Abstract
Fusarium head blight (FHB), caused by the plant pathogen Fusarium graminearum, is a significant threat to small grains production worldwide. Additional knowledge is required to clarify the influence of meteorological conditions on the release of ascospores of F. graminearum. Here, a new application of causality analysis is used to determine how meteorological conditions cause ascospore release. Two types of causality analyses, convergent cross mapping and multivariate state space forecasting, were applied to field measurements of airborne ascospores of F. graminearum over two years. Convergent cross mapping identified relative humidity, solar radiation, wind speed, and air temperature as predictors of ascospore release. Multivariate state space forecasting identified solar radiation and relative humidity as effective predictors of ascospore release. Increased concentration of ascospores in the atmosphere primarily occurred during periods of high relative humidity, low solar radiation, and low wind speed. Results from this study may assist producers in managing FHB in small grains by narrowing the timing and application of fungicides around major ascospore release intervals predicted by meteorological conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fusarium head blight (FHB), produced by the fungus Fusarium graminearum Schwabe, caused damage to small grains yields in the UK (Jennings and Turner 1996) and $3 billion of losses to wheat production in the US alone between 1990 and 2000 (Windels 2000). Grain infected with F. graminearum may contain mycotoxins (Schollenberger et al. 2002) that threaten the health of domestic animals and humans (McMullen and Stack 1983). The fungus forcibly discharges ascospores from perithecia produced from overwintered residues of corn and small grains (Gilbert and Fernando 2004; Paulitz 1999), and these ascospores may be transported through the atmosphere over long distances to susceptible crops (Prussin et al. 2015; Schmale et al. 2012). Prior studies have suggested that the release of ascospores and FHB epidemics are associated with light intensity and spectral energy distribution (Trail et al. 2002), rainfall (Chen and Yuan 1984; Reis 1990; Gilbert and Tekauz 2000), air temperature (Del Ponte et al. 2009; Sutton 1982), wind speed (Prussin et al. 2014b), and relative humidity (Paulitz 1996). A complete understanding of the causal relationship, in which one event is responsible for a second event, between meteorological conditions and the release of ascospores is needed to accurately predict the spread of disease. Detecting causal relationships in natural systems, however, is extremely challenging because of large variability, nonlinear dynamics, and confounding variables (Holling 2001).
A series of innovative and promising methods have been developed to identify causation in weakly connected dynamic systems, such as ecological time series (Sugihara et al. 2012; Deyle et al. 2013). These methods, known collectively as causality analyses, have been applied within the natural (Sugihara et al. 2012; Deyle et al. 2013) and social sciences (Stern and Enflo 2013), but have not yet been applied to the field of plant pathology. The application of causality analysis to plant pathology has the potential to produce new insight into the complex relationships underlying the epidemiology of plant diseases. In contrast to regression analysis, which can identify correlation but not causation, causality analysis is able to determine whether one variable causes an effect on another variable and the direction of the relationship.
The objective of this study was to determine which meteorological variable(s) influence ascospore release through causality analysis. This objective was based on the hypothesis that meteorological variables (relative humidity, solar radiation, and wind speed) are causal agents for ascospore release. This hypothesis was tested using convergent cross mapping analysis (Sugihara et al. 2012; BozorgMagham et al. 2015) and multivariate state space forecasting (Deyle et al. 2013). Results explicitly identified cause-and-effect agents and will allow for more accurate representations of ascospore release in disease forecasting models. Ultimately, results will enable growers of small grains to make informed FHB management decisions based on meteorological predictors of ascospore release.
Materials and methods
Experimental data collection
Field-scale experiments with F. graminearum took place in 2011 and 2012 at the Kentland Farm in Blacksburg, Virginia and have been described previously by Prussin et al. (2014a, b). Briefly, corn stalks were inoculated with the strain Fg_Va_GPS13N4_3ADON and stored at room temperature for approximately 10 weeks to permit colonization of the stalks. After the fungus colonization period, a 3716 m2 wheat field was artificially inoculated with the infected stalks.
The timeline of measurements of airborne ascospore concentration was designed to capture background conditions and the release of ascospores from the infected field. In 2011, the field was inoculated on 2 May, and measurements began 17 days later on 19 May and continued until 3 June (15 days). In 2012, the field was inoculated on 16 April, and measurements started 10 days later on 26 April and were completed on 14 May (18 days). The background concentration of ascospores was established in 2012 via hourly measurements gathered for 7 days prior to artificial inoculation of the field (measurements began on 9 April).
