Abstract
This paper reports the hydrochemistry and activity concentration of the natural radionuclides 238U, 234U, and 210Po for three compartments of the hydrological/hydrogeological system in Araxá city, Minas Gerais State, Brazil: 1) mineral waters from the prominent springs Dona Beja (DBS) and Andrade Júnior (AJS), occurring at Barreiro area; 2) surface waters from Barreiro area and vicinity; and 3) rainwater. According to the Rule for Mineral Waters in Brazil (Register 7841) for temperature, the DBS water is cold (< 25 °C), while AJS is hypothermal (25–33 °C). The TDS (Total Dissolved Solids) concentration of DBS is low (70 mg/L), but high in AJS (2898 mg/L). The hydrogeochemical facies corresponded to sodium–(bi)carbonate for AJS and sodium/potassium–bicarbonate for DBS. The hydrochemical differences of DBS and AJS waters reflect the distinct characteristics of their respective aquifer systems. The DBS classification for TDS is the same of the Barreiro basin surface waters (mean TDS = 102 mg/L). Such value is somewhat higher than that of the rainwater and surface waters used for human consumption at Araxá city (TDS < 50 mg/L). The dataset reported in this paper indicated that fluoride and barium exceeded the WHO limits proposed in 2011 for drinking water. Among the natural radionuclides analyzed here that offer potential hazards for the human health is 210Po, whose WHO’s limiting value of 100 mBq/L in drinking water was exceeded in rainwater, thus, restricting the use of this resource as a possible supply of drinking water for the local community.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Economic constraints and therapeutic purposes have provided the consumption of bottled spring waters as an option to tap water elsewhere. The use of thermomineral waters in Brazil dates back from the early 1700s after arrival of the Portuguese immigrants. The discovery and recognition of the therapeutic values of the mineral waters from Barreiro area happened in 1816. The site is located approximately 8 km distant from the city center of Araxá’s municipality, Minas Gerais State (Magalhães, 1945). In Brazil, the epoch 1930–1950 was the most fortunate for the hydrothermalism, in which several spas were constructed for therapeutical and recreational purposes (Mourão, 1992). The rule for mineral waters in Brazil (BCMW) (Register 7841) was published on August 8, 1945, under French influence (DFPM 1966; Serra, 2009). This legislation is valid in the country yet.
The mineral production department from Brazil (DNPM) administers the manufacturing and business activities dedicated to bottled waters in the country. In 2007, DNPM reported an amount of about 2.1 billion liters of bottled waters, comprising both potable waters and those classified as mineral according to the BCMW guidelines. The states of São Paulo, Rio de Janeiro and Minas Gerais provided about 48% of such production (CPRM 2012; SEBRAE, 2012). That amount was enough to attend the demand of approximately 20 million of people that ingested bottled waters in the country. In the vicinity of the springs discharge areas occurred the development of touristic centers in which hotels and spas have been built for therapeutical and recreational purposes. Thus, visitors in these facilities were also drinking the mineral waters, consisting on additional in loco consumers of these resources.
Araxá city is easily reached from the country capital (Brasília) and from the capitals of the states of Minas Gerais (Belo Horizonte), Rio de Janeiro (Rio de Janeiro), and São Paulo (São Paulo) (Fig. 1). The mineral waters from Barreiro area are well-known since the beginning of the nineteenth century. The thermal waters were first analyzed and used for medicating tuberculosis in 1886–1890. In 1927, Dr. Andrade Júnior found out enhanced radioactivity in gases emanated from some spring waters (Magalhães, 1945). In 1944, the Brazilian President Getúlio Vargas opened the spa "Grand Hotel" (nowadays Tauá) at Barreiro area. This was done for exploiting the mineral waters of the springs Dona Beja (DBS) and Andrade Júnior (AJS).
Barreiro area is also characterized by the prominent exploitation of phosphate fertilizer and niobium ores that have greatly developed since the decades of 1960 and 1970. The mining activities have been realized nowadays by the mining and metallurgical corporation of Brazil (CBMM) and Vale Fertilizantes (Viana et al., 1999, Lemos Jr 2012). Enormous amounts of Nb2O5 and ground apatite have been extracted from Barreiro area for industrial and agricultural purposes, respectively (USGS 2018).
Contaminations of Ba and other metals have occurred at Barreiro area due to the mining industries. For instance, FUNTEC (1984) and Beato et al. (2000) reported an overflow in one tailings dam at CBMM in the early 1980s. Viana et al. (1999), Santos et al. (2011) and Fernandes et al. (2011) remarked that such event caused strong controversy involving different stakeholders, not only of the local level. One could argue that a consequence could be occurrence of the release of pollutants into the DBS and AJS waters, affecting the touristic income for Araxá city.
The surface waters exploited from three streams located away from Barreiro area are also relevant for Araxá city, in addition to the mineral waters of Barreiro. This is done by the Sanitation Company of Minas Gerais State (COPASA), after the waters captation and their chemical treatment. Also, it has been widely recognized that rainfall and surface–groundwater interactions favor the dissemination of possible contaminants in the hydrological resources (Bhardwaj & Singh, 2011; Khayan et al., 2019). Thus, all these aspects have been considered in this paper reporting an integrated hydrogeochemical study held at Araxá city. The main purpose here is to perform a quality diagnosis aiming to contribute for an appropriate management of the waters used by human consumption, i.e., the natural mineral waters of DBS and AJS, and the stream waters exploited by COPASA.
Major features of the study area
In the geological context, Barreiro area is situated in the Alto Paranaíba Igneous Province (APIP) that is a voluminous mafic potassic province, comprising ultrapotassic–potassic, ultramafic–mafic, silica-undersaturated lavas, and hypabyssal intrusions enriched in incompatible trace elements and light rare earth elements (REEs) (Gibson et al., 1995; Gomes & Comin-Chiaramonti, 2005). The whole APIP area corresponds to a well-defined regional high, the Alto Paranaíba arch, about 100 km wide and 300 km long (Traversa et al., 2001). The APIP includes the renowned almost circular (diameter of about 4.5 km) “Araxá carbonatite intrusion” (ACI) that covers an area of approximately 16 km2 at Barreiro area (Fig. 1). Barreiro area is located in a fractured zone tending to a predominant NW general direction. Such fracture zone is hundreds of kilometers long, consisting of several parallel faults in which are situated other APIP alkaline complexes (Tapira, Salitre, Serra Negra, and Catalão) (Issa Filho et al., 1984). The intrusive body at Barreiro area induced the development of concentric and radial fractures in the surrounding rocks and caused intense cracking in the adjacent quartzites (Issa Filho et al., 1984).
Traversa et al. (2001) considered that the APIP occurs since late Precambrian times. Gibson et al. (1995) pointed out that the alkaline magmatic activity occurred in the APIP and Araxá areas over the time interval of 80–90 Ma. The alkaline and country rocks are intruded in Barreiro area by a complex network of carbonatites as concentric and radial dykes quite variable in dimension and also by small veins ranging from few millimeters to several centimeters in thickness, with additional lithologies including mica-rich rocks, phoscorites and lamprophyres (Traversa et al., 2001). The ACI complex developed a very conspicuous dome structure within the country rocks that is associated with the presence of up to 2.5-km-wide contact aureole (Issa Filho et al., 1984).
