal host plants of the international host test range used to identify races of the nematode. This study should be extended also to populations from other Algerian regions, because there is no information on the pathotypes of the potato cyst nematode populations. Such information is necessary to select potato cultivars carrying the correct resistance genes for nematode control.
References
Abidou, H., Valetten, S., Gauthier, P., Rivoal, R., El-Ahmed, A., & Yahyaoui, A. (2005). Molecular polymorphism and morphometrics of species of the Heterodera avenae group in Syria and Turkey. Journal of Nematology, 37, 146–154.
Abrantes, I. M., De, O., Vieira Dos Santos, M. C., da Conceição, I. L. P. M., Cunha, M. J. M. D., Santos, M. S. N., et al. (2004). Biochemical and molecular characterization of plant-parasitic nematodes. Phytopathologia Mediterranea, 43, 232–258.
Altschul, S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Zhang, Z., Miller, W., et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research, 25, 3389–3402.
Andersson, S. (1975). Heterodera hordecalis n. sp. (Nematoda: Heteroderidae) a cyst nematode of cereals and grasses in southern Sweden. Nematologica, 20, 445–454.
Baldwin, J. G., & Mundo-Ocampo, M. (1991). Heteroderinae, cyst and non-cyst-forming nematodes. In W. R. Nickle (Ed.), Manual of agricultural nematology (pp. 275–362). New York: Marcel Dekker.
De Luca, F., Fanelli, E., Di Vito, M., Reyes, A., & De Giorgi, C. (2004). Comparisons of the sequences of the D3 expansion of the 26S ribosomal genes reveals different degrees of heterogeneity in different populations and species of Pratylenchus from the Mediterranean region. European Journal of Plant Pathology, 110, 949–957.
De Luca, F., Vovlas, N., Lucarelli, G., Troccoli, A., Radicci, V., Fanelli, E., et al. (2013). Heterodera elachista the Japanese cyst nematode parasitizing corn in Northern Italy: integrative diagnosis and bionomics. European Journal of Plant Pathology, 136, 857–872.
Den Nijs, L. J. M. F. (1992). Interaction between Globodera rostochiensis and G. pallida in simultaneous infections on potatoes with different resistance properties. Fundamental and Applied Nematology, 15, 173–178.
Douda, O., Zouhar, M., Renčo, M., & Marek, M. (2014). Molecular and morphological of a mixed population of two potato-parasiting nematode species, Globodera rostochiensis and G. pallida. Helminthologia, 51, 3–6.
DSA, Ain Defla (2013)- Statistical data. Direction of Agricultural Services of Ain Defla. Unpublished internal document.
EPPO/OEPP PM 7/40 (2) (2009). Globodera rostochiensis and Globodera pallida. EPPO Bulletin, 39, 354–368.
EPPO/OEPP PM 7/40 (3) (2013). Globodera rostochiensis and Globodera pallida. EPPO Bulletin, 43, 119–111.
Evans, K., Franco, J., & De Scurrah, M. M. (1975). Distribution of species of potato cyst-nematodes in South America. Nematologica, 21, 365–369.
Franklin, M. T. (1969). Heterodera latipons n. sp., a cereal cyst nematode from the Mediterranean region. Nematologica, 15, 535–542.
Frezal, P. (1954). Importance et répercussions de la contamination de l'Algérie par le nématode doré (Heterodera rostochiensis Wooll. [Woll.]. Journal Comptes Rendus des Séances de l'Académie d'Agriculture de France, 40, 71–74.
Fu, B., Yuan, H., Zhang, P., Xing, X., Sun, B., Riley, I. T., et al. (2011). Molecular characterisation of cereal cyst nematodes in winter wheat on the Huang-Huai floodplain of China using RFLP and rDNA-ITS sequence analyses. Australasian Plant Pathology, 40, 277–285.
Golden, A. M. (1986). Morphology and identification of cyst nematodes. In F. Lamberti & C. E. Taylor (Eds.), Cyst Nematodes (pp. 23–45). New York: Plenum Press.
Greco, N., Di Vito, M., Brandonisio, A., Giordano, I., & De Marinis, G. (1982). The effect of Globodera pallida and G. rostochiensis on potato yield. Nematologica, 28, 379–386.