A volumetric, active ascospore sampler (Quest Developments, Brits, South Africa) with a flow rate of 0.22 m3 h−1 was used to measure airborne ascospore concentration as a function of time. The sampler was installed at the center of the field and was outfitted with a circular rotating disk coated with silicone grease to collect ascospores released from the inoculum source. The ascospores on the sample disks were stained using Calberla’s Stain (Multidata Inc., Saint Louis Park, Minnesota), and were counted under a microscope at 400× magnification. The concentration of ascospores per cubic meter of air was calculated as the ascospore collection rate divided by the ascospore sampler’s volumetric flow rate. The concentration was assumed to be a proxy for ascospore release, as the measurement height of 50 cm was well within the canopy, approximately half its height (Prussin et al. 2014b), where the effects of dilution in the atmospheric boundary layer were considered to be minimal. A direct relationship between ascospore release and capture was assumed, as the effects of residence time within the canopy were presumed to be negligible.
Meteorological data
A weather station was situated approximately 250 m northwest and 350 m northwest from the center of the inoculum source (i.e., the location of the Quest sampler) in 2011 and 2012, respectively. It recorded air temperature (°C), soil temperature (°C), relative humidity (%), rainfall (mm), wind speed (m s−1), and solar radiation (W m−2). Absolute humidity was calculated using the ideal gas law and the Magnus-Tetens approximation (Bolton 1980). The 15-min records were averaged over 1-h intervals to match the temporal resolution of the ascospore measurements.
Causality analyses
Convergent cross mapping
The convergent cross mapping (CCM) method (Sugihara et al. 2012; BozorgMagham et al. 2015) was used to investigate the relationship between ascospore concentration and meteorological conditions. This method is based loosely on a parent-child relationship. Information associated with a driver (i.e., the parent) is passed on to a response (i.e., the child). It is anticipated that in examining characteristics of the response (i.e., the child), attributes about a specific driver would be apparent. For our application, the driver was the meteorological variable of interest and the response was ascospore concentration.
Time-lagged components (Abarbanel 1996; Takens 1981; Sauer et al. 1991) of ascospore concentration, the response signal, were used to estimate the dynamics of each meteorological variable, the candidate driver. An improvement in the recovery of a meteorological variable shows that the variable has an influential causal impact on ascospore concentration. The meteorological observations were compared to the CCM-estimated meteorological values obtained using the time-lagged ascospore concentration, resulting in the calculation of a Pearson correlation coefficient. The Pearson correlation coefficient quantifies the strength between a dynamically-connected set of variables. An average of the Pearson correlation coefficients, over subsets of length L of the historical data sets, was obtained and termed the convergent cross mapping coefficient, ρ. Causality is indicated by a ρ value that often increases with an increasing length of subset library length, L. A thorough description of the creation of the reconstructed phase spaces and determination of the CCM coefficient can be found in Online Resource 1.
Multivariate state space forecasting
The meteorological variables determined as influential causal agents by the CCM method were further analyzed using the multivariate state space forecasting method. The multivariate state space forecasting method (Deyle et al. 2013) is inspired by a conceptual combination of the Granger causality method (Granger 1969) and the simplex method (Farmer and Sidorowich 1987). The multivariate state space forecasting method aims to improve the prediction of the ascospore concentration by leveraging the historical information of the ascospore concentrations augmented by the information of the causal meteorological variables. Predicted ascospore concentration was obtained using single- variable (by exploiting only the past information of ascospore concentration and without using the causal meteorological data) and multivariable forecasting methods. The root mean square (RMS) error, defined as the difference between the experimentally-obtained ascospore concentration and the forecasted ascospore concentration, was determined for single variable and multivariate forecasting methods. The improvement resulting from the use of augmented information of the causal meteorological data was quantified by calculating the difference between the two RMS error values. Statistical significance of the improvement was studied for each augmented meteorological variable (for example, temperature and solar radiation). A thorough description of the methodology used to create the reconstructed phase spaces and the calculation of the RMS of forecasting error is available in Online Resource 1.
Statistical analyses
Exploratory statistical analyses were conducted using JMP Pro 10.0.2 (SAS Institute Inc., Cary, NC). Statistical significance was defined at a level of 0.05 for all analyses, unless specified otherwise. A two-sample test for independent groups was conducted on ascospore concentration in 2011 and 2012 to determine whether the concentration differed between the two years. This necessitated the use of a Welch’s t-test because the standard deviations between the populations could not be assumed to be approximately equal. Due to expected differences in ascospore release during daytime v. nighttime, analysis of variance (ANOVA) was used to compare the means of daytime (0700 to 1900 local time, EDT) and nighttime (1900 to 0700) ascospore concentration. The one-way ANOVA used periods of the day as the treatment (categorical variables) with treatment levels of daytime or nighttime.
