Abstract
Groundwater represents the primary source of freshwater for more than 35% of world people, and its contamination became a worldwide challenge. Egypt is suffering from water quantity and quality, especially in desert areas. El Obour city and environs Northeast Cairo face waterlogging owing to the elevated-shallow groundwater table. In the present research work, the water quality of the shallow groundwater aquifer was studied. The remediation efficiency of polluted water using photocatalytic treatment technique in the presence of modified nano-titania and solar radiation has also been investigated. Twenty-eight representative samples have been collected from different locations, and their microbial, physical, and chemical characteristics were determined. The average contents of Pb (214.96 µg/L), As (1517 µg/L), Cd (8.79 µg/L), total bacterial count (2.22 × 105 CFU/ml), and bacterial indicators (MPN-index/100 ml): total coliform (497.4), fecal coliform (358.3), and fecal streptococci (115.9) were higher than WHO permissible limits for drinking water, possibly due to higher industrialization, agricultural, and urbanization rates. The organic pollutants reached critical concentrations (chemical oxygen demand up to 960.8 mg O2/L). Most of the studied samples contained acceptable concentrations of the major ions, (e.g., K+, Mg2+, HCO3−), for drinking and irrigation purposes. The statistical analyses (e.g., principal component analysis and cluster analysis) pointed out the control of water–rock interaction and anthropogenic activities in water composition. The hydrochemical data show that most of the water samples (96.4%) are Na2SO4 and NaHCO3 type, indicating its meteoric origin. The contamination with human and animal fecal substances, NO3¯, and NH4+ was identified in all samples, which pointed out the control of anthropogenic activities in water pollution. The photocatalytic technique efficiently eliminated more than 82–95% of organic contents and microbial pollutants, respectively, but it was inefficient in reducing heavy metal levels. According to the current results, shallow groundwater injection into the deep aquifer must be constrained and reusable after treatment. Finally, more studies are imperative to disseminate the applied treatment techniques to elude bacteria and organic pollutants from water at a pilot scale.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human livelihoods depend mainly on freshwater. Groundwater is one of the most fundamental resources of freshwater on the earth. Groundwater is 30% of the world's freshwater supply and is a significant water source for more than 2.5 billion people worldwide (Ajami, 2021; Shaji et al., 2020). Most African countries are arid to semiarid with limited water resources, where two-thirds of South Africa's population depends on groundwater to provide water (Mthembu et al., 2020; Smithers et al., 2017). The intensive human activities and over-exploitation of groundwater across the globe led to the degradation of groundwater quality (Abdelhafiz et al., 2021; Karunanidhi et al., 2021a; Machiwal et al., 2018). Groundwater pollution became one of the most worldwide issues in the last decades due to its impact on human health (Karunanidhi et al., 2021b; You et al., 2020). Urbanization processes release larger amount of chemicals that can reach the shallow groundwater aquifers. Potentially polluting substance can be released from agricultural activities (fertilizers and pesticides), stored in the underground (gasoline and septic tanks) (El-Fakharany et al., 2017; Howard & Gerber, 2018; Mansour, 2020). Several studies have carried out to determine the adverse effects of contaminated groundwater on human health (He et al., 2021; Karunanidhi et al., 2021b; Ukah et al., 2019; Usman et al., 2020; Zhang et al., 2018a). Cholera, dysentery, diarrhea, hepatitis A, typhoid, and polio are directly related to unhygienic and polluted drinking water. It is estimated that more than 485,000 persons die from diarrhea globally every year (WHO, 2017b, 2019).
