Abstract
In the current study we evaluated the production of potential growth-promoting metabolites (IAA, phosphate, siderophore) for 20 isolates of Trichoderma collected from different geographical locations of Chhattisgarh and assessed their growth response on cucumber, bottle gourd and bitter gourd. All the Trichoderma isolates were able to release inorganic phosphorus from tri-calcium phosphate and showed consistent ability to produce siderophores and indole-3-acetic acid (IAA). The production of these metabolites varied greatly within species. Using the same set of isolates we evaluated the production of potential cell wall degrading enzymes. Confrontation assays of Trichoderma isolates against two soilborne plant pathogens (Scelrotium rolfsii and Rhizoctonia solani) expressed varying degrees of antagonistic responses, in-vitro antagonism being more effective against R. solani than S. rolfsii. The production of metabolites in all the Trichoderma isolates did not correlate with enhanced growth on cucumber, bottle gourd and bitter gourd, and bio control efficacy. However, one of the Trichoderma viride isolate (T14) was identified as highest producer of inorganic phosphate, IAA and siderophore exhibited high antagonistic and plant growth promoting ability. A characteristic aromatic odour resembling coconut in T14 isolate was observed which we speculate is due to 6-Pentyl pyrone (one of the best studied secondary metabolites having both antifungal and plant growth-promoting activities).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungi of the genus Trichoderma are soilborne, green-spored filamentous-ascomycete that can be found all over the world. Trichoderma species are highly opportunistic and have been isolated from a diverse range of natural and artificial substrata, which shows their adaptability to various ecological conditions (Druzhinina et al. 2011, 2012). These fungi are successful colonizers of their habitats and efficiently fight with their competitors by launching their potential degradative machinery for decomposition of the often heterogeneous substrates. They have been studied with respect to their distribution and phylogeny, defence mechanisms, beneficial as well as deleterious interaction with hosts, enzyme production and secretion, production of secondary metabolites and antibiotics, sexual development etc. Trichoderma species are some of the best studied fungal species which are being developed as biocontrol agents commercially (Schuster and Schmoll 2010). Some of the species of Trichoderma are endophytic and effectively colonize the roots of most crops and promote plant growth in the form of increased root density, enhanced nutrient uptake, solubilisation of mineral ions, and induced defence response against abiotic and biotic factors (Harman 1992; Mastouri et al. 2010). Trichoderma spp. play an important role in the three-way interaction with the plant and the pathogen (Woo et al. 2006; Shoresh et al. 2010; Viterbo and Horwitz 2010; Hermosa et al. 2012). Trichoderma spp. are endophytic plant symbionts that can also establish themselves in the rhizosphere and are widely used as seed treatments to control diseases and to enhance plant growth and yield (Mastouri et al. 2010; Harman 2011).
Chhattisgarh in central India is divided into three agro-climatic zones—the Northern Hills, the central Chhattisgarh Plains and Bastar Plateau in the south. Diversified crops and cropping systems are the typical characteristics of Chhattisgarh. Popularly known as “Rice bowl” of country, the state is rich in diversity of biotic, abiotic as well as socio-economic condition, which favour the cultivation of various horticultural crops like vegetables (mostly creeper cucurbitaceous gourds), temperate, tropical and high elevation fruits. Cucurbits produce a vast array of novel bioactive molecules serving as chemical defences against infection and predation. The bitter gourd (Momordica charantia) is used as curative plant for mental health disorders and diabetic treatments. Among cucurbits, the bottle gourd (Lagenaria siceraria) is the only plant which contains highest choline level along with required metabolites/precursors for brain function. Crisp and juicy, cucumbers are the basis of a good pickle, but they are also commonly eaten raw. A warm weather vegetable, cucumbers have a refreshing taste, low in calories and rich in a number of nutrients, making them an excellent nutritional choice.
Sclerotium rolfsii is an economically important pathogen on numerous crops worldwide, and commonly occurs in the tropics, subtropics, and other warm temperate regions (Punja 1985). It has an extensive host range; at least 500 species in 100 families are susceptible, the most common hosts are legumes, crucifers, and cucurbits. Sheath blight caused by Rhizoctonia solani Kühn is a major constraint (second only to rice blast) to rice production, causing 5–10 % yield losses in low land tropical Asia. The pathogen has a wide host range and can infect more than 32 plant families and 188 genera (Srinivasachary et al. 2011), often infecting legume crops grown in rotation with rice (Zou et al. 2000).
The objective of this study was to evaluate isolates of Trichoderma collected from different geographical locations of Chhattisgarh for phenotypes associated with bio-control efficacy, such as hydrolysis of chitin and cellulose, siderophore production and their in vitro antagonistic activity against two phytopathogens, S. rolfsii and R. solani. These isolates were further screened for their ability to stimulate plant growth promotion in cucumber, bottle gourd and bitter gourd and traits or parameters associated to growth (such as indole-3-acetic acid, IAA production and phosphate solubilisation ability). Molecular profiling of the isolates was performed using cleaved amplified polymorphic sequence analysis of internal transcribed spacer (ITS) region.
Materials and methods
Fungal isolates
Twenty isolates of Trichoderma used in the present investigation are listed in Table 1. The isolates are maintained in the culture collections of the Department of Plant Molecular Biology and Biotechnology, Indira Gandhi Krishi Vishwavidyalaya, Raipur, Chhattisgarh, India. Sclerotium rolfsii and Rhizoctonia solani used for confrontation assay were isolated from rice plants with symptoms of infection by these pathogens. Fungal cultures were grown and maintained on potato dextrose agar (PDA-Himedia) slants.
Chitinase activity and cellulose hydrolysis
Chitinase activity was detected using the medium and method as developed by Agrawal and Kotasthane (2012). Chitinase detection medium consisted of a basal medium comprising 0.3 g of MgSO4.7H2O, 3.0 g of (NH4)2SO4, 2.0 g of KH2PO4, 1.0 g of citric acid monohydrate, 15 g of agar, 200 μl of Tween-80, 4.5 g of colloidal chitin and 0.15 g of bromocresol purple per litre; pH 4.7. Fresh culture plugs of the isolates to be tested for chitinase activity were inoculated into the medium petri plates and incubated at 25 ± 2 °C. Chitinase activity was identified due to the formation of purple coloured zone in the inoculated medium. Observations were recorded for the colour intensity and diameter of the purple coloured zone until 3rd day after inoculation.
Cellulose hydrolysis by isolates of Trichoderma was determined by using phosphoric acid swollen cellulose (PASC) as a substrate in Mandel and Weber’s medium (Mandels et al. 1974) supplemented with Congo red. Cellulase detection medium consisted of 7 g of PASC (Phosphoric acid swollen cellulose), 0.3 g of MgSO4.7H2O, 2.1 g of (NH4)2SO4, 2 g of KH2PO4, 1 g of CaCl2(fused), 0.5 g of Yeast extract, 5 mg of FeSO4.7H2O, 156 mg of MnSO4.H2O, 1.40 mg of ZnSO4.7H2O, 2.66 mg of CoCl2.6H2O, 0.1 %(v/v) of Triton X-100, 15 g of Agar and 0.15 g of Congo red per litre. The plates were inoculated with 5-mm agar plugs from the edge of actively growing colonies of Trichoderma and incubated at 28 ± 2 °C for 3 days followed by 18 h incubation at 50 °C. Hypercellulolytic isolates were selected on the basis of diameter of the colonies.