Grenier, E., Bossis, M., Fouville, D., Renault, L., & Mugniery, D. (2001). Molecular approaches to the taxonomic position of Peruvian potato cyst nematodes and gene pool similarities in indigenous and imported populations of Globodera. Heredity, 86, 277–290.
Haddadi, F., Mokabli, A., & Smiley, R. W. (2013). Characterization of virulence reactions for Heterodera avenae populations from two localities in Algeria. Phytoparasitica, 41, 449–456.
Hafez, S. L., Sundararaj, P. S., & Turner, J. (2008). Incursion management of potato cyst nematode and restoration of pest area freedom. Fifth International Congress of Nematology, Brisbane, Australia, 73.
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
Hlaoua, W., Horrigue-Raouani, N., Fouville, D., & Mugniery, D. (2008). Morphological and molecular characterisation of potato cyst nematode populations from Tunisia and survey of their probable geographical origine. Biotechnology, 7, 651–659.
Holgado, R., Andersson, S., Rowe, J. A., & Magnusson, C. (2004). First record of Heterodera filipjevi in Norway. Nematologia Mediterranea, 32, 205–211.
Hooper, D. J. (1970). Handling, fixing, staining and mounting nematodes. In J. F. Southey (Ed.), Laboratory methods for work with plant and soil nematodes (5th ed., pp. 59–80). London: Her Majesty’s Stationery Office.
Ibrahim, S. K., Minnis, S., Barker, A. D. P., Russel, M. D., Haydock, P. P. J., Evans, K., et al. (2001). Evaluation of PCR, IEF and ELISA techniques for the detection and identification of potato cyst nematodes from field soil samples in England and Wales. Pest Management Science, 57, 1068–1074.
Joyce, S.A., Reid, A., Driver, F., & Curran, J. (1994). Application of polymerase chain reaction (PCR) methods to the identification of entomopathogenic nematodes. In: Burnell, A.M., Ehlers, R.-U. & Masson, J.-P. (Eds). COST 812 Biotechnology: Genetics of entomopathogenic nematodes bacterium complexes. Proceedings of symposium and workshop, St Patrick’s College, Maynooth, County Kildare, Ireland. Luxembourg, European Commission, DGXII, pp. 178–187.
Lamberti, F., Greco, N., & Zaouchi, H. (1975). A nematological survey of date palm and other major crops in Algeria. FAO Plant Protection Bulletin, 23, 156–160.
Madani, M., Vovlas, N., Castillo, P., Subbotin, S. A., & Moens, M. (2004). Molecular characterization of cyst nematode species (Heterodera spp.) from the Mediterranean Basin using RFLPs and sequences of ITS-rDNA. Journal of Phytopathology, 152, 229–234.
Madani, M., Word, L. G., & De Boer, S. H. (2008). Multiplex real time polymerase chain reaction for identifying potato cyst nematodes, Globodera pallida and Globodera rostochiensis and the tobacco cyst nematode, Globodera tabacum. Canadian Journal of Plant Pathology, 30, 554–564.
Madani, M., Subbotin, S. A., Ward, L. J., Li, X., & De Boer, S. H. (2010). Molecular characterization of Canadian populations of potato cyst nematodes, Globodera rostochiensis and G. pallida using ribosomal nuclear RNA and cytochrome b genes. Canadian Journal of Plant Pathology, 32, 252–263.
Madzhidov, A. R. (1981). [New species Bidera filipjevi sp. nov. (Heteroderina: Tylenchida) from Tadzhikistan]. Izvestiya Akademii Nauk Tadzikshoi S.S.R., Biobgicheskie Nauki, 83, 40–44. (In Russian).
Mai, W. F. (1977). Worldwide distribution of potato cyst nematodes and their importance in crop production. Journal of Nematology, 9, 30–34.
Manduric, S., Olsson, E., England, J. E., & Andersson, S. (2004). Separation of Globodera rostochiensis and G. pallida (Tylenchida: Heteroderidae) using morphology and morphometrics. Nematology, 6, 171–181.
Marshall, J. W. (1993). Detecting the presence and distribution of Globodera rostochiensis and G. pallida mixed populations in New Zealand using DNA probes. New Zealand Journal of Crop and Horticultural Science, 21, 219–223.
