Abstract
This study was undertaken to explore the role of Trichoderma sp. in phosphate (P) solubilization and antagonism against fungal phytopathogens. All fungal isolates (SE6, KT6, KT28, and BRT11) and a standard culture of T. harzianum (Th-std) were able to antagonize two fungal phytopathogens (Sclerotium rolfsii and Rhizoctonia solani) of chickpea (Cicer arietinum L.) wilt complex. Transmission electron microscopic studies (TEM) further confirmed ultra-cytological changes in the sclerotia of S. rolfsii parasitized by Trichoderma sp. All fungal cultures exhibited production of NH3 and siderophore, but only BRT11, SE6, and Th-std could produce HCN. Among all the cultures tested, isolate KT6 was found to be most effective for solubilization of ferric phosphate releasing 398.4 μg ml−1 phosphate while isolates BRT11 and SE6 showed more potential for tricalcium phosphate (TCP) solubilization releasing 449.05 and 412.64 μg ml−1 phosphate, respectively, in their culture filtrates. Part of this study focused on the influence of abiotic stress conditions such as pH, temperature, and heavy metal (cadmium) on phosphate (TCP) solubilizing efficiency. Two selected cultures KT6 and T. harzianum retained their P solubilizing potential at varying concentrations of cadmium (0–1000 μg ml−1). Isolate KT6 and standard culture of T. harzianum released 278.4 and 287.6 μg ml−1 phosphate, respectively, at 1000 μg ml−1cadmium. Maximum solubilization of TCP was obtained at alkaline pH and at 28°C temperature. Isolate BRT11 was found most alkalo-tolerant releasing 448.0 μg ml−1 phosphate at pH 9.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intensification of agriculture and increase in population demands has reduced the structural steadiness of soil. To manage with low productivity, inorganic fertilizers have been intensively used since long back. The majority of agricultural soils contain large reserves of phosphorous (P), of which a considerable part is accumulated as a consequence of regular applications of phosphatic fertilizers. Phosphate in soil mostly exists in insoluble (bound) forms such as tricalcium phosphate (TCP) in alkaline soil and as ferric phosphate (FePO4) and aluminum phosphate (AlPO4) in acidic soil and the concentration of soluble phosphate in soil solution is very low ranging from 400 to 1,200 mg kg−1 of soil. Plants are able to utilize only a small proportion of phosphatic fertilizers that are applied, as a major fraction of it is rapidly converted into insoluble complexes in soil. Thus soils have a large reserve of insoluble phosphate that needs to be solubilized where phosphate solubilizing microorganisms play an important role [6]. Phosphate solubilizing microbes are able to liberate some of this essential element to improve long-standing crop production.
As the establishment and performance of phosphate solubilizing microorganisms are highly influenced by environmental factors, such as temperature, pH, heavy metal, etc., it is essential to isolate microorganisms from the relevant microbial niches to maximize the chances for their use as a means of improving phosphate solubilizing ability in the field [12]. Cadmium (Cd), a heavy metal of no known nutritional function in soil is associated with metal ores and phosphate rocks. It is introduced into the agricultural system by the application of synthetic fertilizers, pesticides, and sewage sludge and by water and air polluted with industrial effluents. Plants take up and accumulate heavy metal ions which results in stunted growth, with poor rooting and pale leaves. One approach to diminish the lethal effects of heavy metals taken up from the environment by some plants, involves the use of plant growth-promoting rhizobacteria (PGPR) and fungal-like mycorrhizae [8].
The plant growth-promoting bacteria (PGPB) are soil and rhizosphere bacteria that, in addition to mineralization abilities, can benefit plant growth by several different mechanisms, such as ammonia production, production of plant hormones, and control of phytopathogenic microorganisms [16]. Specifically, biological control of plant pathogens and deleterious microbes, through the production of antibiotics, cell wall-degrading enzymes, hydrogen cyanide, and siderophores or through competition for nutrients and space, can improve significantly plant health and promote growth as evidenced by increase in seedling emergence, vigor, and yield [2]. In recent years, Trichoderma sp. is widely used in agriculture as biocontrol agent and inoculants to provide plant growth promotion. They are involved in fundamental activities that ensure the stability and productivity of both agricultural and natural ecosystems [15].