Quantitative assay for siderophore production
For the evaluation of siderophore production, chrome azurol sulphonate (CAS) assay of Payne (1994) was followed. Four discs (5 mm) from the edge of actively growing colonies of Trichoderma were inoculated to 20 ml potato dextrose broth (PDB-HiMedia) and incubated for 3 days at 28 ± 2 °C. The mycelial mats were removed by centrifugation at 3000 rpm for 5 min. 0.5 ml of the culture supernatant was then mixed with 0.5 ml CAS solution and 10 μl shuttling solution (sulphosalicyclic acid). The colour obtained was determined using spectrophotometer at 630 nm after 20 min of incubation. Similarly, a reference solution was prepared by mixing PDB, CAS dye and shuttle solution and absorbance was recorded. Uninoculated PDB served as the blank and values of siderophore released in PDB was expressed as percent siderophore units. It was calculated using the formula: (Ar-As)/Ar × 100 Ar; where Ar is the absorbance of reference solution and As is the absorbance of samples.
In vitro fungal growth inhibition assays (Confrontation assay)
Dual culture confrontation assay was performed on PDA plates as described by Dennis and Webster (1971) to assess the ability of Trichoderma isolates to overgrow and lyse the mycelia of two test plant pathogens, S. rolfsii and R. solani. Five mm agar plugs from the edge of actively growing colonies of Trichoderma and the pathogen respectively were placed approximately 7 cm opposite to each other and incubated at 28 ± 2 °C for 5 days. In vitro antagonistic potential of different isolates of Trichoderma was assessed by calculating percentage inhibition growth of pathogens in presence of Trichoderma using the formula: (C-T)/C × 100 where C and T are radial growth of pathogen in control and in presence of Trichoderma spp. respectively. Isolates were also scored for degree of antagonism as per a scale of classes 1–5 designated by Bell et al. (1982): 1 = Trichoderma completely overgrew the pathogen and covered the entire medium surface (>75 % inhibition); 2 = Trichoderma overgrew at least 2/3 of the medium surface(50–75 % inhibition); 3 = Trichoderma and pathogen each colonized approximately ½ of the medium surface (more than 1/3 and less than 2/3), and neither organism appeared to dominate the other (25–50 % inhibition); 4 = the pathogen colonized at least 2/3 of the medium surface and appeared to withstand encroachment by Trichoderma (<25 % inhibition); 5 = the pathogen completely overgrew the Trichoderma and covered the entire medium surface
Confrontation areas were observed under microscope for coiling structures. Observation was recorded for overgrowth of Trichoderma on the test fungi and for the lysis of the mycelia. Microscopic observations were performed at 100× magnification using Leica ATC 2000 binocular microscope and the selected specimen were micro photographed digitally using COSLAB DCE-2 attachment.
Phosphate solubilisation ability
Qualitative screening of phosphate solubilising Trichoderma was performed on Pikovskaya agar medium (Himedia) containing tricalcium phosphate as a phosphate source and bromocresol purple (0.1 g l−1) as a pH indicator for acidification (Vazquez et al. 2000). After incubation of actively growing colonies of Trichoderma at 28 ± 2 °C for 48 h, phosphate solubilising isolates turned the media colour from purple to yellow in the zones of acidification.
Quantitative estimation of phosphate solubilisation in Pikovskaya broth (Himedia) was performed according to the procedure of Jackson (1973). Freshly grown Trichoderma isolates were inoculated to 50 ml of Pikovskaya’s broth and incubated at 28 ± 2 °C and 100 rpm. The amount of inorganic phosphate (Pi) released in the broth was estimated after 5 days of incubation in comparison with un-inoculated control. The broth culture was centrifuged at 10,000 rpm for 10 min to separate the supernatant from the mycelial growth and insoluble phosphate. To the 0.5 ml of the culture supernatant, 5 ml of chloromolybdic acid was added and mixed thoroughly. Volume was made up to 10 ml with distilled water and 125 μl chlorostannous acid was added to it. Immediately, the final volume was made up to 25 ml with distilled water and mixed thoroughly. After 15 min, the blue colour developed was read in a spectrophotometer at 610 nm using a reagent blank. Corresponding amount of soluble phosphorous was calculated from a standard curve of potassium dihydrogen phosphate (KH2PO4). Phosphate solubilizing activity was expressed in terms of tricalcium phosphate solubilization which in turn was measured by μg/ml of available orthophosphate as calibrated from the standard curve of KH2PO4.
Quantification of indole-3-acetic acid (IAA) production
For the quantitative estimation of IAA, four agar plugs (5 mm) from the edge of actively growing colonies of Trichoderma were inoculated to 20 ml DF salts minimal media (Dworkin and Foster 1958) in 100 ml conical flasks and incubated for 3 days at 28 ± 2 °C. The medium was supplemented with L-tryptophan at a concentration of 1.02 g/l. After incubation for 72 h, the mycelia were removed from the culture medium by centrifugation at 5,000 rpm for 5 min. One ml aliquot of the supernatant was mixed vigorously with 4 ml of Salkowski’s reagent (Gordon and Weber 1951) and allowed to stand at room temperature for 20 min. The absorbance at 535 nm was measured with DF salts minimal media (plus Salkowski’s reagent) as blank. The concentration of IAA in each culture supernatant was determined using an IAA (Himedia) standard curve.
Evaluation for plant growth promoting response
For seed treatment, Trichoderma spores were harvested from 7-day old sporulating cultures of different isolates and re-suspended in 20 ml of sterile distilled water containing 0.5 %(w/v) carboxy methyl cellulose such that the spore concentration was maintained to 108 conidia/ml. Seeds of bottle gourd (Lagenaria siceraria; F1 hybrid from VNR seeds), cucumber (Cucumis sativus; Local variety) and bitter gourd (Momordica charantia; F1 hybrid from VNR seeds) were treated with spore suspension for one hour.
Out of a total of 20 isolates, 12 Trichoderma isolates (showing profuse sporulation on PDA) were selected to evaluate the plant growth promotion activity. Treated seeds were sown in pots containing autoclaved soil under natural sunlight condition with regular irrigation as per requirement (soil was not amended with any fertilizer). Control seeds were treated with 0.5 % (w/v) carboxy methyl cellulose prepared in sterile distilled water. From different treatments seedlings were harvested 1 month after sowing and observations were recorded for shoot length and root length for five plants per treatment with three replications.
Estimation of chlorophyll content
Chlorophyll (total chlorophyll, Chlorophyll a and Chlorophyll b) contents were determined by following the method of Arnon (1949). Fresh fully opened leaves harvested from apical portion of the plants (30 day old) were cut into small pieces of which 100 mg from each sample were homogenized in 80 % acetone and volume was made up to 10 ml. Extract was centrifuged at 5000 rpm for 5 min and supernatant was transferred to another tube and final volume of the extract was made up to 20 ml. Absorbance of the extract was recorded at 645, 652 and 663 nm in a spectrophotometer with 80 % acetone used as blank. Chlorophyll content was estimated using the formula:
where, A = Absorbance at different wavelengths; V = Final volume (20 ml); w = Fresh weight of the sample (0.1 g); a = path length (1 cm)
Molecular profiling of Trichoderma isolates
DNA extraction
DNA extraction was performed by following the method of Agrawal and Kotasthane (2010a, 2010b). A mycelial mat was homogenized to uniform consistency in a re-suspension buffer containing 50 mM Tris–HCl, 10 mM EDTA at pH 7.5 and 1 % SDS. DNA was extracted with 5 M potassium acetate solution (pH 4.8) and chilled isopropanol followed by RNase (Bangalore Genei, 10 mg/ml) treatment. Final precipitation of DNA was done with ice cold absolute ethanol. The pelleted DNA was air dried and re-suspended in 100 μl of sterile TE (10 mM Tris–HCl and 1 mM EDTA at pH 8.0) buffer.