A bivariate analysis of ascospore concentration and individual meteorological variables was performed to determine if there were any significant relationships. All meteorological variables analyzed were continuous unless otherwise stated as nominal. In all correlation analyses, all data points were included unless otherwise noted. A logarithmic base-10 transformation of the ascospore data was applied because of the skewed nature of the distribution. Simple linear regression was employed to model the relationship between the continuous response variable (ascospore concentration) and the continuous predictor variable (meteorological variables).
Results
Causality analysis
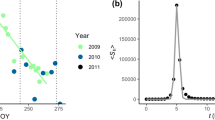
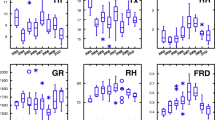
The CCM coefficient, ρ, between the candidate driver (meteorological variables) and the response signal (ascospore concentration) is shown in Fig. 1 (2011) and Fig. S1 (2012) (Online Resource 2); results are similar between the two years. The parameter ρ denotes the strength of the causal relationship with a value of one indicating the strongest causal relationship and lower values signifying a weaker relationship. The convergence of ρ as L increased for air temperature, relative humidity, wind speed, and solar radiation indicated that these four variables were the driving signals. The clustering of ρ for large L for these variables suggested that no single one was dominant. In addition, soil temperature and absolute humidity had the smallest influence on ascospore concentration.
The CCM coefficient, ρ, between driver signals (meteorological variables) and response signals (ascospore concentration) for the 2011 monitoring period. Solar radiation, relative humidity, air temperature, and wind speed show CCM coefficients ρ that increase significantly with increasing library length and are significantly greater than zero for the large library length. Thus, they are the most important drivers influencing ascospore release
The multivariate state space forecasting analysis was performed considering a time difference of 0 to 8 h between the meteorological and ascospore concentration time series, such that the meteorological variable preceded the ascospore concentration signal. At each time step, ascospore concentration was predicted for a 12 h lead time. Forecasting began on 11 May 2012 at 0700 and was performed for hourly intervals. Figure S2 (2012) (Online Resource 2) shows one sample of the RMS of single variable (solid line) and multivariate state space forecasting errors (dashed line). In this example the solar radiation signal, leading the ascospore concentration signal by 4 h, was considered as the augmented variable.
This study investigated whether the augmentation of the meteorological variables significantly improved the forecasting of ascospore concentration. A t-test was applied between the RMS errors for each case. The null hypothesis was that the RMS of errors of the multivariate and single variable forecasting results were identical and the differences were random. Table S1 shows the results of this analysis, where the cases that rejected the null hypothesis are denoted by +1 if the forecast was improved and −1 if the forecast was degenerated. Solar radiation and relative humidity were among the most influential external driving forces in this system. Absolute humidity, as the augmented variable, significantly changed the results, but degenerated the forecast.
Correlation analysis
Atmospheric ascospore concentration was significantly higher after the introduction of inoculated corn stalks to the field (P < 0.0001). In 2012, the average ascospore concentration was 176 ascospores m−3 pre-inoculation and 3066 ascospores m−3 post-inoculation, indicating that a great majority of the ascospores collected originated from the inoculated field and not from background sources.
Atmospheric ascospore concentration varied by period of the day in both 2011 and 2012. Analysis of variance (ANOVA) was employed to determine whether the mean daytime concentration (0700 to 1900 h) was significantly different from the nighttime concentration (1900 to 0700 h). In 2011 (n = 352), the daytime ascospore concentration (mean ± standard error = 278 ± 2810 ascospores m−3) was significantly different (P < 0.0001) from the nighttime ascospore concentration (15,509 ± 2747 ascospores m−3). In 2012 (n = 424), the mean concentration during the nighttime (5542 ± 523) was significantly higher than during the daytime (524 ± 533).
Elevated ascospore concentrations occurred episodically; most of the time, concentrations were zero or close to zero. Each ascospore event documented in the field was categorized as either zero (0 ascospores), low (between 1 and 4999 ascospores m−3), minor (between 5000 and 49,999 ascospores m−3), or major (≥ 50,000 ascospores m−3) (Table 1). In 2011, 90 % (37/41) of all “minor” events and 100 % (12/12) of all “major” events occurred during the nighttime hours. The results for 2012 were similar, with 98 % (64/65) of “minor” events and 100 % (2/2) of “major” events occurring during the nighttime hours.