Egypt is an arid country with water scarcity, especially in the desert areas. El Obour City is one of the sustainable development projects for lateral expansion in the Egyptian deserts. Unfortunately, the hydrogeologic and geomorphologic settings of El Obour city led to the emergence of the subsurface waterlogging problem. The city is divided mainly into three sectors: residential, industrial, and agricultural (Fig. S1). The waterlogging problem has been appeared in lower parts of some locations within the residential sector and adversely has affected the infrastructure (Fig. S2) and the lower groundwater aquifer quality (Abdelhafiz, 2017; Barseem et al., 2015b; Zeid et al., 2018). The government tried to solve this issue by setting up many wells for water collection and draining directly into the underlying groundwater aquifer. It is anticipated that these injected waters will be overloaded with organic, inorganic, and biological pollutants that will adversely influence the underline Miocene aquifer. The previous studies focused on the hydrochemical and engineering aspects of the waterlogging problem without concern about environmental quality and economic value (Barseem et al., 2015a; El-Aassar et al., 2010; Zeid et al., 2018). Zeid et al. (2018) pointed out the quality of this water for irrigation and, to some extent, drinking after simple physical treatment without considering toxic metals and bacteria. The current work will depend on combining hydrochemical, environmental, and photochemical techniques for combating waterlogging.
The most severe water pollutants are heavy metals and bacteria (Osman et al., 2012; Vasudevan et al., 2021; Xiong et al., 2021). Heavy metals naturally occur in small quantities in groundwater and are vital for both human metabolism and the growth of animals and plants. As they are persistent, non-biodegradable, stable, and harmful in the natural ecosystem, heavy metals are known to be essential pollutants affecting groundwater quality and pose a threat to human health. Exposure to polluted water (drinking or domestic use) has been linked to many health problems (Ahmed et al., 2021; He et al., 2021; Li & Wu, 2019). However, long-term exposure to toxic metals can also cause deadly diseases such as Alzheimer's and cancer (WHO, 2017a; Li et al., 2021; Mishra et al., 2010). As pollution is significant contamination of groundwater and affects almost 70 million people worldwide (UNESCO, 2009). The WHO stated that high arsenic intake might cause acute and chronic health effects, including cancer (Jadhav et al., 2015; Wen et al., 2013). Nakadaira et al. (2000) reported that long-term ingestion of low levels of arsenic in drinking water could lead to lung and prostate cancer. High contents of Cd, Cr, and Pb in drinking water can also affect the kidney, liver, and lead to fatal cancer. Moreover, high intake of As, Cd, Cr, and Pb can damage organs due to high-level toxicity and longtime persistence (Chen et al., 2018; WHO, 2017a). Subsequently, As, Cd, and Cr are human carcinogens, according to WHO (2011).
In Egypt, groundwater is the primary source of freshwater in the Eastern and Western Deserts and Sinai. Shallow groundwater provides Egypt with about 6.5 BCM/year and represents the second freshwater source after the Nile River (MWRI, 2014; Omar & Moussa, 2016). The study area is located in the Eastern Desert, representing recently built urban and agricultural lands. El-Aassar et al. (2010) studied the environmental impacts on groundwater quality in El Obour city and its vicinities. The results reflected that the pollution source is from a municipality or public water or leakage from the sewage or industrial wastewater system. Many authors have pointed out groundwater pollution in Egypt by heavy metals (Aamer et al., 2016; Salman & Elnazer, 2020; Salman et al., 2019a, b). Several groundwater samples obtained from the Nile aquifer were observed to contain carbamate, organochlorine, and organophosphorus pesticides (Abdel-Shafy & Kamel, 2016). Masoud et al. (2018) have found several pesticides in the groundwater collected from Al-Gharbiya Governorate. The groundwater at the Delta was polluted with total and fecal coliform bacteria (range 0–300 and 0–72 MPN/100 ml, respectively) (Awad et al., 2015).