Polymerase chain reaction (PCR), cleaved amplified polymorphic sequence (CAPS) and cluster analysis
Amplification of the nuclear rRNA gene cluster from 20 isolates of Trichoderma containing internal transcribed spacer (ITS1), ITS2, and the 5.8S rRNA gene was performed using two different primer pairs: ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) - ITS5 (5′-GGA AGT AAA AGT CGT AAC AAG G-3′) (Miyazaki et al. 2009) and HjITS F (5′-AGC GGA GGG ATC ATT ACC GAG TTT-3′) – HjITS R (5′-TCG CAT TTC GCT GCG TTC TTC ATC-3′). Primers were commercially synthesized from ILS (Imperial Life Sciences limited). HjITS forward and reverse primers were designed using Batch primer3 from EU280094 gene sequence of Hypocrea jecorina strain CIB T118 procured from NCBI database. PCR amplification was performed in a total volume of 20 μl and the reaction mixture contained 1× assay buffer (10 mM Tris–HCl at pH 9.0, 50 mM KCl, 2.5 mM MgCl2), 0.1 mM dNTP mix, 0.5 μM both forward and reverse primers, 60–90 ng of template DNA and 0.5 U Taq polymerase (Axygen) in a Gradient Palm Cycler (Corbett Life Science, Sydney, Australia). After a denaturation step for 3 min at 94 °C, the amplification was carried out for 30 cycles. Each cycle comprised of 1 min each of 94 °C, 55 °C (for ITS4-ITS5) or 59 °C (for HjITSF- HjITSR) and 72 °C. The final elongation step was extended to 7 min.
ITS4-ITS5 and HjITS F-HjITS R derived PCR amplicons were subjected to CAPS analysis using MspI (tetra cutter) and HinfI (penta cutter) (New England Biolabs, Beverly, MA) of all the 20 isolates. Twenty μl enzyme digestion reaction mixtures consisted of 5 μl of PCR product, 1× enzyme specific buffer, 1× bovine serum albumin and 0.5 U of enzymes which was incubated at 37 °C for 1 h. The non digested and digested PCR products along with 100 bp ladder (New England Biolabs, Beverly, MA) were electrophoresed on 5 % native polyacrylamide gel in 1× TBE (Tris borate EDTA) electrophoresis buffer and visualized after silver staining.
Statistical analysis
The design of all phenotype assays and plant growth experiments was a completely randomized block with three replications per treatment. Data of all biochemical tests and plant growth experiments (repeated twice) were subsequently analyzed by ANOVA followed by Duncan’s test using SAS PROC GLM program of SAS 9.2 software (Copyright 2002–2008 by SAS Institute Inc., Cary, NC, USA). Duncan’s test controls the Type I comparison wise error rate and as per Duncan’s grouping, means with the same letter are not significantly different. Duncan’s test can be used irrespective of whether F is significant or not and compares all possible pairs of treatment means.
PCR characters were analyzed using NTSYS-pc V2.2. For each primer pair as well as specific digestion products the presence or absence of bands in each isolate was visually scored and set in a binary matrix. DNA fragments showing polymorphism were scored as present (1) or absent (0) and used for statistical analysis. Genetic similarities among the isolates were determined based on the Jaccard’s coefficient. A dendrogram was then constructed using the un-weighted pair-group method of the arithmetic average (UPGMA) with a SAHN module of NTSYS-pc V2.2 (Rohlf 2002). The statistical analysis was applied to both individual and pooled data matrix of primers and digested products.
Results
Chitinase activity and cellulose hydrolysis
Twenty isolates of Trichoderma screened for their ability to hydrolyse chitin showed differential response (Table 1). Chitin hydrolysis was significantly higher for two isolates, viz. N (Trichoderma virens) and T27 (Trichoderma aureoviride), than the remaining isolates. The same set of Trichoderma isolates showed variable response for cellulose hydrolysis on Mandel and Weber’s medium supplemented with PASC (Table 1). Isolate T1 belonging to section Longibrachiatum exhibited highest cellulose hydrolysing ability (72 mm diameter). Five candidate Trichoderma isolates, T17 (Trichoderma viride), T16 (Trichoderma harzianum), T27 (T. aureoviride), T1 (section Longibrachiatum), and T158a (section Pachybasium), had dual enzymatic potential (high chitinase and cellulase activity). It was also demonstrated that there was variability between individual isolates of the same species for chitinase and cellulose production. There was incongruence between chitin and cellulose hydrolysis of the isolates.
Quantitative assay for siderophore production
Siderophore production by 20 Trichoderma isolates as analysed using CAS assay ranged from 11.09 to 95.31 % (Table 1). Isolates T110 and T15 belonging to section Pachybasium and T. harzianum respectively showed the highest production in siderophore units at 95.31 % whereas isolate T73 (section Pachybasium) showed the least 11.09 % siderophore units. Thirteen out of twenty isolates showed siderophore production above 85.00 %. Fifteen isolates produced % siderophore units ranging between 81.57 and 95.31 %.
In vitro fungal growth inhibition assays (Confrontation assay)
Confrontation assays were performed using 20 Trichoderma isolates against two soilborne plant pathogens (S. rolfsii and R. solani) which showed varying degrees of antagonistic response against both phytopathogens. Growth inhibition ranged from 49.50 to 81.00 % and 60.50 to 100.00 % for S. rolfsii and R. solani respectively (Table 2). More effective antagonistic response of Trichoderma isolates was observed against R. solani than S. rolfsii on agar plates. In the present study, Trichoderma isolates were considered to be more effective and efficient antagonists towards R. solani because, of the 20 isolates, 75 % shared class 1 and 25 % shared class 2 of the scale of antagonism. However, only 20 % Trichoderma isolates fell into class 1 with S. rolfsii.
Isolates showing antagonism class 1 and 100 % inhibition against R. solani were T14 (T.viride), T15 (T.harzianum), T27, T29, T114, T120 (T. aureoviride) and T101b (T. virens: 90 % inhibition). Isolates which showed effective inhibition against the S. rolfsii were T14 (T. viride), T101b (T. virens) and T66 (Sec. Pachybasium). Isolate T66 was significantly more efficient than all other isolates in suppressing linear growth of S. rolfsii. Isolates T15, T16, T27, T114, T120, T73, T93, T110, T158a & T174 showed the same level of efficiency in suppressing the linear growth of S. rolfsii. T. viride isolate T14 was significantly effective against S. rolfsii as well as R. solani. However, isolates T1 and T31 belonging to Sec. Longibrachiatum were poor antagonists for both the phytopathogens. The degree of antagonism was not the same for particular Trichoderma isolate with both the phytopathogens. For example, T. aureoviride isolate showing 100 % apparent inhibition against R. solani was poor against S. rolfsii (only 56.05 % inhibition). Similarly, isolate T66 (Sec. Pachybasium) which exhibited the maximum antagonism against S. rolfsii was not effective towards R.solani. Results of present interaction signify that a particular isolate of the antagonist may be highly effective against one phytopathogen (here R. solani) but may have only minimal effect on other phytopathogen (here S. rolfsii) and vice versa.