Tropical weather dominates Araxá city. The summer (December–March) is wet, while the winter (June–September) is the driest season. In the period 2001–2015, December is the wettest month (mean = 329.8 mm of rainfall), while August is the driest one (9.1 mm of rainfall) (Fig. 2). Such climatic conditions have favored the development of a thick (locally higher than 200 m) weathering profile resulting from the alteration of the alkaline-carbonatite rocks. The weathering profile is enriched in P2O5, Nb2O5, TiO2, BaO, and REE2O3 in the alteration material. Its distribution in the intrusion area is irregular, reflecting the complex lithology of the alkaline association (Braga & Born, 1988). The weathering processes promoted the presence of abundant barium-pyrochlore at Barreiro area, where Ba and Nb dominate, respectively, the A and B positions in the formula A2B2X7. The enriched materials in the weathered mantle occur in the central and NW portions of the Araxá intrusion, originating one of the biggest world niobium deposit and a large (about 2.5 km2) phosphate reserve (Issa Filho et al., 1984).
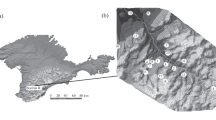
The main geomorphological feature at Barreiro area is the slightly sloping rounded/oval hills with flattened hilltops. The altitude ranges from 935 m (Sal stream bed) to1222 m (southern zone) (FUNTEC 1984). The Barreiro area landscape is dominated by the presence of Grand Hotel lake (GHL), basically formed by DBS waters, and a pond formed by AJS waters (Fig. 3). The drainage area for surface waters occurring at Barreiro area comprises two major portions (Fig. 3): (1) An eastern one, consisting of Baritina and Mata streams and respective tributaries; (2) A western one, consisting of Cascatinha and Borges streams. Cascatinha, Borges, and Baritina streams drain the mining and waste disposal sites of the phosphate mine. Baritina stream also receives waters providing from the niobium mining, which influences Mata stream too. All streams flow into the direction of Grand Hotel Tauá, originating Sal stream that corresponds to the unique Barreiro area exit.

adapted from FUNTEC (1984). In the bottom are more details of Barreiro area
Sampling points in this study plotted in a map
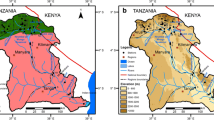
Fourteen boreholes were drilled at Barreiro area during the development of the Pró-Araxá Project for investigating the character of the underground flow (FUNTEC 1984). The potentiometric level was between 950 and 970 m a.s.l. for most boreholes situated in the Grand Hotel area and nearby but reached 1090 m in the higher altitude zones (FUNTEC 1984). Two major aquifer systems were identified at Barreiro area by Beato et al. (2000). The first is a granular, free, and semi-confined aquifer system, mainly occurring in loose sediments of the weathering mantle developed over the rocks of the intrusive body domain (2 km–diameter; variable thickness, reaching up to 200 m at southern portion, close to the niobium mine) (Beato et al., 2000). Such aquifer may be locally semi-confined by clayey levels of the weathered layer. The recharge occurs due to direct infiltration in areas of higher altitude (2–3 km is the maximum estimated recharge-discharge distance) and the flow lines at subsurface converge to the Grand Hotel lake and Sal stream (Fig. 4a). One main recharge area is the 0.7 km2 “green ring” site located upwards of DBS, which allows a significant aquifer replenishment, also contributing to the waters of some local dams and of the phosphate mine dyke (Beato et al., 2000). The second is a fractured, free to semi-confined aquifer system, mainly occurring in rocks around the carbonatite complex (Fig. 4b). The water circulation is deep, flowing out in an area where the rock has not suffered significant alteration. The recharge is due to direct rainwater infiltration, in small areas of the outcropping rocks, as well by waters infiltrating in the quartzite residual soil. Such aquifer system is related to the AJS waters outflow occurring in front of Grand Hotel Tauá (Fig. 4b).
Sampling and analytical methods
The DBS and AJS waters were sampled and analyzed in this study (Tables 1 and 2). Additionally, the same was done for the main surface water bodies in and out of Barreiro area, corresponding to Grand Hotel lake (GHL), Sal stream (SS1 + SS2), Areia stream (ARS), Fundo stream (FUS), and Feio stream (FES) (Fig. 3). Also, sampling was performed in two further monitoring points (Fig. 3): 1) the channel for discharging the GHL waters (GHC); 2) a creek, tributary of Sal stream, flowing close to the Effluents treatment station (ETS). In this paper, BAR corresponds to the mean hydrochemical composition of the surface waters from Barreiro area. Rainwater samples (RW1 and RW2 in Tables 1 and 2) were collected as well at the same stations reported by Bonotto and Thomazini (2019), but in different periods. RW1 was located about 1 km distant of the phosphate fertilizer processing unit of Vale Fertilizantes, while RW2 at the COPASA water treatment and distribution unit. The sampling campaign was in the dry season (DS, winter–spring = end August to middle October) and wet season (WS, spring–summer = middle October to middle March).
Polyethylene flasks (5L to 40L) were washed with HNO3 (10%) and rinsed with Milli Q water for collecting the groundwater, lake, and stream water samples. Bulk collectors for dry and wet deposition (Cresswell & Bonotto, 2008) were coupled to polyethylene flasks (10L to 20L) for sampling the rainfall (RW1 and RW2) in the dry and wet seasons. The same analytical protocol described by Bonotto and Thomazini (2019) was adopted for the field and laboratory readings of the physicochemical and chemical parameters for the whole group of samples: rainwater (7), groundwater (2), and surface waters (32).
Temperature, pH, conductivity, and potential redox (Eh) readings of the water samples were done in situ adopting potentiometric methods as described by Bonotto (2006). The sampling flasks were transported up to the laboratory, where aliquots were divided for evaluating the major/trace elements/compounds, and radionuclides (238U, 234U, and 210Po). Filtered aliquots preserved with HCl or HNO3 were used for obtaining the chemical composition in waters. An aliquot of the filtrate was evaporated to dryness in a previously weighed flask for yielding the total dissolved solids (TDS) data (detection limit, DL = 1 mg/L) (Rice et al., 2012). Titration with 0.02 N H2SO4 (Hach, 1992) allowed determining alkalinity in one 100 mL aliquot of each water sample (DL = 1 mg/L). Additional aliquots provided the data acquisition by the following methods: 1) atomic absorption spectrometry (AAS)—Na (DL = 0.3 µg/L); 2) colorimetry—K, Ca, SiO2, Mg, Cl−, NO3−, SO42−, and PO43− (DL between 6 µg/L and 0.8 mg/L, depending on the parameter); 3) ion selective electrode–fluoride (DL = 20 µg/L); 4) inductively coupled plasma atomic emission spectrometry (ICP-AES)—Fe, Mn, Al, Cd, Cr, Sr, Ba, Co, Cu, Zn, Pb, and Ni (DL of 0.1 mg/L for Cd and 0.1 µg/L for other metals). More details of these analyses are given elsewhere (e.g., Hach, 1992, van de Wiel, 2003, Bonotto & Silveira, 2003).
An aliquot of about 10 L of each water sample was used for measuring the dissolved 210Po activity concentration (209Po spike = 8 dpm), whereas approximately 20 L for acquiring the 238U activity concentration and 234U/238U activity ratio (AR) data (232U spike = 4.4 dpm). Bonotto et al. (2009) and Bonotto (2010) described the procedures adopted for such readings as done by alpha spectrometry, whose analytical uncertainties were often ± 10–15% (1σ standard deviation, 5% significance level; Young, 1962).