The adverse problems of polluted water led to a continuous search for low-expensive and effective treatment techniques for removing biological and chemical pollutants. Traditional water treatment methods (such as ozonation and chlorination) may not completely disinfect the water and also generate harmful by-products. As for photocatalysis, it depends on the oxidation of pollutants, an effective water purification process, and does not produce secondary harmful substances (Das et al., 2020). Photocatalytic techniques have been studied widely for environmental purification in liquid and gas phases. This may be attributed to that under mild conditions (ambient pressure and room temperature), photoactivated semiconductors by certain irradiation could mineralize many bacteria and organic pollutants that are non-biodegradable, toxic, and refractory to carbon dioxide and water. Titania (TiO2), as an eco-friendly (Chobba et al., 2019), less expensive, and highly photoactive catalyst, has been widely applied for remediation of organic pollutants, for air purification, as air filter and as a deodorant, for sterilization (Linsebigler et al., 1995). However, a small UV fraction of solar energy (3–5%) could be utilized due to the wide band gap of titania. Therefore, great research works have been put to develop efficient visible light-sensitive nano-photocatalysts by titania modification. Recently, anion doping of TiO2 films and powders with anions (i.e., nitrogen) has been studied (Batzill et al., 2006; Gole et al., 2004). Hence, the studied technique eliminates dissolved organic and microbial pollutants efficiently at the rates of 86.9% and 100% (El Nazer, 2017).
Without a robust understanding and greater certainty of where shallow groundwater pollution originates in El Obour city, environmental management or effective engineering strategies to find an optimal solution for waterlogging problem cannot be successfully applied. The current study dealt with chemical and bacterial contents and sources in shallow groundwater at northeastern Cairo and the impact of the injection of this shallow groundwater into the deep aquifer to resolve the waterlogging problem. The present study also aimed to investigate the reliability of advanced photocatalytic techniques application for water treatment, using N-doped TiO2 photocatalyst in the presence of UV–visible light as a source of irradiation.
Materials and methods
Study area and sampling sites
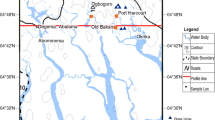
The study area located on the eastern side of the Nile Delta with a total area of 65 km2 is considered the largest newly urbanized communities in Egypt (Details presented in Section 1 of the Supplementary information (SI)). In the southern part of El Obour city, sixth and seventh districts, the waterlogging problem is severely documented because of geological and topographical variations (Fig. S3). Water samples were collected from 28 sampling sites (Fig. 1), out of which 22 water samples from the sixth and seventh districts (where the problem-focused and they face risks in the area). Thus, samples were selected to represent the waterlogging issue in the southern part of the current area.
1 L sterile plastic bottle was rinsed three times for each sample point, and the water samples were filled to the brim before sealing tightly to provide as little air as possible at the top of the bottles. Water samples were acidified by adding analytical grade nitric acid (98% pure) up to pH 2 for heavy metal analysis (Trick et al., 2018). The water samples were collected for bacterial analyses in brown glass bottles, which were used after sterilization and transferred to the laboratory in an icebox within 6 h.
Analytical methods
Twenty-six water variables were measured/determined in each sampling point. The pH, conductivity, and TDS were performed in situ by using a combined electrode (Hanna Hi93300). In the laboratory, the water samples were filtered and analyzed for chemical constituents employing standard procedures (APHA, 2012). Analytical methods and units of chemical water variables are summarized in Table S1. K2Cr2O7 open reflux method was used for Chemical Oxygen Demand (COD) (APHA, 1998; El Nazer et al., 2017). Heavy metals comprise arsenic (As), cadmium (Cd), copper (Cu), chromium (Cr), iron (Fe), lead (Pb), manganese (Mn), and zinc (Zn) were determined by using atomic absorption spectrometer (AAS-Perkin Elmer 400) at the laboratory of the National Research Centre.
To assess the suitability of water for irrigation use, magnesium ratio (MR) and sodium absorption ratio (SAR) were calculated. Excess MR in water affects the soil quality due to increasing alkalinity and, consequently, affects crop yields. The total bacterial counts (TBCs) and the total count for various bacterial indicators comprise total coliform (TC) and fecal coliform (FC), and fecal streptococci (FS) were carried out via the most probable number technique (MPN) (APHA, 2012; Divya & Solomon, 2016).