Selected cultures from pairings of the Trichoderma x pathogen combinations were viewed microscopically to determine state and fate of pathogen. In confrontation assays with S. rolfsii (Fig. 1a), Trichoderma sp. does not coil around S. rolfsii hyphae, but it recognises the host, overgrows and lyses the mycelia of S. rolfsii. There occurs disintegration of host cell wall and dissolution of cytoplasmic contents (Fig. 1b1-3). The host cell is also deprived of nutrition and space required for survival due to overgrowth of Trichoderma. On the other hand, confrontation of Trichoderma sp. with R. solani (Fig. 2a) resulted in hyperparasitic coiling around the hyphae of R. solani (Fig. 2b1-3). The mycelial tip of Trichoderma ran parallel on to the mycelium of R. solani and produced side branches at regular intervals. These side branches were observed to grip the host mycelia at several places. The appressoria-like penetrating structures were produced upon Rhizoctonia by Trichoderma. At several targeted sites of penetration, intense shining was clearly discernable. Such small tapering branches effected penetration and direct entry of Trichoderma hyphae into the lumen of the target fungus resulting in dissolution of the cell walls.
a In vitro antagonistic activity of Trichoderma (T) on S. rolfsii (Sr) carried out on a 90-mm PDA plate and observed 5 DAI; Petri plate has Trichodermea at the top and S. rolfsii at bottom and b its microscopic observations; (B1) mycelia recognition of host (S. rolfsii) by Trichoderma as indicated by arrow heads, (B2) growth of Trichoderma through S. rolfsii mycelia and (B3) complete overgrowth of Trichoderma over S. rolfsii depriving it of nutrition and space required for survival
a In vitro antagonistic activity of Trichoderma (T) on R. solani (Rs) carried out on a 90-mm PDA plate and observed 5 DAI; Petri plate has Trichoderma at the top and R. solani at bottom and b its microscopic observations; (B1) mycelia recognition of host (R. solani) by Trichoderma, (B2) coiling of host mycelia by Trichoderma, (B3) host cell rupture by Trichoderma resulting in complete dissolution of R. solani mycelia
Phosphate solubilisation ability
Each of the 20 isolates were capable of differentially utilizing tri-calcium phosphate in both agar plate and broth assays. Quantitative estimation of soluble phosphate concentrations in Pikovskaya’s broth was expressed as μg/ml and it varied from 139 to 780 μg/ml. The lowest value was observed for T. harzianum isolate T16 (139 μg/ml) and highest for T. viride isolate T14 (780 μg/ml). All the rhizospheric isolates of Trichoderma showed variable phosphate solubilizing potential with T14, T1, and N being the best P solubilizers (Table 3). It was observed that the production of various plant growth-promoting metabolites was a characteristic of an individual isolate and is not consistent within any species of Trichoderma. No single isolate was best for producing all the metabolites.
Quantification of indole-3-acetic acid (IAA) production
Production of IAA was evaluated for 20 isolates of Trichoderma in culture medium amended with 1.02 g/l of L-tryptophan as precursor molecule. Interpolation of the spectrophotometer readings with standard curve were used to quantify the amount of IAA produced by different isolates of Trichoderma in the media which ranged from 1.08 to 30.80 μg/ml (Table 3). Differential production of IAA was observed in different isolates of the same species. For example, T. viride, T. aureoviride and Section Longibrachiatum were represented by isolates with widely varying abilities to produce IAA. A significantly high amount of IAA was produced by T. viride isolate T14 (30.80 μg/ml), whereas T. aureoviride isolate T27 (0.818 μg/ml) was the lowest producer. Trichoderma viride isolate T14 induced highest plant growth promoting activity, and chlorophyll a, b and total content, when applied as seed treatment to cucumber and bottle gourd. Isolate T15 and T16 (T. harzianum) were also identified as promising producers of indole acetic acid 28.87 μg/ml and 24.34 μg/ml respectively. Trichoderma harzianum isolate T16 treated plants expressed highest chlorophyll a and total chlorophyll content in bitter gourd. Isolate T15 was identified as high siderophore producer with plant growth promotion activity. Efficacy of different isolates of Trichoderma used in the present investigation to produce IAA from L-tryptophan as a precursor varied significantly. Trichoderma viride isolate T14 and T. harzianum isolate T15 and T16 were identified as the highest producer of IAA thus indicating their ability to efficiently synthesize IAA from L-tryptophan as a precursor in vitro.
Evaluation for plant growth promoting response
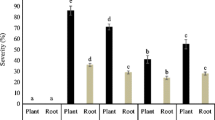
Efficacy of selected isolates of Trichoderma varied to induce root and shoot length (Table 4; Fig. 3). Root length was increased by 75, 25 and 92 % of Trichoderma isolates in cucumber, bottle gourd and bitter gourd respectively; whereas 42, 83 and 66 % of Trichoderma isolates enhanced length of aerial parts in cucumber, bottle gourd and bitter gourd respectively. Trichoderma viride isolate T14 produced significantly higher increase in root and shoot length on cucumber as compared to control and other treatments. Trichoderma viride isolate T14 also enhanced shoot length in bottle gourd. T. aureoviride isolate T27 was responsible for significant shoot development in bitter gourd, whereas root length was enhanced by T. virens isolate T101b. T. virens isolate N was identified as a positive inducer of both shoot and root length for cucumber, bottle gourd and bitter gourd. T. viride isolate T14 significantly enhanced shoot length of cucumber as well as bottle gourd and served as a positive inducer of root length in cucumber. Negative inducers were also identified in the present study. For example, T. virens isolate T101b and T. aureoviride isolate T29 reduced shoot and length in cucumber and an apparent reduction in root length was observed in T101b treated bottle gourd.
Estimation of chlorophyll content
Chlorophyll (Total chlorophyll, chl a and chl b) contents as determined on freshly harvested leaves derived from 30 day old plants which were grown following seed treatment with 12 different isolates of Trichoderma (Table 5) showed variation. Total chlorophyll content in cucumber ranged from 6.73 mg/g to 29.55 mg/g and chlorophyll a and b ranged from 4.11 to 13.96 mg/g and 2.17 to 13.21 mg/g, respectively. Chlorophyll a, b and total chlorophyll content for control plants was 5.31, 3.04 and 7.64 mg/g, respectively. T. viride T14 treated plants measured highest chlorophyll a (13.96 mg/g), b (13.21 mg/g) and total chlorophyll (29.55 mg/g) content as compared to the control. Total chlorophyll content in bottle gourd ranged from 11.52 mg/g to 24.13 mg/g. Chlorophyll a and b ranged from 7.68 to 15.72 mg/g and 3.51 to 8.24 mg/g, respectively. The chlorophyll a, b and total chlorophyll content for control plants was 7.98, 3.51 and 11.52 mg/g, respectively. Trichoderma viride T14 treated plants measured highest chlorophyll a, b and total content as 15.72, 8.24 and 24.13 mg/g, respectively as compared to chlorophyll content of control. Total chlorophyll content in bitter gourd ranged from 10.46 to 19.40 mg/g. Chlorophyll a and b ranged from 5.36 to 13.28 mg/g and 4.03 to 6.23 mg/g, respectively. Chlorophyll a, b and total chlorophyll content for control plants was 5.36, 4.39 and 11.43 mg/g, respectively. T. harzianum T16 treated plants measured highest chlorophyll a and total content as 13.28 and 19.40 mg/g, as compared to the control. T. aureoviride T120 treated plants measured highest chlorophyll b content as 6.23 as compared to the control.