Results
The results of the hydrochemical data of this study are reported in Tables 1 and 2. Table 1 shows that the DBS waters are cold (< 25 °C) and the AJS waters are hypothermal (25–33 °C), according to the BCMW guidelines for temperature (DFPM 1966). Table 1 indicates that acid rain (mean pH = 5.5) characterizes the precipitation in the study area. This is mainly highlighted by the pH value of 3.8 found in the dry season at COPASA station (RW2; Table 1). On the other hand, all groundwaters and surface waters are alkaline (pH > 7) (Table 1).
The criterion frequently adopted to check the reliability and completeness of the hydrogeochemical data is that the deviation of the neutral ion balance should be lower than 5–10% (Schoeller, 1962, Custodio and Llamas 1976, Appelo & Postma, 2004). Aquachem 4.0 software (Waterloo Hydrogeologic, 2003) allowed find such values in some water samples, possibly due to errors in the hydrogen carbonate determination, effects of waters containing very low to low mineral concentration and accentuated SiO2 contribution in the hydrochemistry. Silica is high in several waters analyzed and despite SiO2 is not taken into account on the “neutrality condition” evaluation (Schoeller, 1962, Custodio and Llamas 1976, Appelo & Postma, 2004), the quartz solubility increases rapidly at pH ~ 9 and above, causing the silicic acid (H4SiO4) deprotonation and forming the anion H3SiO4−. Ritter (2012) identified this process when used the PHREEQC software for calculating the concentration of dominant solutes in spring waters of Poços de Caldas spa, Minas Gerais State, Brazil.
Generally, groundwaters exhibit TDS values higher than those of surface waters. The results obtained in this paper confirm such tendency for the AJS waters, which exhibited the highest EC (6390 µS/cm) and TDS (2898 mg/L) values (Table 1). Such waters circulate in the deeper fractured aquifer (Fig. 4), reaching depths above 200 m (Beato et al., 2000). van der Aa (2003) proposed four TDS classes, ranging from very low mineral concentration (< 50 mg/L) to high mineral concentration (> 1500 mg/L). According to this classification, the spring waters from Barreiro area contain low (DBS) and high (AJS) mineral concentration, differing too much as a consequence of the distinct characteristics of their aquifer systems. The DBS classification (low mineral concentration) is the same of the Barreiro basin surface waters (mean TDS = 102 mg/L, Table 1), while the rainwater and waters of the streams Areia, Fundo, and Feio possess very low mineral concentration (mean TDS < 50 mg/L) (Table 1).
Hem (1985), among other, pointed out a direct relationship of TDS with EC in surface waters and groundwaters, also recognizing that sodium and chloride are the most common dominant ions that justify the TDS-EC relationship. Figure 5 shows plots of EC, TDS, and major hydrochemical data of rainwater and surface waters of Barreiro basin, Areia stream, Feio stream, and Fundo stream in the dry (DS) and wet (WS) seasons. In rainwater, the values of EC, TDS, Na+, Cl−, Ca2+, Mg2+, and NO3− are higher in the dry season than in the wet season. For the same parameters, highest values were also found in the dry period for the hydrochemical composition of the surface waters of Barreiro basin (but changing Mg2+ by SO42−) and Areia stream (but changing Ca2+ by K+ in addition to Mg2+ by SO42−) (Fig. 5). In Feio stream, the values of EC, TDS (except in one sample), Na+, Cl−, NO3−, and SO42− are also higher in the dry season than in the wet season (Fig. 5). Compared to the hydrochemical composition of rainwater and other surface waters, Fundo stream did not follow the same general trends as pH instead of the EC-TDS relationship took a major role. In this case, higher alkalinity (pH = 8.8) was associated with higher concentrations of dissolved Na+, Ca2+, and Cl− in samples collected in the dry season rather than in the wet season (Fig. 5). In general terms, the chemical composition of the surface waters is similar in the different parts of the hydrologic system investigated, despite these little exceptions, possibly associated with some lithological variation in the drainage basins, among other factors.
Discussion
Major hydrogeochemical features
Aquachem 4.0 software (Waterloo Hydrogeologic, 2003) allowed plotting the hydrochemical data of Table 1 in the Piper (1944) and Schoeller (1962) diagrams as shown in Fig. 6. Several hydrochemical facies have been identified from both diagrams, which are summarized in Table 3. Calcium is the dominant cation in the majority of the rainwater samples, while bicarbonate, chloride, or sulfate may occur as prevailing anion. Neither the fresh mineral water (DBS) nor the thermal mineral water (AJS) possess calcium as dominant cation in their composition because sodium and potassium are the most abundant ones. However, in terms of dissolved anions, bicarbonate and carbonate take a major role in their composition. The enhanced concentration of sodium, potassium, and bicarbonate in the DBS and AJS waters certainly contributes to the hydrochemical facies of the surface waters of the Barreiro basin in terms of these dissolved constituents. However, it cannot be also disregarded the rainwaters aid as evidenced by the predominant presence of the anions bicarbonate and chloride in them. The streams Areia, Fundo, and Feio are out of the Barreiro area, exhibiting hydrochemical facies which often highlights the mixed character involving all major cations and anions. The rainwater contribution in the chemical composition of the waters of Areia, Fundo, and Feio streams is mainly suggested by the coincident presence in them of the dominant anions bicarbonate, chloride, and sulfate, whereas prevailing sodium, potassium, and magnesium are possibly indicating that lithological (geogenic) influences also occur. Therefore, both Piper (1944) and Schoeller (1962) diagrams highlight the accentuated dominance of sodium and (bi)carbonate in the AJS waters (Fig. 6). The diagram of Schoeller (1962) also reveals that sodium, potassium, magnesium, bicarbonate, chloride, nitrate, and sulfate exhibit the lowest concentration value in a rainwater sample collected at COPASA monitoring station (Fig. 6).
The physicochemical parameters and major/minor/trace chemical dissolved constituents measured in RW1 and RW2 (dry season; Tables 1 and 2) are plotted in Fig. 7. Most of the data are plotted above or below the equiline, as also indicated the ratio RW1/RW2. For instance, the ratio is approximately 0.5 for SiO2, it is about 4 for HCO3− and it is 37 for PO43−. The Pearson correlation coefficient among all data obtained for RW1 and RW2 is r = 0.89 (Fig. 7), suggesting a linear relationship in spite of the difference in the ratios and of the monitoring points distance. The null hypothesis significance testing by calculating the p-value from Pearson correlation coefficients has been traditionally adopted for checking the reliability of the results obtained (Wasserstein & Lazar, 2016). The p-value is the probability of obtaining test results at least as extreme as the results actually observed, under the assumption that the null hypothesis is correct. A very small p-value means that such an extreme observed outcome would be very unlikely under the null hypothesis. For the RW1 and RW2 dataset (r = 0.89; n = 26 parameters), the two-tailed p-value estimated by GraphPad software is less than 0.0001 and, by conventional criteria, this difference is considered to be extremely statistically significant.
The supplementary material shows the results of the same tests applied to the hydrochemical data of the mean Rainwater composition (RWM), groundwaters (DBS and AJS), and surface waters (BAR, ARS, FUS and FES) (Tables 1 and 2). For most parameters, the values in rainwater are lower than in groundwaters and surface waters, as expected due to the increased concentration of dissolved constituents in groundwaters and surface waters as a consequence of the water/rock–soil interactions. The statistically significant correlations reported in the supplementary material suggest that the analytical methods are coherent, also indicating congruency of the lower and higher concentration values of the dissolved constituents in rainwater, groundwaters and surface waters.