Correlation analysis identified significant relationships between ascospore concentration and the following: time of day, wind speed, solar radiation, air temperature, relative humidity, and soil temperature in both years. Results for absolute humidity, rainfall, and soil temperature were not consistent between the two years. However, due to pronounced issues with heteroscedasticity following the log transformation, the correlation results were indeterminate. There was no rainfall during the field campaign in 2011, so analysis of the relationship between rainfall and ascospore release was not possible.
There was a clear relationship between relative humidity and ascospore concentration (Fig. 2 (2011) and Fig. S3 (2012)). The box plots for each nominal relative humidity value in Fig. 3 (2011) and Fig. S4 (2012) depict the spread of ascospore concentration. A prominent wedge pattern depicts higher ascospore concentration at elevated levels of relative humidity, shown by the higher median values and wider intervals between the first and third quartile of the box plots. The equation of a line defining the upper boundary of the wedge was created by binning relative humidity in 1 % increments and fitting a line to the maximum ascospore concentration in each bin. The linear relationships determined for both years were significant (P < 0.0001), with calculated slopes of 0.049 and 0.018 in 2011 and 2012, respectively.
Hourly ascospore concentration (black bar graph) and relative humidity (RH, dashed red line) for a field source of F. graminearum between 1700 h 19 May 2011 and 800 h 3 June 2011. A 1-acre wheat field plot was inoculated with a strain of F. graminearum (FGVA4) and subsequently monitored for airborne ascospore concentration using a Quest volumetric ascospore sampler
F. graminearum ascospore concentration versus relative humidity for a field-scale source of F. graminearum during the monitoring period in 2011. The shading distinguishes nighttime (black and white) from daytime (grey variants) events. The black line fits the highest concentration of ascospores for each 1 % range in relative humidity to illustrate where an apparent threshold exists
The relationship between ascospore concentration and air temperature is shown in Fig. 4 and Fig. S5 (Online Resource 2). The black and white markers in the figures illustrate ascospore concentration during the nighttime period (1900 to 0700), and the grey markers indicate daytime concentration (0700 to 1900). A wedge pattern was evident in 2011, when ascospore concentrations were higher at the lower temperatures. In 2012, there was more scatter in the data, and no clear trend was evident. Temperature was binned in 1 °C increments, and the resulting linear fit to the maximum concentration in each bin is shown in Table S2. The resulting linear fit was significantly different from the null hypothesis in 2011 (P < 0.0001) but not in 2012 (P = 0.70).
F. graminearum hourly ascospore concentration versus air temperature in 2011. The shading distinguishes nighttime (black and white) from daytime (greys variants) events. The black line fits the highest concentration of ascospores within each 1 °C range in temperature to illustrate where an apparent threshold exists
Discussion
The impact of meteorological conditions on the release of ascospores of F. graminearum into the atmosphere is understudied. This study aimed to extend previous field (Inch et al. 2005; Paulitz 1996; Fernando et al. 2000; Ayers et al. 1975) and laboratory experiments (Gilbert et al. 2008; Tschanz et al. 1975; Trail et al. 2002) by examining two seasons of ascospore concentration data from the same farmland, unique for the large size (3716 m2) of inoculum generated under controlled conditions (Prussin et al. 2014a; Prussin et al. 2014b; Prussin et al. 2015). Associations between meteorological conditions and ascospore concentration were investigated through causality analyses developed to provide information about systems using small ecological data sets (Sugihara et al. 2012; Deyle et al. 2013; Clark et al. 2015).
To the authors’ knowledge, this is the first study to employ causality analyses to predict the release of fungal ascospores into the atmosphere (convergent cross mapping and multivariate state space forecasting). Figure 1 and Fig. S1 (Online Resource 2) show that solar radiation, air temperature, wind speed, and relative humidity are causally linked to release of F. graminearum ascospores. Two causal analysis methods found that: (1) solar radiation and relative humidity were the most influential causal factors; (2) soil temperature and absolute humidity were not causal; and (3) wind speed and air temperature, which may be indicators of atmospheric stability, were causal by one method but not the other. The results further indicated that the identified relationships provide information beyond correlation and actually show that certain meteorological conditions lead to ascospore release. Because of the coupled nonlinear dynamics present within ecological systems, apparent relationships can swing by varying degrees due to minor changes in parameters (Sugihara et al. 2012); thus knowledge of the direction and strength of the relationships is valuable when predicting future conditions.