Molecular profiling of Trichoderma isolates
PCR amplification of the ITS region of 5.8S rRNA gene with primer ITS4 - ITS5 yielded an ITS fragment of approximately 700 bp in length in all 20 isolates of Trichoderma. No distinct inter- or intra-specific ITS length diversity was detected. This is due to the fact that the 5.8S rRNA gene is known to be highly conserved at genus level and this only confirmed that all the isolates belonged to a single genus. However, digestion of ITS amplicons with restriction endonucleases MspI and HinfI revealed polymorphism in the ITS1-5.8S-ITS2 region (gel picture not shown). HinfI digestion of ITS4-ITS5 amplicons produced three fragments of 305, 180 and 110 bp specific to isolates of Sec. Longibrachiatum; two fragments of size 305 and 255 bp were specific for two T. virens isolates; fragments of 310 and 250 bp were specific to overlapping isolates T. harzianum and T. viride. Digestion of ITS4-ITS5 amplicons with MspI enzyme produced two fragments of sizes 300 & 110 bp specific for the three isolates of Sec. Longibrachiatum, whereas 180 bp fragment was specific to the two virens isolates. Digestion of amplicons of HjITSF-HjITSR primers with HinfI produced 550, 500 and 180 bp bands specific to three isolates of Sec. Longibrachiatum and fragments of 450 and 400 bp were observed specifically in two T. harzianum isolates; whereas fragments of 490 and 460 bp were observed only in two T. virens isolates. These specific ribosomal DNA restriction patterns generated by the enzymes were responsible for categorizing and specifically differentiating most of the isolates of Trichoderma. Individual binary data of restriction digestion (MspI & HinfI) for ITS4-ITS5 and HjITSF-HjITSR primers were used to obtain genetic similarity coefficients using the Jaccard algorithm. The same statistical approach was repeated for the pooled data set from bothprimers together.
Cluster analysis performed on the binary matrix derived from CAPS analysis of ITS4-ITS5 primer based amplicons with HinfI enzyme divided the 20 isolates into four distinct clusters: Cluster 1 of three isolates of Sec. Longibrachiatum (T1, T7 & T31), Cluster 2 of two T. virens isolates (N & T101b), Cluster 3 consisted of mixed groups of isolates of Sec Pachybasium (T66, T73, T93, T110, T132, T158a & T174) and T. aureoviride (T27, T29, T114 & T120), and Cluster 4 of overlapping T. harzianum and T. viride isolates (T14, T15, T16 & T17). Clustering based on this dendrogram was not able to discriminate isolates of Sec. Pachybasium and T. aureoviride and the overlapping nature of T. harzianum and T. viride could not be resolved (Fig. 4). However, cluster analysis performed on the binary matrix derived from CAPS analysis of HjITSF-HjITSR primer based amplicons with HinfI enzyme could resolve overlapping isolates of T. harzianum and T. viride into two separate groups but could not differentiate isolates of Sec. Pachybasium from T. aureoviride (Fig. 5). Cluster analysis performed on the binary matrix derived from CAPS analysis of ITS4-ITS5 primer based amplicons with MspI enzyme also differentiated overlapping isolates of T. harzianum and T. viride, but T. viride T14 isolate was placed away from the other isolates (Fig. 6). Cluster analysis performed on the binary matrix derived from CAPS analysis of pooled data set from both primers together further resolved the clustering, but was still unable to clearly discriminate isolates of Sec. Pachybasium and T. aureoviride (Fig. 7).
Discussion
Fungi belonging to the genus Trichoderma are well known producers of cell-wall degrading enzymes and used commercially as sources of these proteins (Harman et al. 1993, 2008; Domingues et al. 2000; Kubicek 2013). Of all of the mycoparasitic Hypocrea/Trichoderma species studied, the large holomorphic species H. lixii/T. harzianum likely contains superior chitinase-producing strains. Nagy et al. (2007) observed that the superior chitinase producers may not necessarily exhibit similarly increased cellulase formation and vice versa. Similar observations were recorded in the present work with T. virens isolates N and T101b and T. aureoviride T27 which produced significantly high chitinase activity but cellulose hydrolysis was low. This justifies the fact that production of any metabolite or biomolecule may be specific for certain individual or strain within a species. Trichoderma isolates with high enzymatic activity, in particular chitinase activity, are the isolates of choice for their application as biological control agent therefore screening procedures should include a more diverse population of a given species from particular environment.
Normally, bacteria and fungi coexist with each other in the rhizosphere soil and produce siderophore and related compounds which are capable of sequestering the available soluble iron (Becker et al. 1985; Johnson 2008). Dutta et al. (2006) studied production of siderophores by nine different soil fungi and wood-decay fungi following CAS-assay. Microbial siderophores benefit the plants by increasing solubility of otherwise unavailable ferric iron (Fe III) for plant nutrition (Renshaw et al. 2002). In vitro production of soluble forms of manganese, metallic zinc, calcium phosphate and metabolites that reduce ferric iron (III) to the ferrous form, which are readily assimilated by plants, has been reported for T. harzianum strain T22 by Altomare et al. (1999). Trichoderma siderophores are not only the components of growth stimulation but are also involved in control of plant pathogens (Anusuya and Jayarajan 1998; Harman 2006; Woo et al. 2006). Differential ability to produce a high affinity chelator for Fe III may be responsible for the variability in siderophore production as observed among Trichoderma isolates in the current study.
The present study determined the potential antagonistic variation of isolates of Trichoderma to the two pathogens. Similar results were reported by Bell et al. (1982) in their antagonistic study of Trichoderma spp. against six phytopathogens. They considered an isolate of Trichoderma to be antagonist to the pathogen if the mean score for a given comparision was ≤2, but not highly antagonist if the number was ≥3. In-vitro study measures the varying susceptibility of pathogens to Trichoderma; however, it has been realized that in vitro screening with the arbitrary rating system for a biological antagonist effective against soil-borne plant pathogens is a simplistic approach to understand a small sector of biological system in disease control. The interactions also suggest that it may be more prudent to evaluate blends of antagonist isolates for wider application against a greater number of phytopathogens. In vitro assays may not be predictive of the potential antagonist because pathogenicity of the phytopathogen or inhibitory effect of the antagonist is strongly affected by soil conditions and microenvironment, therefore simultaneous in vivo inhibition assays should also be performed.
Mechanisms of mycoparasitism have been studied with several model Trichoderma spp. which involve lysis of host’s cell wall due to both physical as well as enzymatic activity (Benítez et al. 1998). It was observed that the mycoparasite drained out the host mycelia contents thus leaving behind the empty host carcass (Agrawal and Kotasthane 2010a, b). Mycelial growth of Sclerotinia sclerotiorum (Kapil and Kapoor 2005; Lee and Wu 1984) was inhibited by metabolites produced by T. viride. Mukherjee et al. (2003) and Rawat and Tewari (2011) reported that in contrast to hyperparasitic coiling in R. solani, hyphal overgrowth occurs during S. rolfsii interaction by Trichoderma sp.. Molecular mechanisms involving expression of different enzymes, metabolites and differentiation between self versus non-self fungal cell-wall degradation has been well documented and reviewed (Mukherjee et al. 2012a, b; Baker et al. 2012; Gruber and Seidl-Seiboth 2012). This opens new avenues towards understanding of the use and careful choice of these necrotrophs as potential biocontrol agents to replace chemical control agents.
Several metabolic factors such as phosphate solubilisation, siderophore and auxin production may be responsible for growth regulation in different agricultural and vegetable crops. Raghothama (1999) reported that phosphorus frequently is the least accessible macronuntrient in many ecosystems and its low availability is often limiting to plant growth. In vitro phosphate solubilization efficacy of Trichoderma isolates as performed on Pikovaskaya agar by acidification showed positive results for all the 20 isolates tested in the current study. However, acidification of the media indicated by production of a yellow pigment was not necessarily an effective indicator of phosphate solubilisation as reported by Hoyos-Carvajal et al. (2009). Dunaitsev et al. (2008) demonstrated Trichoderma spp. as promising inducers of phosphate mobilization. The ability to release phosphorus from mineral raw materials can be considered as promising producers of a biological preparation of combined an operating bio-fungicide and inducer of phosphate mobilization. Altomare et al. (1999) observed that in the natural habitats, the phytopathogens such as Pythium and Rhizoctonia can be suppressed easily by the high competitive efficiency of T. harzianum through P-uptake because the former are unable to solubilize phosphates. Phosphate solubilization potential may be an attribute contributing to biocontrol properties of Trichoderma.