A wide range of mixing models have been developed in the literature for investigating influences among different components of the hydrological cycle (sea water, rainwater, surface waters, and groundwaters) like the Gibbs boomerang diagram (Gibbs, 1970) and the van Wirdum (1991) diagram. The van Wirdum (1991) diagram was primarily developed for ecological purposes, taking into account rainfall, groundwater, and oceanic water data for its construction. It will not be used in this paper due to the different hydrological/ hydrogeological context for which it was designed.
Gibbs (1970) proposed diagrams plotting hydrogeochemical data for discussion of the several mechanisms that control the world surface water chemistry: (1) atmospheric precipitation; (2) salts dissolution of the rocks and soils of their basins; and (3) evaporation-fractional crystallization processes. Later, those diagrams started to be used for invoking groundwater processes (Marandi & Shand, 2018). Figure 8(a) shows the analytical data reported in Table 1 as plotted in an original version of the Gibbs (1970) boomerang diagram. This diagram suggests that the chemical composition of the low-salinity waters of the streams Areia (ARS), Fundo (FUS), and Feio (FES) is mainly controlled by the amount of dissolved salts furnished by precipitation. Additionally, Fig. 8(a) also indicates that there is a stronger influence of the composition of the rocks and soils of the Barreiro basin in the chemistry of their waters (BAR).
Marandi and Shand (2018) pointed out some restrictions on the use of the boomerang contour of the Gibbs (1970) diagram in groundwater chemistry. Despite the chemical composition of precipitation remains fixed, Marandi and Shand (2018) considered that in order to apply a plot with same axes to describe groundwater processes, the major concepts driving the evolution of the water quality differ from those originally proposed by Gibbs (1970) for surface waters.
In this study, the hydrochemical data reported in Table 1 for fresh and thermal waters DBS and AJS are plotted in the diagram depicted by Marandi and Shand (2018) as shown in Fig. 8(b). It is possible to verify that the DBS waters composition conforms with the one related to processes involving the water–rock interaction of fresh waters recharged from precipitation or fresh surface water, agreeing with the characteristics of the aquifer system related to the DBS waters outflow (Fig. 4a).
Furthermore, Fig. 8(b) also indicates that the AJS hypothermal waters would be more geochemically evolved than the fresh DBS waters due to further water–rock reactions along flow paths or exchange with aquitards when they circulate at greater depths of the local aquifer system (Fig. 4b). In order to explain the enriched mineralization in Na compared to Ca of the AJS waters, Beato et al. (2000) suggested that the infiltrating waters percolate through deep cracks, joints, and fractures in the quartzites, dissolving sodic minerals such as amphiboles, sodic pyroxenes and alkaline feldspars from the fenitized rocks. Then, upon reaching the contact with the intrusive rocks, the water flow would occur through this zone, dissolving the minerals present in calciocarbonatites and ferrocarbonatites (Na2O levels of up to 8–11 wt.%; Traversa et al., 2001) until discharging onto surface with enhanced Na levels.
Several authors in the literature such as Jacks (1973), Stallard and Edmond (1983), Kumar et al. (2009), Wanda et al. (2011), Rajesh et al. (2012), Kumar (2014), Okiongbo and Akpofure (2014), among other, have adopted other hydrogeochemical diagrams aiming a better understanding of the lithological influences due to the wide range of factors involved, for instance, silicates weathering, dissolution of carbonates and evaporates, etc. Kumar (2014) suggested the use of plots involving ratios of the major ions HCO3−, Na+, Ca2+ and Mg2+ as the graph of HCO3−/Na+ vs. Ca2+/Na+ shown in Fig. 9, in which it was inserted the dataset obtained in this study. Additionally to carbonatites, mica-rich rocks bearing silicates widely occur in the study area, yielding SiO2 concentrations ranging from 14.3 to 35.5% (Traversa et al., 2001). Such abundance would explain the importance of the silicates weathering processes on controlling the presence of solutes dissolved in surface waters and groundwaters DBS and AJS (Fig. 9). Nevertheless, Fig. 9 also shows that the fresh mineral water DBS and several surface water samples exhibit HCO3/Na ratios indicating relevant contribution of carbonatites dissolution processes on their chemical composition. The high Na+ concentration compared to Ca2+ in the AJS thermal mineral water implies on a very low Ca2+/Na+ ratio as shown in its plotting at the left side of Fig. 9.
Water use in agriculture
The grade and type of dissolved constituents define the groundwater use by municipalities, industries, and agriculture, under different regulation at the national and international levels. Starting with Wilcox (1955), the B, Na, and EC levels in water samples have been used in order to define the adequate type for irrigating specific crops (Mirabbasi et al., 2008).
The EC and sodium adsorption ratio (SAR) data have been plotted in a chart developed initially for evaluating salinity in the USA (USSL 1954). Such diagram (sometimes reported as “USSL diagram”) combines the sodium and salinity hazards by plotting SAR in the vertical axis and EC in the horizontal axis, allowing to group the waters into 16 types (Fig. 10). The kind of class will define the water suitability for the different crops and soils. USSL (1954) defined the following equation for calculating SAR:
Data of the waters analyzed in this study plotted in the diagram for salinity hazard classification (USSL 1954). Salinity levels according to the EC values: low (C1), medium (C2), high (C3), and very high (C4). Sodium levels according to the SAR values: low (S1), medium (S2), high (S3), and very high (S4). The water usefulness for irrigation is based on such classes (Lokhande & Mujawar, 2016; Mirabbasi et al., 2008)
SAR = Na+ / [ ½ (Ca2+ + Mg2+)]1/2.
The plots of rainwater, groundwaters, and surface waters chemistry of the monitored points in this study are shown in Fig. 10. The stream waters exploited by COPASA (Areia, Fundo, and Feio streams) lie in the C1–S1 group, indicating that they are low saline and sodic, thus, suitable for irrigating most of the soil types. This category is equivalent to that of the rainwater as also shown in Fig. 10, which is often suitable under normal irrigation practices. The DBS groundwater and some surface waters from Barreiro basin are inserted in the C2–S1 and C3–S1 groups (medium/high-salinity and low-sodium water), suggesting possible use for cultivating some species of plants. The AJS groundwater exhibits a notorious different classification that is beyond the category C4–S4 of the waters possessing very high-salinity and very high-sodium concentration. Its use for irrigation only can be sporadic, but accompanied by some specific actions like applying additional products as soil amendments, for instance, calcium sulfate (Lyerly & Longenecker, 1957).
Water quality and health
Access to safe drinking water is important as a health and development issue at national, regional and local levels. Since 1958, the World Health Organization (WHO) has published “International standards for drinking water”, considering, among other aspects, those key chemicals responsible for small/large-scale health effects through drinking water exposure. Thus, in order to avoid diseases for humans, guidance levels have been proposed by the WHO for the ingestion of dissolved constituents in potable waters, including the mineral waters and those provided by the public water distribution systems.
For Cl, taste (not health) limits between 200 mg/L and 300 mg/L have been suggested by WHO (2011). Other reference values relevant in this study as proposed by WHO (2011) for public water supplies are: NO3− = 50 mg/L, F− = 1.5 mg/L, Ba2+ = 0.7 mg/L, Cr = 50 µg/L, Pb = 10 µg/L, Cd = 3 µg/L and U = 30 µg/L.