Thus, it is highly desirable to have a bioagent with dual potential for biocontrol and plant growth promotion. Moreover, to develop and use it as a bioinoculant for supporting plantation in industrial effluent affected unfertile soil, it should have high metal resistance and also capability of retaining its plant growth promotory potential under other abiotic stress such as pH as well as biotic stress conditions. However, very little information is available in literature on the biocontrol fungus (Trichoderma sp.) having high pH (alkaline) and heavy metal tolerance and influence of these abiotic stress conditions on its phosphate solubilizing potential. The present investigation was carried out to characterize the Trichoderma strains for solubilizing inorganic phosphate under various abiotic stress conditions and to antagonize the fungal phytopathogens using transmission electron microscopy of the parasitized sclerotia of S. rolfsii with the view of utilizing them as biofertilizer sources to correspondence to boost the fertility of soils.
Materials and Methods
Fungal Isolates and Growth Conditions
Four fungal cultures viz., Trichoderma virens (SE6, KT28), T. viridae (KT6), and Aspergillus flavus (BRT11) were evaluated for their plant growth promotory potential and antagonism against two root rot/wilt causing fungal phytopathogens of chick pea (Cicer arietinum L.) viz., Rhizoctonia solani and Sclerotium rolfsii. Fungal cultures were compared for their efficiencies, with the standard culture of Trichoderma harzianum Rifai, MTCC792 (Th-std). All fungal isolates were obtained from Departmental culture collection (Department of Microbiology, G.B. Pant University of Agric. & Tech, Pantnagar, India) and maintained routinely on Potato dextrose agar (PDA) medium at 4°C.
In Vitro Screening of Fungal Cultures for Parasitization of Fungal Phytopathogens
In vitro antagonistic potential of all fungal cultures against two root rot/wilt causing fungal phytopathogens of chick pea (S. rolfsii and R. solani) was tested on PDA by dual culture plate technique [5]. Parasitized and non-parasitized sclerotia of S. rolfsii were collected either from pure culture or dual culture plates and were examined by transmission electron microscopy for structural changes [17].
Screening of Fungal Cultures for in Vitro Plant Growth Promotory Potential
The fungal cultures were evaluated for various plant growth promotory activities such as (i) HCN production following the method given by Bakker and Schipper [3]; (ii) Ammonia production from organic compound following the method given by Cappuccino and Sherman [4]; (iii) Siderophore production using chromeazurol S (CAS) assay [19]; and (iv) Phosphate solubilization on Trichoderma selective minimal medium (TSMM) (gl−1, MgSO4·H2O 0.2, NH4NO3 1.0, KCl 0.15, Glucose 3.0, Agar-20.0) containing 10 gl−1 of TCP or FePO4 separately as sole phosphorus source [12]. The plates inoculated individually with a fungal mycelial disc (5 mm) of active culture in each case, were incubated at 28°C for 5 days and evaluated for above mentioned activities at an interval of 24 h.
Influence of Abiotic Stress on in Vitro Phosphate Solubilization
All fungal isolates were evaluated for their in vitro solubilization of TCP and ferric phosphate in TSMM broth medium. Concentration of phosphate released upon solubilization of these insoluble (fixed) phosphorous sources in culture filtrates was estimated for a period of 5 days at regular interval of 24 h. In order to study heavy metal (cadmium) tolerance of fungal cultures and its influence on P solubilizing capacity, the cultures were grown in TCP containing TSMM broth having variable concentrations of cadmium ranging from 200 to 1000 μg ml−1 with an interval of 200 μg ml−1 as CdSO4·8H2O, while 0 μg ml−1 served as control. For selecting an isolate capable of solubilizing phosphate at broader pH values, the pH of the medium containing TCP as sole P-source was adjusted to different pH values (3.0, 5.0, 6.8, 8.0, 9.0, and 10.0). The effect of temperature was studied by incubating the cultures at different temperatures (12, 28, 37, and 45°C) at pH 6.8 and concentration of solubilized phosphate were estimated up to 5 days at a periodical interval of 24 h.
Statistical Analysis
All the experiments were conducted in triplicates and data were stored in Microsoft Excel. Completely randomized design (CRD) with two or three factors was used. Data were statistically analyzed using programme Stpr2 and Stpr15.