Trichoderma spp. produce auxins that are able to stimulate plant growth and root development. Auxins are key hormones effecting plant growth and development that can be produced by fungi in both symbiotic and pathogenic interactions with plants (Gravel et al. 2007; Losane and Kumar 1992; Shayakhmetov 2001). IAA produced by T. virens and T. atroviride were found to stimulate the growth of Arabidopsis plants in vitro (Contreras-Cornejo et al. 2009). Potential indole acetic acid-producing isolate (T14) was identified in the current study.
In this study Trichoderma isolates were evaluated for their ability to stimulate growth in seeds of cucumber, bottle gourd and bitter gourd wherein variable responses were observed. It was observed that both positive and negative inducers for root and shoot length can be found in the isolates of single species. The present investigation shows that use of any particular Trichoderma sp. as a plant growth promoter cannot be generalised for all the test materials. Tucci et al. (2011) demonstrated the effects of the plant genetic background on the outcome of the interaction between different tomato lines and two biocontrol strains of T. atroviride and T. harzianum. They observed that in at least one tomato cultivar the Trichoderma treatment did not exert any plant growth promotion effect and was even seen to be detrimental. However, Trichoderma are multifunctional plant symbionts responsible for enhanced nutrient uptake, increased root and shoot growth, improved plant vigour and biotic/abiotic stress tolerance (Inbar et al. 1994; Yedidia et al. 2001; Harman et al. 2008; Harman 2011). Several lines of evidence indicate that Trichoderma induce phytohormones-like effect in the treated plants which is responsible for root and shoot development. Several auxin-like secondary metabolites produced by Trichoderma strains were able to induce plant growth and are required for development of lateral roots in Arabidopsis (Contreras-Cornejo et al. 2009; Vinale et al. 2008). Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to absorb energy from light. Variability was observed in chlorophyll content of the three cucurbits with different treatments.
Seed treatment with T. viride T14 induced comparatively larger effects similar to hormonal application for plant growth promotion as compared to other isolates used in the present investigation which was measurable in terms of high chlorophyll content, indole acetic acid production (29.09 μg/ml), phosphate solubilisation (780 μg/ml) and siderophore production (90.12 %). Apparent correlation between plant growth and metabolite production was observed for T. viride isolate T14. T. viride isolate T14 in the present study had a characteristic aromatic odour resembling coconut reported to be produced commonly by strains of T. viride, and sometimes also by T. atroviride (Kubicek and Harman 1998). 6-Pentyl pyrone (6-PP), the ‘coconut aroma’ volatile compound produced by Trichoderma spp., is one of the best-studied secondary metabolites from a biocontrol perspective having both antifungal and plant growth-promoting activities. (Mukherjee et al. 2012a). Vinale et al. (2008) reported an auxin-like effect in etiolated pea stems when treated with harzianolide and 6-pentyl pyrone, the major secondary metabolites produced by different Trichoderma strains. Plant greenness is also increased by Trichoderma as observed in maize by application of Trichoderma-T22 (Mastouri et al. 2010; Shoresh et al. 2010). Higher photosynthetic rates and systemic increases in the uptake of CO2 in leaves have been induced in maize due to rhizosphere colonization by T. virens (Vargas et al. 2009). We report here in for the first time the growth response conducted on the three cucurbits using native isolates of Trichoderma which were collected from different geographical locations of Chhattisgarh.
Morphological descriptors of Trichoderma are often confusing therefore molecular tools are employed for identification. Size differences of the intact ITS1 fragments can be used for distinguishing the strains into groups that correspond to sections and clades (Lord et al. 2002; Kindermann et al. 1998). Digestion patterns of the intact ITS1 fragments can be obtained with restriction enzymes, and the advantage of using the digestion patterns is that the differences of the ITS fragments are more clear (Bulat et al. 1998; Lübeck et al. 1999, 2000). However, ribotyping must be used with caution as the recognition site for the enzymes only requires one base substitution in order to be lost. Elke et al. (1999) and Latha et al. (2002) performed restriction analysis of the amplified ITS1–5.8S–ITS2 region of the nuclear ribosomal DNA for molecular profiling and reclassification of Trichoderma spp. However, the overlapping in species identification of strains was observed. This intron has been used as a reliable phylogenetic marker for Hypocrea/Trichoderma (Jaklitsch et al. 2006; Samuels et al. 2006). Paz et al. (2010) used sequences of the internal transcribed spacers 1 and 2 (ITS1 and ITS2) of the rRNA gene cluster and the fourth intron of the translation elongation factor 1-alpha-encoding gene (tef1) to attribute three of their strains to the two putatively new phylogenetic species Trichoderma sp. O.Y. 14707 and Trichoderma sp. O.Y. 2407, respectively. However, it is recommended that when using heterogenous populations of Trichoderma, different phylogenetic markers such as ITS, tef1, cal, RPB2 should be used simultaneously along with TrichOKEY 2 for molecular profiling, in-situ diversity studies and species identification (Friedl and Druzhinina 2012).
It can be concluded that for the development of a biological control program it is important to consider together the interaction of the three components, plant-Trichoderma-pathogen. Dramatic changes may be induced by this beneficial fungus in the plant-microbial community as a result of the mutual molecular dialogue, occasionally establishing a positive interaction within plant roots as an endophyte. Such association has significant implications on the selection of specific strains that can be used as bio-inculants to improve crop health and productivity (Harman 2006; Woo et al. 2006; Hermosa et al. 2012). In the present study, we identified a potential Trichoderma isolate (T. viride T14) that could stimulate growth in cucumber and bottle gourd and act as antagonist against S. rolfsii and R. solani under in-vitro conditions. However this research program needs to be, and will be, further extended for disease suppression studies under natural field conditions.
References
Agrawal, T., & Kotasthane, A. S. (2010a). A simplified method for extraction of genomic DNA from filamentous fungus belonging to the genus Trichoderma. International Journal of Biotechnology and Bioengineering Research, 1(1), 47–52.
Agrawal, T., & Kotasthane, A. S. (2010b). Mycoparasitism and AFLP characterization of Trichoderma spp. isolated from Chhattisgarh in Central India effective against Rhizoctonia solani infecting rice. Journal of Mycology and Plant Pathology, 40(4), 532–539.
Agrawal, T., & Kotasthane, A. S. (2012). Chitinolytic assay of indigenous Trichoderma isolates collected from different geographical locations of Chhattisgarh in Central India. Springer Plus, 1, 73.
Altomare, C., Norvell, W. A., Björkman, T., & Harman, G. E. (1999). Solubilization of phosphates and micronutrients by the plant-growth promoting and biocontrol fungus Trichoderma harzianum rifai 129522. Applied and Environmental Microbiology, 65, 2926–2933.
Anusuya, D., & Jayarajan, R. (1998). Solubilization of phosphorus by Trichoderma viride. Current Science, 74, 464–466.
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts: Polyphenol oxidise in Beta vulgaris. Plant Physiology, 24, 1–15.