The health hazards from consuming water with NO3− are related to the direct toxicity of NO2−. NO2− is able to directly oxidize hemoglobin, changing it to methemoglobin, which cannot bind oxygen (Manassaram et al., 2006). Accumulation of methemoglobin (methemoglobinemia) occurs if this oxidation process overwhelms the protective reduction capacity of the cells (Manassaram et al., 2006). High levels of NO3− can pose a risk to babies younger than six months, but growing body of literature has indicated potential associations between NO3−/NO2− exposure and other health effects on adults such as increased heart rate, nausea, headaches, and abdominal cramps (Manassaram et al., 2006).
High F− concentration in drinking water may cause a dental enamel of higher porosity and lower mineral content (dental fluorosis) (Alvarez et al., 2009, Edmunds and Smedley 2013). The ion Ba2+ is responsible by the barium toxicity in mammals as they are promptly absorbed from the gastrointestinal tract due to the high permeability of the intestinal mucosa (DiBello et al., 1991).
Health effects related to Cr6+ exposure include diarrhea, stomach and intestinal bleedings, cramps, and liver and kidney damage (WHO 2011). Adults exposed to Pb can suffer from cardiovascular effects, increased blood pressure and incidence of hypertension, decreased kidney function, reproductive problems (in both men and women) (WHO 2011). Cd has the chronic potential to cause kidney, liver, bone, and blood damage from long-term exposure at levels above the maximum contaminant level (WHO 2011). The kidneys are the main human organs affected by the U compounds dissolved in drinking water as confirmed the studies held in Finland and Canada (Birke et al., 2010).
The waters analyzed in this study exceeded the guidance levels proposed by WHO (2011) for F− and Ba2+ (Table 2). F− concentration > 1.5 mg/L was found at AJS groundwater (Table 2), as well in other Brazilian natural mineral waters (Bonotto 2016), and in some aquifer systems worldwide (Smedley et al., 2002; Reddy et al., 2010, Edmunds and Smedley 2013, etc.). Additionally, Fordyce et al. (2007) and Edmunds and Smedley (2013) pointed out that the waters enriched in Na+, K+, and Cl− and poor in Ca2+ tend to contain high F− concentration. Coincidentally, this is a typical feature of the AJS groundwater as Na+ preponderates in its hydrochemical facies. Apatite [Ca5(PO4)3(OH, F, Cl] may be a major source of F− in those waters as it is a common mineral found in ferrocarbonatites of the Araxá complex (Traversa et al., 2001). The national health agencies have proposed distinct actions for controlling the disease due to excessive F− levels ingested in waters, but this is not a significant problem for the AJS waters as their ingestion is only occasional by tourists walking around the Grand Hotel Tauá zone.
Ba2+ concentration surpassed 0.7 mg/L in the groundwaters and surface waters of the Barreiro basin area, but also in the surface waters exploited by COPASA, and even in the rainwater (Table 2). Such widely spread Ba2+ dissemination should not be attributed to the environmental accident in the beginning of the 1980 decade, when Ba2+ discharges happened due to a leak of a CBMM tailings dam (Pinto et al., 2011), affecting the water quality at Barreiro area. In 1997, the groundwater monitoring pointed out Ba2+ levels of 0.5–2 mg/L, 14–44.7 mg/L, and 1.4–1.6 mg/L, respectively, in the aquifer, contamination plume and DBS waters (Beato et al., 2000). After that casualty and until 2006, CBMM provided significant actions for solving the problem such as the use of synthetical film in the barrage bottom, improvement in the steps for the neutralization of the residues aiming the barite precipitation, changes in the leach procedure, and enhancement of the groundwater surveys (Beato et al., 2000; Pinto et al., 2011, Lemos Jr 2012).
The enhanced Ba2+ levels in the waters of the study area may be geogenic because Ba is a key element in pyrochlore that is a highly abundant mineral there. One possible formula of this mineral is A2B2O7 (A = Ca,Na,K,Ba,U; B = Ti,Nb,Ta), corroborating that such complex mineral group may be the major source for the accentuated Ba2+ presence in the different waters of Araxá city. Ba2+ transported in aerosols together with other elements suffer dissolution during rainfall, causing their enrichment in rainwater. Baran and Tarnawski (2015), among other, have evaluated the Ba2+ presence in the waters and related ecology, but such more integrative studies still not took place at Araxá city.
Natural radionuclides and human health
Once natural radionuclides occur in solution, their presence in drinking water may be a cause of diseases for humans. This justifies the WHO (2011) guidance levels for their ingestion in potable waters, including the mineral waters and those provided by the public water distribution systems. Uranium (238U) and its descendants in the (4n + 2) decay series are of special interest in the hydrologic environment due to economic constraints, chemistry and radiological toxicity (Birke et al., 2009, 2010; Dreesen et al., 1982; Reimann & de Caritat, 1998). Uranium is a natural lithophile trace element of broad distribution in rocks and soils of the Earth’s crust, but anthropic releases of this element have occurred in water bodies due to works in mines, mineral beneficiation, agribusiness, burning of fossil fuels, and development of the nuclear fuel cycle, causing its presence in bottled and tap waters (Birke et al., 2010).
Uranium is widely distributed in crustal rocks, and under reducing conditions uranium tends to precipitate as uranous ion (U4+), but under oxidizing conditions it is often transported as uranyl ion (UO22+) that commonly forms complexes with the anions HCO3− and CO32− in alkaline contexts. Similarly to Nb and Ba, uranium occurring at Barreiro area is also associated with pyrochlore as pointed out the chemical analysis held by Castro and Souza (1970). It can be dispersed in the mineral matrices achieving levels of up to ~ 100 ppm U3O8, but also can be concentrated in the pyrochlore (up to 2% U3O8) (Castro & Souza, 1970).
The U concentration cannot exceed 30 µg/L in drinking water according to the WHO (2011) guideline reference value. Most of the dissolved U concentration in surface waters (rivers and lakes) and groundwaters is within the interval of 0.1–10 μg/L (Fritz & Fontes, 1980; Ivanovich & Harmon, 1992). The 238U concentration data reported in Table 2 indicate that the highest value was found at Sal stream 2 (SS2, 1.7 µg/L), which is almost 20 times under the WHO (2011) guidance level for this radionuclide. In terms of activity concentration, this 238U value (1.7 µg/L) corresponds to 0.02 Bq/L (Table 2) that is 500 times lesser than the WHO (2011) guideline reference value of 10 Bq/L.
The 238U decay series starts with 238U and finishes at the stable 206Pb, arising the radionuclides 234U, 222Rn, and 210Po along this path. Thus, 234U is a radiogenic 238U-daughter in this series, also existing health constraints related to its ingestion in waters. Because of its shorter half-life (0.248 Ma) compared to that of 238U (4.49 Ga), WHO (2011) proposed a more restrictive activity concentration limit for 234U (1 Bq/L) relatively to that of 238U (10 Bq/L). The secular equilibrium condition between 234 and 238U can be attained in ~ 1 Ma for systems that remained locked for U, yielding 234U/238U activity ratio (AR) = 1. However, the AR values may be above 1 in waters when they interact with the rocks and soils (Baskaran, 2012; Ivanovich & Harmon, 1992; Osmond & Cowart, 1976).