McDonald, A. H., & Nicol, J. M. (2005). Nematodes parasites of cereals. In M. Luc, R. A. Sikora, & J. Bridge (Eds.), Plant parasitic nematodes in subtropical and tropical agriculture (Second ed., pp. 131–191). Wallingford: CABI publishing.
Mokabli, A. (2002). Biologie des nématodes à kystes (Heterodera) des céréales en Algérie (66 p). INA, Algier: Virulence de quelques populations à l’égard de diverses variétés et lignées de céréales. Doctorat thesis.
Mokabli, A., Valette, S., Gauthier, J. P., & Rivoal, R. (2001). Influence of the temperature on the hatch of Heterodera avenae Woll. Populations from Algeria. Nematology, 3, 171–178.
Mokrini, F., Abbad Andaloussi, F., Alaoui, Y., & Troccoli, A. (2009). Importance and distribution of the main cereal nematodes in Morocco. In Cereal cyst nematodes: status, research and outlook (pp. 45–50). Antalya, Turkey: Proceedings of the First Workshop of the International Cereal Cyst Nematode Initiative.
Mugnièry, D. (1984). Les nematodes de la pomme de terre. Agronomie, 3, 45–50.
Namouchi-Kachouri, N., & B’Chir, M. M. (2005). Identification morphométrique et moléculaire de quelques populations tunisiennes d’Heterodera avenae associées aux céréales. Nematologia Mediterranea, 33, 3–9.
Nunn, G. B. (1992). Nematode molecular evolution. Ph.D. Thesis, University of Nottingam, Nottingham, UK.
Plantard, O., Picard, D., Valette, S., Scurrah, M., Grenier, E., & Mugniéry, D. (2008). Origin and genetic diversity of Western European populations of the potato cyst nematode (Globodera pallida) inferred from mitochondrial sequences and microsatellite loci. Molecular Ecology, 17, 2208–2218.
Quader, M., Nambiar, L., & Cunnigton, J. (2008). Conventional and real-time PCR-based species identification and diversity of potato cyst nematodes and diversity of potato cyst nematodes (Globodera spp.) from Victoria, Australia. Nematology, 10, 471–478.
Rammah, A. (1994). Cereal cyst nematode (Heterodera avenae) in Morocco. Arab and Near East Plant Protection Newsletter, 19, 40.
Rivoal, R. , Valette, S., Bekal, S. , Gauthier, J.P., & Yahyaoui, A. (2003). Genetic and phenotypic diversity in the graminaceous cyst nematode complex, inferred from PCR-RFLP of ribosomal DNA and morphometric analysis. European Journal of Plant Pathology, 109, 227–241.
Schlùter, K. (1976). The potato cyst eelworm Heterodera rostochiensis Woll. in Morocco: its distribution and economic importance. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz, 83, 401–406.
Scotto La Massese, C. (1961). Aperçu sur les problèmes posés par les nématodes phytoparasites en Algérie. Journée d’Etude et d’Information. Association de Coordination Technique Agricole, F.N.G.P.C., Paris, 1-27.
Scotto La Massese, C. (1962). Aperçu sur les problèmes posés par les nématodes phytoparasites en Algérie. In: Les nématodes. Paris, France: Association de Coordination Technique Agricole. pp. 83–109.
Scurrah, M., Niere, B., & Bridge, J. (2005). Nematode parasites of potato and sweet potato. In M. Luc, R. Sikora, & J. Bridge (Eds.), Plant parasitic nematodes in subtropical and tropical agriculture (pp. 193–221). Wallingford, UK: CAB International.
Skantar, A. M., Handoo, Z. A., Carta, L. K., & Chitwood, D. J. (2007). Morphological and molecular identification of Globodera pallida associated with potato in Idaho. Journal of Nematology, 39, 133–144.
Skantar, A. M., Handoo, Z. A., Zasada, I. A., Ingham, R. E., Carta, L. K., & Chitwood, D. J. (2011). Morphological and molecular identification of Globodera populations from Oregon and Idaho. Phytopathology, 101, 480–491.
Skarbilovich, T. S. (1959). On the structure of the systematics of nematode order Tylenchida Thorne, 1949. Acta Parasitologica Polonica, 7, 117–132.