Baker, S. E., Perrone, G., Richardson, N. M., Gallo, A., & Kubicek, C. P. (2012). Phylogenetic analysis and evolution of polyketide synthase-encoding genes in Trichoderma. Microbiology, 158, 147–154.
Becker, J. O., Hedges, R. W., & Messens, E. (1985). Inhibitory effect of pseudobactin on the uptake of iron by higher plants. Applied and Environmental Microbiology, 49, 1090–1093.
Bell, D. K., Wells, H. D., & Markham, C. R. (1982). In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathology, 72, 379–382.
Benítez, T., Delgado-Jarana, J., Rincón, A. M., Rey, M., & Limón, M. C. (1998). Biofungicides: Trichoderma as a biocontrol agent against phytopathogenic fungi. In S. G. Pandalai (Ed.), Recent research developments in microbiology (Vol. 2, pp. 129–150). Trivandrum: Research sign post.
Bulat, S. A., Lübeck, M., Mironenko, N., Jensen, D. F., & Lübeck, P. S. (1998). UP-PCR analysis and ITS1 ribotyping of Trichoderma and Gliocladium. Mycological Research, 102, 933–943.
Contreras-Cornejo, H. A., Macias-Rodríguez, L., Cortés-Penagos, C., & López-Bucio, J. (2009). Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiology, 149, 1579–1592.
Dennis, C. J., & Webster, J. (1971). Antagonism properties of species groups of Trichoderma, III hyphal interaction. Transactions of the British Mycological Society, 57, 363–369.
Domingues, F. C., Queiroz, J. A., Cabral, J. M. C., & Fonseca, L. P. (2000). The influence of culture conditions on mycelial structure and cellulase production by Trichoderma reesei Rut C-30. Enzyme and Microbial Technology, 26, 394–401.
Druzhinina, I. S., Seidl-Seiboth, V., Herrera-Estrella, A., Horwitz, B. A., Kenerley, C. M., Monte, E., Mukherjee, P. K., Zeilinger, S., Grigoriev, I. V., & Kubicek, C. P. (2011). Trichoderma: the genomics of opportunistic success. Nature Reviews Microbiology, 9, 749–759.
Druzhinina, I. S., Shelest, E., & Kubicek, C. P. (2012). Novel traits of Trichoderma predicted through the analysis of its secretome. FEMS Microbiology Letters, 337, 1–9.
Dunaitsev, I. A., Kolombet, L. V., Zhigletsova, S. K., Bystrova, E. V., Besaeva, S. G., Klykova, M. V., & Kondrashenko, T. N. (2008). Phosphate releasing microorganisms with antagonistic activity against phytopathogenic microorganisms. Mikologiya i Fitopatologiya, 42(3), 264–269.
Dutta, S., Kundu, A., Chakraborty, M. R., Ojha, S., Chakraborty, J., & Chatterjee, N. C. (2006). Production and Optimization of Fe(III) specific ligand, the siderophore of soil inhabiting and wood rotting fungi as deterrent to plant pathogens. Acta Phytopathologica Et Entomologica Hungarica, 41(3–4), 237–248.
Dworkin, M., & Foster, J. (1958). Experiments with some microorganisms, which utilize ethane and hydrogen. Journal of Bacteriology, 75, 592–601.
Elke, L., Samuels, G. J., Nirenberg, H. I., & Petrini, O. (1999). A morphological and molecular perspective of Trichoderma viride: Is it one or two species? Applied and Environmental Microbiology, 65(6), 2418–2428.
Friedl, M. A., & Druzhinina, I. S. (2012). Taxon-specific metagenomics of Trichoderma reveals a narrow community of opportunistic species that regulate each other’s development. Microbiology, 158, 69–83.
Gordon, S. A., & Weber, R. P. (1951). Colorimetric estimation of indole-acetic acid. Plant Physiology, 26, 192–195.
Gravel, V., Antoun, H., & Tweddell, R. J. (2007). Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride : possible role of indole acetic acid (IAA). Soil Biology and Biochemistry, 39, 1968–1977.
Gruber, S., & Seidl-Seiboth, V. (2012). Self versus non-self: fungal cell wall degradation in Trichoderma. Microbiology, 158, 26–34.
Harman, G. E. (1992). The development and benefits of rhizosphere competent fungi for biological control of plant pathogens. Journal of Plant Nutrition, 15, 835–843.
Harman, G. E. (2006). Overview of mechanisms and uses of Trichoderma spp. Phytopathology, 96(2), 190–194.
Harman, G. E. (2011). Multifunctional fungal plant symbionts: new tools to enhance plant growth and productivity. New Phytologist, 189, 647–649.
Harman, G. E., Hayes, C. K., Lorito, M., Broadway, R. M., Di Pietro, A., Peterbauer, C., & Tronsmo, A. (1993). Chitinolytic enzymes of Trichoderma harzianum: purification of chitobiosidase and endochitinase. Phytopathology, 83, 313–318.
Harman, G. E., Bj¨orkman, T., Ondik, K., & Shoresh, M. (2008). Changing paradigms on the mode of action and uses of Trichoderma spp. for biocontrol. Outlooks on Pest Management, 19, 24–29.
Hermosa, R., Viterbo, A., Chet, I., & Monte, E. (2012). Plant-beneficial effects of Trichoderma and of its genes. Microbiology, 158, 17–25.
Hoyos-Carvajal, L., Orduz, S., & Bissett, J. (2009). Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biological Control, 51, 409–416.
Inbar, J., Abramsky, M., Cohen, D., & Chet, I. (1994). Plant growth enhancement and disease control by Trichoderma harzianum in vegetable seedlings grown under commercial conditions. European Journal of Plant Pathology, 100, 337–346.
Jackson, M. L. (1973). Soil chemical analysis (pp 392). New Delhi: Printice hall of India.
Jaklitsch, W. M., Samuels, G. J., Dodd, S. L., Lu, B. S., & Druzhinina, I. S. (2006). Hypocrea rufa /Trichoderma viride : a reassessment, and description of five closely related species with and without warted conidia. Studies in Mycology, 56, 135–177.
Johnson, L. (2008). Iron and siderophores in fungal-host interactions. Mycological Research, 112, 170–183.
Kapil, R., & Kapoor, A. S. (2005). Management of white rot of pea incited by (Sclerotinia sclerotiorum) using Trichoderma spp. and biopesticides. Indian Phytopathology, 58(1), 10–16.
Kindermann, J., El-Ayouti, Y., Samuels, G. J., & Kubicek, C. P. (1998). Phylogeny of the genus Trichoderma based on sequence analysis of the internal transcribed spacer 1 of the rDNA cluster. Fungal Genetics and Biology, 24, 298–309.
Kubicek, C. P. (2013). Systems biological approaches towards understanding cellulase production by Trichoderma reesei. Journal of Biotechnology, 163(2), 133–142.
Kubicek, C. P., & Harman, G. E. (1998). Trichoderma and Gliocladium. Vol.I and II.. London: Taylor and Francies Ltd.
Latha, J., Verma, A., & Mukherjee, P. K. (2002). PCR-fingerprinting of some Trichoderma isolates from two Indian type culture collections – a need for re-identification of these economically important fungi. Current Science, 83(4), 372–374.
Lee, Y., & Wu, W. (1984). The antagonisms of Trichoderma spp. and Gliocladium virens against Sclerotinia sclerotiorum. Plant Protection Bulletin (Taiwan ROC), 26, 293–304.
Lord, N. S., Kaplan, C. W., Shank, P., Kitts, C. L., & Elrod, S. L. (2002). Assessment of fungal diversity using terminal restriction fragment (TRF) pattern analysis: comparison of 18S and ITS ribosomal regions. FEMS Microbiology Ecology, 42, 327–337.