In this study, the highest 234U activity concentration was 0.02 Bq/L, also at SS2 (Sal stream 2, Table 2), which is 50 times lesser than the WHO (2011) limit of 1 Bq/L. Most of the AR values corresponded to 1, within experimental errors, suggesting that both U-isotopes 234U and 238U were equally leached during the water/rock–soil interactions. However, ARs higher than 1 were found in the DBS and AJS groundwaters, indicating preferential 234U-transfer to the liquid phase due to alpha recoil effects, among other factors (Ivanovich & Harmon, 1992; Osmond & Cowart, 1976). This also occurred for some samples of the Areia and Fundo streams as sometimes reported in the literature for surface waters (e.g., Baskaran, 2012; Ivanovich & Harmon, 1992).
The 222Rn released from the rocks and minerals escapes to the atmosphere where produces 210Po that is the penultimate nuclide in the 238U decay series. 210Po in drinking water may cause diseases for the human health, reason by which WHO (2011) proposed an activity concentration limit of 0.1 Bq/L (100 mBq/L) for its ingestion. In this study, the 210Po removed from atmosphere by precipitation yielded an activity concentration range of 1–375 mBq/L (mean = 101 mBq/L) in rainwater (Table 2; Fig. 11).
The dissolved 210Po activity concentration in the rainwater samples collected at the municipality of Araxá, Minas Gerais State, Brazil. The WHO (2011) guideline reference value of 100 mBq/L is also shown. DS = Dry season (winter–spring = end August to middle October). WS = Wet season (spring–summer = middle October to middle March)
In addition to rainwater, rocks and soils are also sources for 210Po in groundwaters and surface waters. The following dissolved 210Po activity concentration values were found in this study (Table 2): 1) groundwaters of Barreiro area = 8–10 mBq/L; 2) surface waters of Barreiro basin = 1.3–5.4 mBq/L; 3) surface waters of Areia, Fundo, and Feio streams = 1.6–1.8 mBq/L.
Figure 11 shows that the 210Po activity concentration in four rainwater samples surpassed the WHO (2011) limit of 100 mBq/L. Because pyrochlore is the main U-source at Barreiro area, such mineral is also responsible by its descendants, like 210Po. Radon escaping from these U-enriched materials into the atmosphere could cause the Po enrichment in rainwater. But, similarly to Ba2+, 210Po transported in aerosols could be dissolved during precipitation, also enhancing its presence in rainwater. The preponderant mechanism responsible for such high 210Po levels is an interesting topic for further investigations in the study area.
Among the major world issues nowadays is the task of providing enough suitable water for drinking. In some African countries, Australia, and Indonesia, many people are drinking roof-harvested rainwater (Chubaka et al., 2017). However, anthropogenic activities have acutely contaminated the atmosphere, causing acidity and cloudiness into rainwater, also adding several heavy metals like Pb, Cd, Ni, etc., that possibly will affect the people healthiness ultimately (Khayan et al., 2019). Therefore, the findings of this paper are a relevant contribution to this recent research topic, claiming attention to the natural 210Po. Such radionuclide is incorporated in high levels in the rainwater occurring at Araxá city. As a consequence, there are restrictions for the rainwater use as a possible supply of drinking water for the local community. Notably, 210Po absorption by plants and soils before reaching the surface drainage and aquifer systems in that area causes significant reduction in its level in the groundwaters and water courses (Table 2), implying on 210Po levels lesser than 100 mBq/L as proposed by WHO (2011).
Conclusion
In this study, hydrochemical and radiometric (natural radionuclides 238U, 234U, and 210Po) data are reported for three different types of water samples providing from Araxá city, Minas Gerais State, Brazil: (1) Groundwaters from two outstanding springs named Dona Beja (DBS) and Andrade Júnior (AJS) that supply mineral waters at Barreiro area; (2) Surface waters from Barreiro area and outskirts; (3) Rainwater. All samples were subjected to the same analytical protocols that allowed an integrated and comparative evaluation of these distinct compartments of the hydrological cycle. One major finding was the accentuated difference in the hydrochemical composition of the AJS waters that are hypothermal (29 °C) as they circulate at greater depths (above 200 m). According to the total dissolved solids (TDS) classification, they contain high mineral concentration (> 1500 mg/L), while the remaining waters possess low (50–500 mg/L) or very low mineral concentration (< 50 mg/L). In rainwater, the values of the electrical conductivity (EC), TDS, Na+, Cl−, Ca2+, Mg2+, and NO3− are higher in the dry season than in the wet season. The Piper and Schoeller diagrams highlighted the accentuated dominance of Na+ and CO32− in the AJS waters compared to other, implying on their very alkaline character (pH = 9.6). Ca2+ is the dominant cation in the majority of the rainwater samples, while Na+, K+, Mg2+, HCO3−, Cl−, NO3− and SO42− exhibited the lowest concentration value in a rainwater sample. The Gibbs boomerang diagram pointed out that the chemical composition of the waters of Areia, Fundo and Feio streams is mainly related to the precipitation composition, also indicating an outstanding influence of the Barreiro basin rocks/soils composition in the Barreiro waters chemistry. However, the plot of HCO3−/Na+ vs. Ca2+/Na+ data revealed that silicates weathering processes are important on controlling the presence of solutes dissolved in surface waters and groundwaters DBS and AJS. Additionally, the HCO3−/Na+ ratio in the fresh mineral water DBS and several surface water samples suggested relevant contribution of carbonatites dissolution processes on their chemical composition. The USSL diagram, based on the EC and sodium adsorption ratio (SAR) values, pointed out that the DBS groundwater and some surface waters from Barreiro basin are inserted in the C2-S1 and C3-S1 groups (medium/high-salinity and low-sodium water). The AJS thermal waters are in the C4-S4 category, suggesting their use for irrigation only can be sporadic, in opposition to other waters that are suitable for irrigating most of the soil types or for cultivating some species of plants. Some elements and compounds that should be avoided for intake in drinking water under specified limits of concentration as proposed by WHO in 2011 were analyzed in this paper, such as nitrate, chloride, fluoride, Ba, Cr, Pb, Cd, and U. The fluoride concentration surpassed the limit of 1.5 mg/L only in the AJS waters, conforming with some high F− levels found in other occurrences of mineral waters/groundwaters in Brazil and somewhere. The maximum value proposed by WHO for Ba in drinking water is 0.7 mg/L, which was surpassed in all water samples analyzed in this paper. Such widely spread Ba dissemination in the study area cannot be only attributed to the environmental accident that happened there in the early 1980s as this element is commonly associated with the pyrochlore generations occurring at Barreiro area. Neither 238U nor 234U activity concentration exceeded the WHO guideline reference values of 10 Bq/L and 1 Bq/L, respectively, whereas four rainwater samples exhibited values above 100 mBq/L for 210Po that is the WHO’s limit for this radionuclide, constituting a relevant topic of further detailed investigation to be held in the study area.
Data availability
The author declares that all available data are reported in this paper.
References
Alvarez, J. A., Rezende, K. M. P. C., Marocho, S. M. S., Alves, F. B. T., Celiberti, P., & Ciamponi, A. L. (2009). Dental fluorosis: exposure, prevention and management. Journal of Clinical and Experimental Dentistry, 1(1), e14-18.
Appelo, C. A. J., & Postma, D. (2004). Geochemistry, groundwater and pollution. Balkema.
Baran, A., & Tarnawski, M. (2015). Assessment of heavy metals mobility and toxicity in contaminated sediments by sequential extrac-tion and a battery of bioassays. Ecotoxicology, 24(6), 1279–1293.