Stone, A. R. (1973). Heterodera pallida n. sp. (Nematoda: Heterodoridae) a second species of potatocyst nematode. Nematologica 18, 591–606.
Subbotin, S.A., Waeyenberge, L., Molokanova, I.A., & Moens, M. (1999). Identification of Heterodera avenae group species by morphometrics and rDNA-RFLP. Nematology1, 195–207.
Subbotin, S. A., Halford, P. D., Warry, A., & Perry, R. N. (2000). Variations in ribosomal DNA sequences and phylogeny of Globodera parasitising Solanaceous plants. Nematology, 2, 591–604.
Subbotin, S. A., Sturhan, D., Rumpenhorst, H. J. & Moens, M. (2003). Molecular and morphological characterisation of the Heterodera avenae complex species (Tylenchida: Heteroderidae). Nematology, 5, 515–538.
Subbotin, S. A., Mundo-Ocampo, M., & Baldwin, J. G. (2010a). Systematics of cyst nematodes (Nematoda Heteroderinae). Nematology monographs and perspectives. A. In D. J. Hunt & R. N. Perry (Eds.), Brill Nv (p. 351). The Netherlands: Leiden.
Subbotin, S. A., Mundo-Ocampo, M., & Baldwin, J. G. (2010b). Systematics of cyst nematodes (Nematoda Heteroderinae). Nematology monographs and perspectives B. In D. J. Hunt & R. N. Perry (Eds.), Brill Nv (p. 512). The Netherlands: Leiden.
Subbotin, S. A., Cid Del Prado Vera, I., Mundo-Ocampo, M., & Baldwin, J. G. (2011). Identification, phylogeny and phylogeography of circumfenestrate cyst nematodes (Nematoda: Heteroderidae) as inferred from analysis of ITS-rDNA. Nematology, 13, 805–824.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729.
Tanha Maafi, Z., Subbotin, S. A., & Moens, M. (2003). Molecular identification of cyst forming nematodes (Heteroderidae) from Iran and a phylogeny based on ITS-rDNA sequences. Nematology, 5, 99–11.
Tanha Maafi, Z., Sturhan, D., Kheiri, A., & Geraert, E. (2007). Species of the Heterodera avenae group (Nematoda: Heteroderidae) from Iran. Russian Journal of Nematology, 15, 49–58.
Tanha Maafi, Z., Ahmadi, A. R., Hajihasani A., Karimipour Fard, H. & Nicol, J.M. (2010). Current progress on cereal cyst nematodes (Heterodera filipjevi, H. avenae type B and H. latipons) of importance on wheat in Iran, 30th international symposium of the European Society of Nematologists, Vienna, Austria, 19–23 September: 226.
Thiéry, M., & Mugniéry, D. (1996). Interspecific rDNA restriction fragment length polymorphismin Globodera species, parasites of Solanaceous plants. Fundamental and Applied Nematology, 19, 471–479.
Turner, S. J. (1996). Population decline of potato cyst nematodes (Globodera rostochiensis, G. pallida) in field soils in Northern Ireland. Annals of Applied Biology, 129, 315–322.
Wollenweber, H. W. (1923). Krankheiten und Beschädigungen der Kartoffel. Arb. Forschungsinst. Kartoffelbau, 7, 1–56.
Wollenweber, H. W. (1924). Zur Kenntnis der Kartoffel-Heteroderen. Illustrierte Landwirtschaftliche Zeitung, 44, 100–101.
Wouts, W. M., & Sturhan, D. (1995). Heterodera aucklandica sp. n. (Nematoda: Heteroderidae) from a New Zealand native grass, with notes on the species of the H. avenae group. New Zealand Journal of Zoology, 22, 199–207.
Acknowledgments
The authors wish to thank Dr. Nicola Greco for critical review of the manuscript and helpful suggestions and discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s10658-016-1100-x.
Rights and permissions
About this article
Cite this article
Tirchi, N., Troccoli, A., Fanelli, E. et al. Morphological and molecular identification of potato and cereal cyst nematode isolates from Algeria and their phylogenetic relationships with other populations from distant theirgeographical areas. Eur J Plant Pathol 146, 861–880 (2016). https://doi.org/10.1007/s10658-016-0965-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-0965-z