The results of the 2011 and 2012 field trials illustrated a strong tendency for ascospore concentration to be higher at nighttime (1900 to 0700), agreeing with previous research (Schmale and Bergstrom 2004; Schmale et al. 2005b; Schmale et al. 2006; Inch et al. 2005; Fernando et al. 2000; Ayers et al. 1975; Paulitz 1996). Mycosphaerella fijiensis exhibits similar afternoon or nighttime-dominated release events (Meredith et al. 1973; Burt et al. 1997), while other fungal pathogens, such as Bremia lactucae (Su et al. 2000), Venturia inaequalis (Gadoury et al. 1998), and Venturia pirina (Spotts and Cervantes 1994) tend to release ascospores during the daytime or exhibit no apparent temporal preference, such as Sclerotinia sclerotiorum (Clarkson et al. 2003).
The largest ascospore concentrations occurred at the highest relative humidity levels. Both types of causality analyses (convergent cross mapping and multivariate state space forecasting) identified relative humidity as an important controlling signal as opposed to factors such as soil temperature and absolute humidity. This study adds to previous research that has identified a relationship between relative humidity and ascospore release (Paulitz 1996; Tschanz et al. 1975; Inch et al. 2005; Reis 1990). Researchers have hypothesized that relative humidity may be the trigger responsible for ascospore release (Paulitz 1996; Tschanz et al. 1975). Turgor pressure within the ascus may be the mechanism for forcibly discharging ascospores (Trail et al. 2002; Trail et al. 2005; Trail 2007), and the balance between water inside the ascus and water in the vapor phase in the atmosphere may help to determine ascospore release.
Convergent cross mapping identified air temperature to be a causal agent of ascospore concentration and a negative correlate per linear regression (Fig. 4 and Fig. S5 (Online Resource 2)). The results agree with a field trial that found a negative correlation between ascospore release and temperature (Paulitz 1996). Similarly, controlled laboratory studies found that lower temperatures favored ascospore discharge events (Tschanz et al. 1975). The apparent preference for low temperature aligns with studies of F. graminearum ascospores showing that germination rates increased with decreasing temperatures (Gilbert et al. 2008), highlighting that low temperatures may favor both release and ascospore survival.
Wind speed was identified as a causal agent of ascospore release with the highest concentration observed during periods with the lowest wind speeds (Fig. 1 and Fig. S1 (Online Resource 2)). Researchers have encountered similar daily periodicity in other field data and have hypothesized that the higher number of ascospores could be associated with ascospore release events during the afternoon coupled with the transition to more stable overnight conditions (Fernando et al. 2000). Higher nighttime ascospore concentration may be at least partially explained by more stable atmospheric conditions. We speculate that turbulent mixing associated with an unstable atmosphere during the daytime results in fewer ascospores settling to the ground, whereas stable nighttime conditions produce less mixing in the atmosphere and are more favorable for ascospore deposition and capture (Maldonado-Ramirez et al. 2005; Schmale and Bergstrom 2004; Schmale et al. 2005a). Additional research is needed to determine the nature of the relationship between wind speed, ascospore release, and ascospore deposition.
The causality analyses identified a relationship between solar radiation and ascospore concentration. A chamber study showed that light quality and intensity similar to that encountered during mid-day rain events was associated with the largest ascospore release events (Trail et al. 2002). In a field study in New York, the highest ascospore deposition events within a corn canopy were encountered during the nighttime (Schmale and Bergstrom 2004), while another field study with a 60-m sampling height found no significant difference between daytime and nighttime ascospore counts (Maldonado-Ramirez et al. 2005). The release of ascospores during low levels of solar radiation may not be an advantageous strategy to achieve long distance transport (Schmale et al. 2006; Oke 1987), but may prolong ascospore survival (Rotem and Aust 1991; Sung and Cook 1981). Research on the effect of UV radiation on Mycosphaerella fijiensis ascospores showed that exposure to UV radiation beyond 6 h reduced spore viability, so the timing of a release event could influence the potential for long-distance transport (Parnell et al. 1998). The strong correlation of solar radiation with other meteorological variables makes it challenging to isolate its effect. Additional laboratory experiments are required to further determine how solar radiation may induce ascospore release or if the relationship may be a byproduct of other conditions.
Future research within the area of ascospore release and meteorological conditions would benefit from an understanding of the limitations of this study. An atmospheric transport model of ascospores that accounts for the large difference in atmospheric stability between the day and night would help define the vertical concentration gradient of ascospores and resolve some of these questions, although reliable information about ascospore emission rate as a function of conditions is also needed for such a model to be useful. The number of ascospores measured during the field experiments was always less than the number released because the sampling method captured only those ascospores that reached the instrument’s inlet height; many ascospores were not sampled and deposited back to the ground (Buttner and Stetzenbach 1993; Hart et al. 1994). This disparity between released and captured ascospores has been identified in M. fijiensis field investigations as well (Burt et al. 1999; Rutter et al. 1998).