Baskaran, M. (2012). Handbook of Environmental Isotope Geochemistry. Springer.
Beato, D. A. C., Viana, H. S., & Davis, E. G. (2000). Evaluation and hydrogeological diagnosis of mineral waters aquifers from Barreiro, Araxá, MG, Brazil. In ABAS (Brazilian Association of Groundwater) (Ed.), Proc. I Joint World Congress on Groundwater (pp 1–20). Fortaleza: ABAS.
Bhardwaj, V., & Singh, D. S. (2011). Surface and groundwater quality characterization of Deoria district, Ganga plain, India. Environmental Earth Sciences, 63, 383–395.
Birke, M., Rauch, U., & Lorenz, H. (2009). Uranium in stream and mineral water of the Federal Republic of Germany. Environmental Geochemistry and Health, 31(6), 693–706.
Birke, M., Rauch, U., Lorenz, H., & Kringel, R. (2010). Distribution of uranium in German bottled and tap water. Journal of Geochemical Exploration, 107, 272–282.
Bonotto, D. M. (2006). Hydro(radio)chemical relationships in the giant Guarani aquifer, Brazil. Journal of Hydrology, 323, 353–386.
Bonotto, D. M. (2010). The Poços de Caldas Hot Spot: A Big Blast for Nuclear Energy in Brazil. Nova Science.
Bonotto, D. M., Caprioglio, L., Bueno, T. O., & Lazarindo, J. R. (2009). Dissolved 210Po and 210Pb in Guarani aquifer groundwater, Brazil. Radiation Measurements, 44, 311–324.
Bonotto, D. M., & Silveira, E. G. (2003). Preference ratios for mercury and other chemical elements in the Madeira river, Brazil. Journal of South American Earth Sciences, 15, 911–923.
Bonotto, D. M., & Thomazini, F. O. (2019). Comparative study of mineral and surface waters of Araxá spa, Minas Gerais State, Brazil. Environmental Earth Sciences, 78, 542.
Braga, J. R. K., & Born, H. (1988). Geological and mineralogical characteristics of the apatite mineralization at Araxá. In SBG (Brazilian Society of Geology) (Ed.), Proc. XXXV Brazilian Congress of Geology (pp 219–226). Belém: SBG.
Castro, L. O., & Souza, J. M. (1970). Study of uranium and rare earths associated to niobium from Araxá – MG. Belo Horizonte: IPR (Institute for Researches in Radioactivity).
Chubaka, C. E., Ross, K. E., & Edwards, J. W. (2017). Rainwater for drinking water: a study of household attitudes. WIT Transactions on Ecology and the Environment, 216, 299–311.
CPRM (Brazilian Geological Survey) (2012). The Brazilian industry of mineral waters. http://www.cprm.gov.br/. Accessed 20 February 2018.
Cresswell, R. G., & Bonotto, D. M. (2008). Some possible evolutionary scenarios suggested by 36Cl measurements in Guarani aquifer groundwaters. Applied Radiation and Isotopes, 66, 1160–1174.
Custodio, E. G., & LLamas, M. R. (1976). Underground hydrology. Omega.
DFPM (Division for Supporting the Mineral Production). (1966). The mining code, the mineral waters code and how applying research in a mineral deposit. DFPM.
DiBello, P. M., Manganaro, J. L., & Aguinaldo, E. R. (1991). Kirk-Othmer Encyclopedia of Chemical Technology: Barium compounds. John Wiley and Sons.
DNPM (National Department of Mineral Production) (1987). Major mineral deposits of Brazil. Brasília: DNPM.
Dreesen, D. R., Williams, J. M., Marple, M. L., Gladney, E. S., & Perrin, D. R. (1982). Mobility and bioavailability of uranium mill tailings contaminants. Environmental Science Technology, 16, 702–709.
Edmunds, W. M., & Smedley, P. L., et al. (2013). Fluoride in natural waters. In O. Selinus, B. Alloway, J. A. Centeno, R. B. Finkelman, R. Fuge, & U. Lindh (Eds.), Essentials of Medical Geology (pp. 311–336). Springer.
Fernandes, F. R. C., Enriquez, M. A., & Alamino, R. C. J. (2011). Mineral resources & territorial sustainability. CETEM/MCTI.
Fordyce, F. M., Vrana, K., Zhovinsky, E., Povoroznuk, V., Toth, G., Hope, B. C., et al. (2007). A health risk assessment for fluoride in Central Europe. Environmental Geochemistry and Health, 29, 83–102.
Fritz, P., & Fontes, J. C. (1980). Handbook of Environmental Isotope Geochemistry. Elsevier.
FUNTEC (Minas Gerais Technological Center Foundation) (1984). Ecological conflict diagnosis report including recommended works and measures for mitigation of the ecological impact due to mining. Technical Report. Araxá: ECOS–Geology Consulting and Services Ltd.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science, 170, 1088–1090.
Gibson, S. A., Thompson, R. N., Leonardos, O. K., Dickin, A. P., & Mitchell, J. G. (1995). The late cretaceous impact of the Trindade mantle plume - evidence from large-volume, mafic, potassic magmatism in SE Brazil. Journal of Petrology, 36, 189–229.
Gomes, C. B., & Comin-Chiaramonti, P. (2005). Some notes on the Alto Paranaíba Igneous Province. In P. Comin-Chiaramonti, & C. B. Gomes (Eds.), Mesozoic to Cenozoic Alkaline Magmatism in the Brazilian Platform (pp 317–340). São Paulo: EDUSP.
Hach (1992). Water Analysis Handbook. Loveland: Hach Co.
Hem, J. D. (1985). Study and interpretation of the chemical characteristics of natural waters. U.S.G.S. Water-Supply Paper, 2254.
Waterloo Hydrogeologic (2003). AquaChem User´s Manual: Water Quality Data Analysis, Plotting & Modeling. Waterloo: Waterloo Hydrogeologic.
Issa Filho, A., Lima, P. R. A. S., & Souza, O. M. (1984). Geological aspects of Barreiro carbonatite complex, Araxá, MG, Brasil. In C. S. Rodrigues, & P. R. A. S. Lima (Eds.), Carbonatite complexes in Brazil: Geology (pp 20–44). São Paulo: CBMM.
Ivanovich, M., & Harmon, R. S. (1992). Uranium Series Disequilibrium: Applications to Environmental Problems. Clarendon Press.
Jacks, G. (1973). Chemistry of groundwater in a district in Southern India. Journal of Hydrology, 18, 185–200.
Khayan, K., Husodo, A. H., Astuti, I., Sudarmadji, S., & Djohan, T. S. (2019). Rainwater as a source of drinking water: health impacts and rainwater treatment. Journal of Environmental Public Health. https://doi.org/10.1155/2019/1760950
Kumar, M., Kumari, K., Singh, U. K., & Ramanathan, A. (2009). Hydrogeochemical processes in the groundwater environment of Muktsar, Punjab: conventional graphical and multivariate statistical approach. Environmental Geology, 57, 873–884.
Kumar, P. J. S. (2014). Evolution of groundwater chemistry in and around Vaniyambadi industrial area: differentiating the natural and anthropogenic sources of contamination. Chemie Der Erde, 74, 641–651.
Lemos Jr, M. A. (2012). Studies for evaluating the capacity of the niobium wastes reservoir. M.Sc. Dissertation. Ouro Preto: School of Mines, Federal University of Ouro Preto.