Losane, B. K., & Kumar, P. K. R. (1992) Fungal plant growth regulators. In, D. K. Arora, Elander K. G., & K. G. Mujerji (Eds.), Handbook of Applied Mycology: Fungal Biotechnology, (Vol 4, pp. 565–602).
Lübeck, M., Alekhina, I. A., Lübeck, P. S., Jensen, D. F., & Bulat, S. A. (1999). Delineation of Trichoderma harzianum into two genetic entities by a highly robust fingerprinting method, UP-PCR, and UP-PCR product cross-hybridization. Mycological Research, 103, 289–298.
Lubeck, M., Poulsen, S. K., Lubeck, P. S., Jensen, D. F., & Thrane, U. (2000). Identification of Trichoderma strains from building materials by ITS1 ribotyping, UP-PCR fingerprinting and UPPCR cross hybridization. FEMS Microbiology Letters, 185, 129–134.
Mandels, M., Hontz, I., & Nystrom, J. (1974). Enzymatic hydrolysis of waste cellulose. Biotechnology and Bioengineering, 16, 1471–1493.
Mastouri, F., Björkman, T., & Harman, G. E. (2010). Seed treatment with Trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedlings. Phytopathology, 100(11), 1213–1221.
Miyazaki, K., Tsuchiya, Y., & Okuda, T. (2009). Specific PCR assays for detection of T. harzianum causing green mold disease during mushroom cultivation. Mycoscience, 50, 94–99.
Mukherjee, P. K., Latha, J., Hadar, R., & Horwitz, B. A. (2003). TmkA, a mitogen-activated protein kinase of Trichoderma virens, is involved in biocontrol properties and repression of conidiation in the dark. Eukaryotic Cell, 2, 446–455.
Mukherjee, P. K., Buensanteai, N., Moran-Diez, M. E., Druzhinina, I. S., & Kenerley, C. M. (2012a). Functional analysis of non-ribosomal peptide synthetases (NRPSs) in Trichoderma virens reveals a polyketide synthase (PKS)/NRPS hybrid enzyme involved in the induced systemic resistance response in maize. Microbiology, 158, 155–165.
Mukherjee, P. K., Horwitz, B. A., & Kenerley, C. M. (2012b). Secondary metabolism in Trichoderma – a genomic perspective. Microbiology, 158, 35–45.
Nagy, V., Verena, S., Szakacs, G., Kubicek, C. P., Komoń-Zelazowska, M., & Druzhinina, I. S. (2007). Application of DNA bar codes for screening of industrially important fungi: the haplotype of Trichoderma harzianum sensu stricto indicates superior chitinase formation. Applied and Environmental Microbiology, 73(21), 7048–7058.
Payne, S. M. (1994). Detection, isolation and characterization of siderphores. Methods in Enzymology, 235, 329–344.
Paz, Z., Komon-Zelazowska, M., Druzhinina, I. S., Aveskamp, M. M., Schnaiderman, A., Aluma, Y., Carmeli, S., Ilan, M., & Yarden, O. (2010). Diversity and potential antifungal properties of fungi associated with a Mediterranean sponge. Fungal Diversity, 42, 17–26.
Punja, Z. K. (1985). The biology, ecolology, and control of Sclerotium rolfsii. Annual Review of Phytopathology, 23, 97–127.
Raghothama, K. G. (1999). Phosphate acquisition. Annual Reviews in Plant Physiology and Molecular Biology, 50, 665–693.
Rawat, R., & Tewari, L. (2011). Effect of abiotic stress on phosphate solubilization by biocontrol fungus Trichoderma sp. Current Microbiology, 62, 1521–1526.
Renshaw, J., Robson, G., Trinci, P., Wiebe, M., Livens, F., Collison, D., & Taylor, R. (2002). Fungal siderophores: Structures, functions and applications. Mycological Research, 106, 1123–1142.
Rohlf, F. J. (2002). NTSYSpc- Numerical taxonomy and multivariate analysis system, Version 2.2. Setauket: Exeter Software.
Samuels, G. J., Dodd, S., Lu, B., Petrini, O., Schroers, H. J., & Druzhinina, I. S. (2006). The Trichoderma koningii morphological species. Studies in Mycology, 56, 67–133.
Schuster, A., & Schmoll, M. (2010). Biology and biotechnology of Trichoderma. Applied Microbiology and Biotechnology, 87(3), 787–799.
Shayakhmetov, I. F. (2001). Biological activity of metabolites from culture filtrate of Cochliobolus sativus and Fusarium oxysporum in connection with in vitro cellular selection of crop plants for resistance to phytopathogens. Mycology and Phytopathology, 35, 66–71.
Shoresh, M., Harman, G. E., & Mastouri, F. (2010). Induced systemic resistance and plant responses to fungal biocontrol agents. Annual Reviews of Phytopathology, 48, 21–43.
Srinivasachary, S., Willocquet, L., & Savary, S. (2011). Resistance to rice sheath blight (Rhizoctonia solani Kühn) [(teleomorph: Thanatephorus cucumeris (A.B.. Frank) Donk.] disease: current status and perspectives. Euphytica, 178(1), 1–22. doi:10.1007/s10681-010-0296-7.
Tucci, M., Ruocco, M., De Masi, L., De Palma, M., & Lorito, M. (2011). The beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Molecular Plant Pathology, 12, 341–354.
Vargas, W. A., Mandawe, J. C., & Kenerley, C. M. (2009). Plant-derived sucrose is a key element in the symbiotic association between Trichoderma virens and maize plants. Plant Physiology, 151, 792–808.
Vazquez, P. G., Holguin, M. E., Puente, A., Cortes, L., & Bashan, Y. (2000). Phosphate-solubilising microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biology and Fertility of Soils, 30, 460–468.
Vinale, F., Sivasithamparam, K., Ghisalberti, E. L., Marra, R., & Barbetti, M. J. (2008). A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiology and Molecular Plant Pathology, 72, 80–86.
Viterbo, A., & Horwitz, B. A. (2010). Mycoparasitism. In K. A. Borkovich & D. J. Ebbole (Eds.), Cellular and molecular biology of filamentous fungi (Vol 42 (pp. 676–693). Washington: American Society for Microbiology.
Woo, S. L., Scala, F., Ruocco, M., & Lorito, M. (2006). The molecular biology of the interactions between Trichoderma spp., phytopathogenic fungi, and plants. Phytopathology, 96, 181–185.
Yedidia, I., Srivastva, A. K., Kapulnik, Y., & Chet, I. (2001). Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant and Soil, 235, 235–242.
Zou, J. H., Pan, X. B., Chen, Z. X., Xu, J. Y., Lu, J. F., Zhai, W. X., & Zhu, L. H. (2000). Mapping quantitative trait loci controlling sheath blight resistance in two rice cultivars (Oryza sativa L.). Theoretical and Applied Genetics, 101(4), 569–573. doi:10.1007/s001220051517.
Conflict of interest
Authors declare that they have no conflict of interest.
Author’s contributions
ASK and TA prepared the manuscript, TA performed research work and statistical analysis, RK and OVR performed research work. ASK and TA contributes equal share for the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kotasthane, A., Agrawal, T., Kushwah, R. et al. In-vitro antagonism of Trichoderma spp. against Sclerotium rolfsii and Rhizoctonia solani and their response towards growth of cucumber, bottle gourd and bitter gourd. Eur J Plant Pathol 141, 523–543 (2015). https://doi.org/10.1007/s10658-014-0560-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-014-0560-0