These results highlight meteorological variables that are drivers of increased ascospore concentration. By enabling refinements to models of ascospore transport and dissemination, these findings could help improve predictions of the relative risk of infection (Prussin et al. 2015; Schmale and Ross 2015). While the parameters describing ecological, biological, and meteorological conditions are continuously changing and are coupled in complex ways, the ability to describe their interactions can inform decision-making with respect to species management, plant and animal protection, and human health.
References
Abarbanel, H. (1996). Analysis of observed chaotic data. Berlin: Springer-Verlag.
Ayers, J., Pennypacker, S., Nelson, P., & Pennypacker, B. (1975). Environmental factors associated with airborne ascospores of Gibberella zeae in corn and wheat fields. Phytopathology, 65:835.
Bolton, D. (1980). The computation of equivalent potential temperature. Monthly Weather Review, 108(7), 1046–1053.
BozorgMagham, A. E., Motesharrei, S., Penny, S. G., & Kalnay, E. (2015). Causality analysis: Identifying the leading element in a coupled dynamical system. PLoS One, 10(6), e0131226. doi:10.1371/journal.pone.0131226.
Burt, P., Rutter, J., & Gonzales, H. (1997). Short-distance wind dispersal of the fungal pathogens causing Sigatoka diseases in banana and plantain. Plant Pathology, 46(4), 451–458.
Burt, P., Rosenberg, L., Rutter, J., Ramirez, F., & Gonzales, O. H. (1999). Forecasting the airborne spread of Mycosphaerella fijiensis, a cause of black Sigatoka disease on banana: estimations of numbers of perithecia and ascospores. Annals of Applied Biology, 135(1), 369–377.
Buttner, M. P., & Stetzenbach, L. D. (1993). Monitoring airborne fungal spores in an experimental indoor environment to evaluate sampling methods and the effects of human activity on air sampling. Applied and Environmental Microbiology, 59(1), 219–226.
Chen, X., & Yuan, C. (1984). Application of microcomputer in studying wheat scab epidemiology and forecasting. Zhejiang Agricultural Science, 2, 55–60.
Clark, A. T., Ye, H., Isbell, F., Deyle, E. R., Cowles, J. M., Tilman, D., et al. (2015). Spatial 'convergent cross mapping' to detect causal relationships from short time-series. Ecology, 96(5), 1174–1181.
Clarkson, J. P., Staveley, J., Phelps, K., Young, C. S., & Whipps, J. M. (2003). Ascospore release and survival in Sclerotinia sclerotiorum. Mycological Research, 107(02), 213–222.
Del Ponte, E. M., Fernandes, J. M. C., Pavan, W., & Baethgen, W. E. (2009). A model-based assessment of the impacts of climate variability on fusarium head blight seasonal risk in southern Brazil. Journal of Phytopathology, 157(11–12), 675–681.
Deyle, E. R., Fogarty, M., Hsieh, C.-H., Kaufman, L., MacCall, A. D., Munch, S. B., et al. (2013). Predicting climate effects on Pacific sardine. Proceedings of the National Academy of Sciences, 110(16), 6430–6435.
Farmer, J. D., & Sidorowich, J. J. (1987). Predicting chaotic time series. Physical Review Letters, 59(8), 845.
Fernando, W. G., Miller, J., Seaman, W., Seifert, K., & Paulitz, T. (2000). Daily and seasonal dynamics of airborne spores of Fusarium graminearum and other Fusarium species sampled over wheat plots. Canadian Journal of Botany, 78(4), 497–505.
Gadoury, D. M., Stensvand, A., & Seem, R. C. (1998). Influence of light, relative humidity, and maturity of populations on discharge of ascospores of Venturia inaequalis. Phytopathology, 88(9), 902–909.
Gilbert, J., & Fernando, W. (2004). Epidemiology and biological control of Gibberella zeae/Fusarium graminearum. Canadian Journal of Plant Pathology, 26(4), 464–472.
Gilbert, J., & Tekauz, A. (2000). Review: Recent developments in research on fusarium head blight of wheat in Canada. Canadian Journal of Plant Pathology-Revue Canadienne De Phytopathologie, 22(1), 1–8.