Lokhande, P. B., & Mujawar, H. A. (2016). Graphic interpretation and assessment of water quality in the Savitri River basin. International Journal of Scientific & Engineering Research, 7(3), 1113–1123.
Lyerly, P. J., & Longenecker, D. E. (1957). Salinity control in irrigation and agriculture. Texas Agricultural Extension Service Bulletins, 876, 1–20.
Magalhães, M. C. (1945). The spa of Araxá. Buenos Aires: Medical Association of Argentina.
Manassaram, D. M., Backer, L. C., & Moll, D. M. (2006). A review of nitrates in drinking water: maternal exposure and adverse reproductive and developmental outcomes. Environmental Health Perspective, 114, 320–327.
Marandi, A., & Shand, P. (2018). Groundwater chemistry and the Gibbs diagram. Applied Geochemistry, 97, 209–212.
Mirabbasi, R., Mazloumzadeh, S. M., & Rahnama, M. B. (2008). Evaluation of irrigation water quality using Fuzzy Logic. Research Journal of Environmental Sciences, 2, 340–352.
Mourão, B. M. (1992). Hydrological medicine – modern therapy of mineral waters and healing spas. Poços de Caldas: Municipal Secretary of Education.
Okiongbo, K. S., & Akpofure, E. (2014). Identification of hydrogeochemical processes in groundwater using major ion chemistry: a case study of Yenagoa and environs, southern Nigeria. Global Journal of Geological Sciences, 12, 39–52.
Osmond, J. K., & Cowart, J. B. (1976). The theory and uses of natural uranium isotopic variations in hydrology. Atomic Energy Review, 14, 621–679.
Pinto, C. L. L., Dutra, J. I. G., Salum, M. J. G., Ganine, J. F., & Oliveira, M. S. (2011). Study case: Major center producing phosphate and niobium in the country. In F. R. C. Fernandes, M. A. Enriquez, & R. C. J. Alamino (Eds.), Mineral resources & territorial sustainability (pp. 283–305). CETEM/MCTI.
Piper, A. M. A. (1944). A graphic procedure in the geochemical interpretation of water-analyses. Transactions of the American Geophysical Union, 25, 914–928.
Rajesh, R., Brindha, K., Murugan, R., & Elango, L. (2012). Influence of hydrogeochemical processes on temporal changes in groundwater quality in a part of Nalgonda district, Andhra Pradesh, India. Environmental Earth Sciences, 65, 1203–1213.
Reddy, D. V., Nagabhushanam, P., Sukhija, B. S., Reddy, A. G. S., & Smedley, P. (2010). Fluoride dynamics in the granitic aquifer of the Wailapally watershed, Nalgonda District, India. Chemical Geology, 269, 278–289.
Reimann, C., & de Caritat, P. (1998). Chemical elements in the environment: Factsheets for the geochemist and environmental scientist. Springer-Verlag.
Rice, E. W., Baird, R. B., Eaton, A. D., & Clesceri, L. S. (2012). Standard methods for the examination of water and wastewater. American Public Health Association/American Water Works Association/Water Environment Federation.
Ritter, S. M. (2012). Geothermometry on natural spring waters from Poços de Caldas, Minas Gerais, Brazil. Monograph (pp 1–56). Heidelberg: University of Heidelberg.
Santos, M. S., Carneiro, L. G., Medeiros, G., Sampaio, C., Martorell, A. B.T., Gouvea, S., et al. (2011). PIXE analyses applied to characterize water samples. In ABEN (Brazilian Association of Nuclear Energy) (Ed.), Proc. International Nuclear Atlantic Conference – INAC (pp 1–6). Belo Horizonte: ABEN.
Schoeller, H. (1962). Groundwaters. Masson & Cie.
SEBRAE (Service for Supporting the Small Businesses in São Paulo State) (2012). Mineral waters business. http://www.sebrae-sc.com.br/ideais/default.asp?vcdtexto= 31586&%5E%5E. Accessed 20 February 2018.
Serra, S. H. (2009). Mineral waters from Brazil. Campinas: Millenium Editora.
Smedley, P. L., Nicolli, H. B., Macdonald, D. M. J., Barros, A. J., & Tullio, J. O. (2002). Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Applied Geochemistry, 17, 259–284.
Stallard, R. F., & Edmond, J. M. (1983). Geochemistry of the Amazon: 2. The influence of geology and weathering environment on the dissolved load. Journal of Geophysical Research, 88, 9671–9688.
Traversa, G., Gomes, C. B., Brotzu, P., Buraglini, N., Morbidelli, L., Principato, M. S., et al. (2001). Petrography and mineral chemistry of carbonatites and mica-rich rocks from the Araxá complex (Alto Paranaíba Province, Brazil). Anais Da Academia Brasileira De Ciências, 73, 71–98.
USGS (U. S. Geological Survey) (2018). Mineral Resources On-Line Spatial Data: Araxá. https://mrdata.usgs.gov/mrds/show-mrds.php?dep_id=10068132. Accessed 29 January 2018.
USSL (U.S. Salinity Laboratory Staff) 1954. Diagnosis and improvement of saline and alkali soils. Handbook No. 60, U. S. Department of Agriculture.
van de Wiel, H. J. (2003). Determination of elements by ICP-AES and ICP-MS. Bilthoven, The Netherlands: National Institute of Public Health and the Environment (RIVM).
van der Aa, M. (2003). Classification of mineral water types and comparison with drinking water standards. Environmental Geology, 44, 554–563.
van Wirdum, G. (1991). Vegetation and hydrology of floating rich-fens. PhD Thesis. Amsterdam: University of Amsterdam.
Viana, H. S., Davis, E. G., Beato, D. A. C., & Cabral, J. A. L. (1999). Araxá Project: geoenvironmental study of mineral springs. Belo Horizonte: CPRM (Brazilian Geological Survey).
Wanda, E., Monjerezi, M., Mwatseteza, J. F., & Kazembe, L. N. (2011). Hydrogeochemical appraisal of groundwater quality from weathered basement aquifers in Northern Malawi. Physics and Chemistry of the Earth, Parts A/b/c, 36, 1197–1207.
Wasserstein, R. L., & Lazar, N. A. (2016). The ASA’s statement on p-values: context, process, and purpose. The American Statistician, 70(2), 129–133.
WHO (World Health Organization). (2011). Guidelines for drinking water quality. WHO Press.
Wilcox, L. V. (1955). Classification and use of irrigation waters. USDA Circular No. 969, U.S. Department of Agriculture.
Young, H. D. (1962). Statistical treatment of experimental data. McGraw Hill.
Acknowledgements
Three anonymous reviewers are greatly thanked for helpful comments that improved the readability of the manuscript.
Funding
This study was funded by the Brazilian agencies CNPq-Conselho Nacional de Desenvolvimento Científico e Tecnológico (Grants 400700/2016–6 and 301992/2016–9) and FAPESP- Fundação de Amparo à Pesquisa do Estado de São Paulo (Grants 2014/50945–4 and 2018/25332–0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Ethical approval
This is an observational study that did not involve human participants or biological materials, thus, not requiring ethical approval of the Research Ethics Committee of the authors' institution.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bonotto, D.M. Hydrochemical and radiometric evaluation of fresh and thermal waters from Araxá city (Minas Gerais, Brazil). Environ Geochem Health 44, 2163–2186 (2022). https://doi.org/10.1007/s10653-021-01058-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-01058-y