Results
Antagonistic Potential Against Fungal Phytopathogens
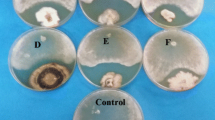
All fungal isolates when evaluated for their in vitro antagonistic potential against S. rolfsii and R. solani successfully inhibited the growth of both the pathogens. Isolate SE6 colonized R. solani in 120 h while isolate BRT11 took 176 h for complete colonization of the same pathogen on dual culture plates. However, the colony of S. rolfsii was completely overgrown by isolate SE6 and BRT11 in 168 h and 224 h, respectively (Fig. 1). The ultramicroscopic observations of the healthy sections revealed that unparasitized sclerotia were comprised of healthy hyphal cells that were surrounded by thick regular, electron dense cell wall. The antagonistic fungus Trichoderma sp. led to break the outer shell of sclerotia causing its destruction along with several histological changes such as degeneration and decay of cytoplasmic content, deformation and lysis of cell wall of hypha (Fig. 2).
In Vitro Plant Growth Promotory Potential of the Biocontrol Fungus
All fungal cultures showed in vitro production of siderophore (chelating agent) and ammonia but none of them could produce IAA. However, only three cultures viz. SE6, KT6, and BRT11 could produce the volatile toxic compound HCN. Qualitative analysis for the phosphate solubilizing capacity of all fungal cultures revealed good mycelial growth but no halo zone formation on the solid medium, devoid of any available phosphorus but contained only insoluble inorganic phosphorous source (Table 1).
In Vitro Solubilization of Inorganic Bound Phosphates [FePO4 and TCP]
All fungal cultures showed heavy mycelial growth when grown in mineral broth medium containing 1% inorganic bound phosphate (TCP or FePO4). During solubilization of FePO4, a gradual increase in phosphate concentration was recorded up to 96 h in all the cases except KT6, which showed maximum phosphate solubilization at 72 h. Among all the isolates tested, isolate KT6 released maximum phosphate (398.4 μg ml−1) in the culture filtrate (Fig. S1a). During solubilization of tricalcium phosphate, all the test cultures converted the insoluble phosphate into the soluble form with an increase in phosphate concentration up to 96 h followed by a gradual decline. All the insoluble TCP completely disappeared in the cultures within 72 h in all the cases. The isolates BRT11 and SE6 showed higher phosphate solubilizing capacity in comparison to others, releasing 449.05 and 412.64 μg ml−1 phosphate, respectively (Fig. S1b). The isolates showed a pH drop (from 6.8 to 2.86) along with increasing phosphate concentration up to 96 h followed by a gradual rise.
Effect of Heavy Metal (Cd) on P Solubilization
Both tested Trichoderma cultures (T. harzianum and KT6) were able to grow in the minimal medium containing heavy metal cadmium. The cultures showed heavy mycelial growth up to 600 μg ml−1 cadmium concentration. Still, they were capable of solubilizing inorganic phosphates in the presence of heavy metal though with lessen potential. Concentration of solubilized phosphate in culture filtrates decreased gradually with increasing cadmium concentration from 0 to 1000 μg ml−1 (Fig. 3a, b). T. harzianum showed significantly higher solubilization releasing 287.6 μg ml−1 phosphate in comparison to KT6 which produced 278.4 μg ml−1 phosphate at highest metal concentration (1000 μg ml−1).
Effect of pH and Temperature on P solubilization
Five fungal cultures were tested for their ability to solubilizing inorganic phosphate at different pH values and temperatures as described above. All isolates were able to grow and solubilize phosphate at tested acidic (3.0–5.0) and alkaline (8.0–9.0) pH values but they showed lowest phosphate concentration at pH 10 (Fig. S2). At pH 9.0, all isolates except SE6 and KT28, showed higher P solubilization. Among all the isolates tested, isolate BRT11 released maximum phosphate (448.0 μg ml−1) followed by isolate KT6 which produced 404 μg ml−1 phosphate in the culture filtrate at pH 9 after 72 h. At pH 6.8 with TCP as a substrate, a significant increase in the solubilization of phosphate substrate was also recorded in the cultures at various temperatures 12–45°C. Maximum phosphate solubilization by all strains occurred at 28°C while 45°C is the least suited temperature for the same (Fig. 4).