Gilbert, J., Woods, S., & Kromer, U. (2008). Germination of ascospores of Gibberella zeae after exposure to various levels of relative humidity and temperature. Phytopathology, 98(5), 504–508.
Granger, C. W. (1969). Investigating causal relations by econometric models and cross-spectral methods. Econometrica: Journal of the Econometric Society, 424–438.
Hart, M., Wentworth, J., & Bailey, J. (1994). The effects of trap height and weather variables on recorded pollen concentration at Leicester. Grana, 33(2), 100–103.
Holling, C. S. (2001). Understanding the complexity of economic, ecological, and social systems. Ecosystems, 4(5), 390–405.
Inch, S., Fernando, W., & Gilbert, J. (2005). Seasonal and daily variation in the airborne concentration of Gibberella zeae (Schw.) Petch spores in Manitoba. Canadian Journal of Plant Pathology, 27(3), 357–363.
Jennings, P., & Turner, J. (1996) Towards the prediction of Fusarium ear blight epidemics in the UK-the role of humidity in disease development. In Brighton Crop Protection Conference: Pests & Diseases-1996. Volume 1. Proceedings of an International Conference, Brighton, UK, 18–21 November, 1996 (pp. 233–238): British Crop Protection Council.
Maldonado-Ramirez, S. L., Schmale III, D. G., Shields, E. J., & Bergstrom, G. C. (2005). The relative abundance of viable spores of Gibberella zeae in the planetary boundary layer suggests the role of long-distance transport in regional epidemics of fusarium head blight. Agricultural and Forest Meteorology, 132(1–2), 20–27.
McMullen, M. P., & Stack, R. W. (1983). Head blight (scab) of small grains. North Dakota Cooperative Extension Service Circular(NPP-8), 1–2.
Meredith, D., Lawrence, J., & Firman, I. (1973). Ascospore release and dispersal in black leaf streak disease of bananas (Mycosphaerella fijiensis). Transactions of the British Mycological Society, 60(3), 547–554.
Oke, T. R. (1987). Boundary layer climates, 2nd edn. London: Methuen.
Parnell, M., Burt, P. J. A., & Wilson, K. (1998). The influence of exposure to ultraviolet radiation in simulated sunlight on ascospores causing Black Sigatoka disease of banana and plantain. International Journal of Biometeorology, 42(1), 22–27.
Paulitz, T. (1996). Diurnal release of ascospores by Gibberella zeae in inoculated wheat plots. Plant Disease, 80(6), 674–678.
Paulitz, T. (1999). Fusarium head blight: a re-emerging disease. Phytoprotection, 80(2), 127–133.
Prussin II, A. J., Li, Q., Malla, R., Ross, S. D., & Schmale III, D. G. (2014a). Monitoring the long distance transport of Fusarium graminearum from field-scale sources of inoculum. Plant Disease, 98(4), 504–511.
Prussin II, A. J., Szanyi, N. A., Welling, P. I., Ross, S. D., & Schmale III, D. G. (2014b). Estimating the production and release of ascospores from a field-scale source of Fusarium graminearum inoculum. Plant Disease, 98(4), 497–503.
Prussin II, A. J., Marr, L. C., Schmale III, D. G., Stoll, R., & Ross, S. D. (2015). Experimental validation of a long-distance transport model for plant pathogens: Application to Fusarium graminearum. Agricultural and Forest Meteorology, 203(0), 118–130.
Reis, E. (1990). Effects of rain and relative humidity on the release of ascospores and on the infection of wheat heads by Gibberella zeae. Fitopatologia Brasileira, 15, 339–343.
Rotem, J., & Aust, H. (1991). The effect of ultraviolet and solar radiation and temperature on survival of fungal propagules. Journal of Phytopathology, 133(1), 76–84.
Rutter, J., Burt, P. J., & Ramirez, F. (1998). Movement of Mycosphaerella fijiensis spores and sigatoka disease development on plantain close to an inoculum source. Aerobiologia, 14(2–3), 201–208.
Sauer, T., Yorke, J. A., & Casdagli, M. (1991). Embedology. Journal of Statistical Physics, 65(3–4), 579–616.
Schmale III, D. G., & Bergstrom, G. C. (2004). Spore deposition of the ear rot pathogen, Gibberella zeae, inside corn canopies. Canadian Journal of Plant Pathology-Revue Canadienne De Phytopathologie, 26(4), 591–595.
Schmale III, D. G., & Ross, S. D. (2015). Highways in the sky: Scales of atmospheric transport of plant pathogens. Annual Review of Phytopathology, 53(1), 591–611.