The remediation of organic pollutants and bacteria present in four collected representative samples has been performed using the photocatalytic treatment technique. Nitrogen-doped titania photocatalyst was synthesized by sol–gel hydrothermal technique using urea and titanium n-butoxide (TNBT) as precursor. TNBT solution and urea were taken in the molar ratio of 5:1. The prepared urea solution was added drop wise to a mixture of ethanol and titanium n-butoxide upon stirring for ~ 24 h at room temperature. The prepared catalyst was then dried at 60 °C and calcined at 400 °C for 4 h (Darwish et al., 2015). The Photocatalytic processes were performed using a (PHOCAT 120 W) solar photoreactor. The dispersion of polluted water containing (1 g/L Nitrogen-doped titania) was sonicated in water medium for 5 min. The polluted water contacting suspended photocatalyst was then irradiated using ten visible light lamps (λmax 400–700 nm) with a total power of 80 W. After 60 min of irradiation, three milliliters aliquots of the reaction mixture were withdrawn and analyzed. When titania photocatalyst suspension is exposed to the visible light, the excited titania could then absorb light energy resulting in the excitation of electrons from the valence band (VB) to conduction band (CB) (Fig. S4) (process is provided in the supplementary information). Subsequent oxidative degradation of dissolved organics and bacterial processes take place at the surface of the exciting photocatalyst (Gole et al., 2004; Pelaez et al., 2012).
Calculation
Sodium absorption ratio assesses the risk of sodium in irrigation water (Salman & Elnazer, 2015). SAR and MR were calculated using Eqs. 1 and 2.
where all the ionic values are expressed in meq/L. The water is considered suitable for irrigation when MR is greater than 50% (Raghunath, 1987). The water can be categorized based on SAR values into excellent (less than 10), good (10 ≤ SAR < 18), doubtful (18 ≤ SAR < 26), and unsuitable (SAR ≥ 26) (Richards, 1954).
Data analyses and quality control
Multivariate statistical analyses “Principal Component Analysis (PCA), cluster analysis, box plot, and descriptive statistics” were performed using SPSS 21, XLSTAT (Addinsoft, USA), and Origin Pro 8.5 software. Principal components with eigenvalues greater than 1 could be selected. The correlations between the factors and the variables were classified as strong, moderate, and weak when the absolute loading values more than 0.75, 0.75–0.5, and 0.5–0.3, respectively. Ward’s method (Ward, 1963) was chosen to combine the variables using the squared Euclidean distance as a similarity measurement to create the dendrogram for cluster analysis. It is widely used in environmental studies to discriminate, interpret geochemical data, identify, and variables grouping.
In order to control the accuracy and precision of the analytical procedure, recovery analysis was performed. The element concentrations in triplicate samples were determined. Calibration curves for each heavy element were drawn by running appropriate concentrations of the standard solutions. The blank absorbance was taken before sample analysis, and blended samples were inserted in the analyses. Analytical grade chemicals were utilized throughout the analysis procedures.
Results and discussion
Major characteristics and quality
Descriptive statistics of the water samples are summarized in Table 1. Approximately, 50% of samples (median) contains less than 796.6, 254.1, 69.7, 22.5, 247.5, 6, 235.9, 223.5, 130.6, 16, 0.16, and 168 mg/L of TDS, TH, Ca2+, Mg2+, Na+, K+, HCO3−, SO42–, Cl–, NO3−, NH4+, and alkalinity, respectively. The high concentration of ions may be attributed to the dissolution of sediments in the study area and industrial, agricultural, and municipal effluents. The highest concentrations were recorded in the wells of the very shallow water table (near the surface). This may be attributed to the contact with the near surface constructions and filling materials. The high concentration of HCO3– and its –ve correlation with other ions indicated the high concentration of CO2 and consequently the surface runoff into the aquifer; the vulnerability of the aquifer (Elnazer et al., 2021). The comparison with WHO (2017a) shows that more than 90% of samples can be used for drinking based on pH, alkalinity, K+, and Mg2+ values. Seventy percent could use for drinking based on TDS, NH4+, Cl–, and NO3−; moreover, 49% based on Ca2+, Na+, EC, and SO42– values. The selected samples for the organic load survey (as indicated by COD) contained 230.4 and 960.8 mg O2/L. SAR and MR were varied in the ranges of 0.74–23.9 and 14–62, respectively. Approximately 93% and 86% of water samples belong to a suitable category and can be used for irrigation for all soils and crops based on SAR and MR values, respectively (Table S2).