Discussion
Recent years have witnessed a worldwide swing to the use of eco-friendly methods for protecting the crops from pests and diseases and to enhance the plant growth and productivity. Plant disease suppression by the biocontrol agent Trichoderma sp. in soil has been widely documented, the isolates used in this study were first evaluated for their antagonistic potential against the two soil borne fungal phytopathogens (S. rolfsii and R. solani). The variations in the magnitude of mycoparasitization revealed their differential antagonistic potential. All fungal cultures showed better capacity to parasitize R. solani. Our results are in agreement with the previous investigation of Mishra [11], who has also reported R. solani to be more susceptible to inhibition by the isolates of Trichoderma as compared to S. rolfsii and Fusarium oxysporium. Parasitization of fungal phytopathogens (S. rolfsii) was further confirmed by transmission electron microscopy. The transmission electron micrographs of parasitized sclerotia showed clearly the deformation and lysis of fungal cell wall along with disappearance of compact cytoplasmic granules as also reported by Nicole and Chet [14]. Several histological changes as malformation, lysis and decay of the outer wall and the inner content in infected sclerotia of the pathogenic fungi was also reported by Sayed and Embaby [18].
All the cultures were also found to produce siderophore like chelating metabolites showing the orange halo zone around the colony but the nature of chelation was different. T. harzianum showed diffusible zone formation all over the plate while other isolates showed localized halo zone around the colony. The role of siderophore in plant growth promotion and biological control has been well established [10]. Various biocontrol agents are equipped with multiple mechanisms for biocontrol of phytopathogens and they produce a wide variety of antibiotics, chitinolytic enzymes, phytohormones, siderophores, HCN, and ammonia. Although, cultures grew well on TCP amended solid medium but none of them showed halo zone formation around the colony. This indicates that the halo zone formation as criteria is not enough for qualitative screening and selection of phosphate solubilizing micro-organisms, as many isolates that did not produce any visible halo zone on agar plates could conversely mobilize significant amount of phosphate in liquid media [6].
The soluble P content in the medium marks the difference between the solubilization of insoluble phosphate and the consumption of the solubilized phosphate by fungal isolates for their growth. During solubilization of ferric phosphate, different fungal isolates showed different growth patterns. KT6 produced the large globular bead like structures while KT28 exhibited homogenous granular appearance. BRT11 showed the suspended mycelial growth pattern whereas heavy mycelial growth with dark brown and light brown color appeared in case of Th-std and SE6, respectively. The results revealed that the rate of solubilization by fungal cultures varied with the nature of the bound phosphorus source. Similar results were also obtained by Gadagi and Tongmin [7] working with Penicillium oxalicum. Ferric phosphate as well as TCP was found to support better fungal growth and higher acid production (reduction in pH) during present investigation. Heavy mycelium growth observed during solubilization of ferric phosphate and TCP might be due to better absorption of phosphate as well as availability of iron in significant amount [1]. Although, in most of the cases phosphate solubilization has been reported due to organic acid production but other mechanisms such as production of phosphatase enzymes, chelating metabolites (siderophores) and redox activity have also been attributed to solubilization [20].
Cadmium is a non essential toxic element and one of the most hazardous of the trace elements for plant growth [13]. Therefore, in the industrial effluent affected areas, the soil is highly polluted with heavy metals which in turn diminish nutrient (P) availability in soil due to their cation chelating actions and thus obstruct plant growth and rhizosphere microbial population. In case of cadmium influenced phosphate solubilization, we observed clearly the induced P solubilization by fungal cultures at higher (>800 μg ml−1) cadmium concentration. Pseudomonads are abundant in a variety of contaminated environments including cadmium and mercury polluted water and sediments and have been reported for phosphate solubilization under the stress of heavy metals. However, no report in literature is available on metal tolerance and stress induced phosphate solubilization by the biocontrol fungus Trichoderma. It has been suggested that heavy metal tolerant fungi could protect plants against harmful effects of excessive heavy metals. Alleviation of Zn and Cd toxicity by fungi has also been reported by Gaur and Adholeya [9]. pH is the vital factor in phosphate solubilization. The results demonstrated that Trichoderma can grow as well as solubilize phosphate efficiently in acidic as well as alkaline environment. BRT11 was found superior at acidic (pH 3.0), neutral (pH 6.8) as well as at alkaline (pH 9.0) conditions. P solubilization at alkaline pH (12.0) by bacteria has been reported in literature but very few reports are available on alkalotolerant phosphate solubilizing Trichoderma. Although different temperatures have been reported by earlier workers for optimum P solubilization by fungal cultures, most of the studies have shown 28°C as the optimum temperature [20].