Schmale III, D. G., Arntsen, Q. A., & Bergstrom, G. C. (2005a). The forcible discharge distance of ascospores of Gibberelia zeae. Canadian Journal of Plant Pathology-Revue Canadienne De Phytopathologie, 27(3), 376–382.
Schmale III, D. G., Shah, D. A., & Bergstrom, G. C. (2005b). Spatial patterns of viable spore deposition of Gibberella zeae in wheat fields. Phytopathology, 95(5), 472–479. doi:10.1094/phyto-95-0472.
Schmale III, D. G., Shields, E. J., & Bergstrom, G. C. (2006). Night-time spore deposition of the fusarium head blight pathogen, Gibberella zeae, in rotational wheat fields. Canadian Journal of Plant Pathology-Revue Canadienne De Phytopathologie, 28(1), 100–108.
Schmale III, D. G., Ross, S. D., Fetters, T. L., Tallapragada, P., Wood-Jones, A. K., & Dingus, B. (2012). Isolates of Fusarium graminearum collected 40–320 meters above ground level cause fusarium head blight in wheat and produce trichothecene mycotoxins. Aerobiologia, 28(1), 1–11.
Schollenberger, M., Jara, H. T., Suchy, S., Drochner, W., & Müller, H.-M. (2002). Fusarium toxins in wheat flour collected in an area in southwest Germany. International Journal of Food Microbiology, 72(1), 85–89.
Spotts, R., & Cervantes, L. (1994). Factors affecting maturation and release of ascospores of Venturia pirina in Oregon. Phytopathology, 84(3), 260–263.
Stern, D. I., & Enflo, K. (2013). Causality between energy and output in the long-run. Energy Economics, 39(0), 135–146, doi:10.1016/j.eneco.2013.05.007.
Su, H., Van Bruggen, A., & Subbarao, K. (2000). Spore release of Bremia lactucae on lettuce is affected by timing of light initiation and decrease in relative humidity. Phytopathology, 90(1), 67–71.
Sugihara, G., May, R., Ye, H., Hsieh, C.-h., Deyle, E., Fogarty, M., et al. (2012). Detecting causality in complex ecosystems. Science, 338(6106), 496–500.
Sung, J.-M., & Cook, R. (1981). Effect of water potential on reproduction and spore germination by Fusarium roseum 'Graminearum,' 'Culmorum,' and 'Avenaceum'. Phytopathology, 71(5), 499–504.
Sutton, J. (1982). Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Canadian Journal of Plant Pathology, 4(2), 195–209.
Takens, F. (1981). Detecting strange attractors in turbulence. Berlin Heidelberg: Springer.
Trail, F. (2007). Fungal cannons: explosive spore discharge in the Ascomycota. FEMS Microbiology Letters, 276(1), 12–18.
Trail, F., Xu, H., Loranger, R., & Gadoury, D. (2002). Physiological and environmental aspects of ascospore discharge in Gibberella zeae (anamorph Fusarium graminearum). Mycologia, 94(2), 181–189.
Trail, F., Gaffoor, I., & Vogel, S. (2005). Ejection mechanics and trajectory of the ascospores of Gibberella zeae (anamorph Fusarium graminearum). Fungal Genetics and Biology, 42(6), 528–533.
Tschanz, A. T., Horst, R. K., & Nelson, P. E. (1975). Ecological aspects of ascospore discharge in Gibberella zeae. Phytopathology, 65, 597.
Windels, C. E. (2000). Economic and social impacts of fusarium head blight: changing farms and rural communities in the Northern Great Plains. Phytopathology, 90(1), 17–21.
Acknowledgments
This research was supported by the National Science Foundation (NSF) under Grant Numbers DGE-0966125 (IGERT: MultiScale Transport in Environmental and Physiological System (MultiSTEPS)) and CMMI-1150456 (Integrating Geometric, Probabilistic, and Topological Methods for Phase Space Transport in Dynamical Systems). A portion of this work was also supported by a grant through the Virginia Small Grains Board (449281, Improving the Management of FHB through an Increased Understanding of how the Pathogen Releases its Spores). The authors thank Dr. Aaron J. Prussin, II for his input on this project. The authors thank the Virginia Tech Laboratory for Interdisciplinary Statistical Analysis for assistance with statistical methods and interpretation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
David, R.F., BozorgMagham, A.E., Schmale, D.G. et al. Identification of meteorological predictors of Fusarium graminearum ascospore release using correlation and causality analyses. Eur J Plant Pathol 145, 483–492 (2016). https://doi.org/10.1007/s10658-015-0832-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0832-3