Hydrochemical classification and genesis of groundwater
Water types in the current area were noticed based on their chemical composition using piper diagram (Piper, 1944). Shallow groundwater samples are fall in three fields; 15 samples in Na–Cl field indicating weathering of halite, 7 samples in the field of mixed Ca–Mg–Cl, and 5 samples fall in the field of calcium bicarbonate. In addition, one sample falls in CaMgClSO4 type (Fig. 2a). Shifting some samples into the center of diamond shape means that these watercourses have been subjected to contamination by fertilizers and sewage (Ikhlil, 2009). Also, the diamond shaped can be divided into two equal upper and lower triangular fields, where the samples which appear in the upper triangle have secondary salinity properties (where SO42− and Cl− exceed Na+ and K+), and the samples which appear in the lower triangular have primary salinity properties (where carbonates exceed Ca2+ and Mg2+) (Awad et al., 1995). The plotting of the sample within the upper half of the diamond indicates the secondary salinity resulting from waterlogging issue. The rising water level is bringing salts from the surface as well as seepage of wastewater and irrigation excess water led to the accumulation of salts in groundwater (Krishan, 2019).
The Sulin's graph (Fig. 2b) was used to detect the origin of the water through two equal squares (Sulin, 1946). The upper right one displays the marine water genesis (MgCl2 and CaCl2). While the lower-left square shows the water of meteoric origin (NaHCO3 and Na2SO4). The two squares are diagonally subdivided into two triangles, where the diagonal line represents Cl− (K + Na)/Mg = 1and Cl− (K + Na)/SO4 = 1 for upper and lower squares, respectively. The output results indicate that the water samples are characterized by recent (NaHCO3) and old meteoric water (Na2SO4) type. Recent meteoric water (NaHCO3) type (10 samples) is formed by freshening processes where the fresh groundwater formed due to the seepage processes from the surface layers (Zeid et al., 2018) and irrigation. The old meteoric water (Na2SO4) type (15 samples) could be related to the groundwater formed before the seepage processes, where the soil salinity might be critical in controlling shallow groundwater composition. Two samples (Nos. 2 and 3) could be considered as a transitional stage between NaHCO3 type and Na2SO4 type (Fig. 2b). On the other hand, only one sample (No. 21) is classified as of marine origin and has MgCl2 composition content.
Heavy metals in water
The descriptive statistics of heavy metals results are described in Table 2 and compared with standard limits recommended by WHO (2017a) for drinking and United States Department of Agriculture (USDA) for irrigation (USDA, 2011). The results indicated the pollution of 100% of water samples with Pb, and more than 89% of samples exceeded the permissible limits of As and Cd recommended by WHO (2017a) (Fig. 3).
The arsenic content estimated in the studied water samples fluctuates between BDL and 4849 μg/L with an average of 1517 μg/L (Table 2). Several intermixed processes control arsenic content, as noticed from the wide range between Q1and Q3 (Fig. 4a), including the natural water–rock interaction and the anthropogenic activities such as leakage from industrial and domestic wastewater (Boonkaewwan et al., 2021; El-Aassar et al., 2010). As which is strongly correlated with Ca2+, Mg2+ and SO42− (Figs. 4b & S5) reflects the role of natural source, maybe dissolution of anhydrite and gypsum from the country rocks. These results are in line with Abdelbakey (2014), who found gypsum in the study area's lithology. On the other hand, its + ve correlation with NO3¯ and NH4+ pointed out the anthropogenic contribution of As content (Fig. S6). Application of nitrogen fertilizers, livestock farms, and wastewater leakage considered the main sources of nitrogen compounds in groundwater (Bahrami et al., 2020).