This study indicates clearly that Trichoderma viridae (KT6) and T. harzianum are potent alkalotolerant plant growth promotory biocontrol agents which retain their phosphate solubilizing capacity at alkaline pH and heavy metal stress conditions.
References
Altomare C, Norvell WA, Bjorkman T, Harman GC (1999) Solubilization of phosphates and micronutrients by the plant-growth promoting and biocontrol fungus Trichoderma harzianum Rifai 1295–22. Appl Environ Microbiol 65:2926–2933
Antoun H, Kloepper JW (2001) Plant growth-promoting rhizobacteria (PGPR). In: Brenner S, Miller JF (eds) Encyclopedia of genetics. Academic Press, New York, pp 1477–1480
Bakker AW, Schipper B (1986) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas sp. mediated plant growth stimulation. Soil Biol Biochem 19:451–457
Cappucino JC, Sherman N (1992) Microbiology: a laboratory manual. Benjamin/Cummings Publishing Company, New York, pp 125–179
Dennis C, Webster J (1971) Antagonistic properties of species-groups of Trichoderma. I. Production of non-volatile antibiotics. Trans Br Mycol Soc 57:25–39
Fankem H, Nwaga D, Deubel A, Dieng L, Merbach W, Etoa FX (2006) Occurrence and functioning of phosphate solubilizing microorganisms from oil palm tree (Elaeis guineensis) rhizosphere in Cameroon. Afr J Biotechnol 5(24):2450–2460
Gadagi RS, Tongmin S (2002) New isolation method for microorganisms solubilizing iron and aluminum phosphates using dyes. Soil Sci Plant Nutr 48:615–618
Ganesan V (2008) Rhizoremediation of cadmium soil using a cadmium-resistant plant growth-promoting rhizopseudomonad. Curr Microbiol 56:403–407
Gaur A, Adholeya A (2004) Prospects of arbuscular mycorrhizal fungi in phytoremediation of heavy metal contaminated soils. Curr Sci 86(4):528–534
Hass D, Defago G (2005) Biological control of soil borne pathogens by fluorescent Pseudomonas. Nat Rev Microbiol 3:307–319
Mishra DS (1998) Comparative efficacy of some biocontrol agents against R. solani Kuhn, the cause of sheath blight of rice. M.Sc.(Ag.) Thesis. G.B.P.U.A & T. India, p 242
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270
Nicholas MD, Pulford ID (2004) Cadmium phytoextraction using short rotation coppice salix: the evidence trail. Environ Int 31:609–613
Nicole B, Chet I (1996) Parasitism of sclerotia of Sclerotium rolfsii by Trichoderma harzianum: Ultrastructural and cytochemical aspects of the interaction. Phytopathology 86:405–416
Pozo MJ, Baek JM, García JM, Kenerley CM (2004) Functional analysis of tvsp1, a serine protease-encoding gene in the biocontrol agent Trichoderma virens. Fungal Genet Biol 41:336–348
Rangarajan S, Saleena LM, Vasudevan P, Nair S (2003) Biological suppression of rice disease by Pseudomonas spp. under saline soil condition. Plant Soil 251:73–82
Rawat R, Tewari L (2010) Transmission electron microscopic study of the cytological changes in Sclerotium rolfsii parasitized by a biocontrol fungus Trichoderma sp. Mycology 1(4):237–241
Embaby SM (2006) Using a biofungicide (Coniothyrum minitans Campbell.) in controlling some soilborne plant pathogenic fungi in Egypt. Res J Agric Biol Sci 2(6):423–432
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Shahab S, Ahmed N (2008) Effect of various parameters on the efficiency of zinc phosphate solubilization by indigenous bacterial isolates. Afr J Biotechnol 7(10):1543–1549
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rawat, R., Tewari, L. Effect of Abiotic Stress on Phosphate Solubilization by Biocontrol Fungus Trichoderma sp.. Curr Microbiol 62, 1521–1526 (2011). https://doi.org/10.1007/s00284-011-9888-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-011-9888-2