In this study, the measured Cd concentrations in water ranged from BDL to 20 μg/L with a mean value of 8.79 μg/L. The insignificant difference between both mean and median, and Q1 and Q3 (Table 2; Fig. 4a) points to the uniform source of Cr in water. Cluster analysis confirmed this finding where Cd is strongly correlated with K+, indicating that Cd comes mainly from fertilizers use effect (Fig. 4b) (Abdel Latif & El Kashouty, 2010; Taha et al., 2004). Chaney (2010) reported the primary Cd source due to the anthropogenic, whereas a few geogenic Cd enrichment sources were identified. The + ve correlation between Cd and Zn can be attributed to the role of traffic emissions in the distribution of these metals. Cd and Zn present in many parts of vehicles and can be realized through abrasion and spills out of those parts (Elnazer et al., 2015). About 20–90 ppm Cd was recorded in car tires (Nan et al., 2006) in addition to the importance of Zn in tire vulcanization (Adachi & Tainosho, 2004). The wells are commonly found inside the roads with some unsealed well-holes led to the leaching of surface substances into the wells.
The minimum, maximum, and mean Pb values in the water samples are 18, 820, and 214.96 μg/L, respectively (Table 2). Similar to As and Cd, the Pb concentration is a major threat in the studied area, which greatly exceeded the WHO permissible limit (10 μg/L) in all samples (Fig. 3c). Due to its presence in small amounts in the earth’s crust, Pb comes from various anthropogenic sources, such as industrial wastes and household products (detergents) (Kabata-Pendias & Mukherjee, 2007; Nadeem-ul-Haq, 2009; Tchounwou et al., 2012). Thus, the contribution of numerous sources can also be depicted by significant differences between mean and median in a box plot (Fig. 4a). The presence of NO3¯ indicates the effect of human activity in the distribution of Pb in the studied area (Fig. 4b). The negative relationship between water depth and As, Cd, Pb concentrations predicted the influence of surficial substances for groundwater contamination by these metals (Fig. S7).
The measured chromium content in the water ranged from below the detection limit to 140 μg/L. Eighty-nine percent of analyzed water samples had Cr < 50 μg/L (Fig. 3d). Only two samples (7.1% of total samples) exceeded the permissible limit of 300 μg/L recommended by the World Health Organization for (Fe) in water samples (WHO, 2017a). All the analyzed water samples had Cu, Mn, and Zn within safe limits.
By comparing the analyzed data of trace constituents in the water samples with the recommended limits of heavy metals for irrigation, the concentrations of Cd, Cu, Cr, Fe, Mn, Pb, and Zn were within permissible limits for irrigation (USDA, 2011). Nevertheless, approximately 39.3% of the water samples exceeded the maximum allowable limit of As for the irrigation (Fig. 3a). Previous studies have shown a significant association between the contamination of heavy metals and the prevalence of chronic diseases such as kidney failure, hair loss, and chronic anemia in Egypt (Mandour & Azab, 2011; Melegy et al., 2014; Salem et al., 2000; Salman et al., 2019b). It can be seen from the assessment of the water parameters for drinking and irrigation purposes that arsenic concentration is found to be a significant threat in the study area.
Bacterial populations in water
Bacterial pollution worldwide is systematically assessed using fecal indicator bacteria contents, including E. coli and enterococci, as proxies for pathogenic organisms (Zhang et al., 2020). The bacterial results comprising total bacterial counts (TBCs), fecal streptococci, total coliform, and fecal coliform are shown in Tables 2, S2, and Fig. 5. TBCs ranged from 2.9 × 104 to 91 × 104 with average 22.2 × 104 (CFU/ml). All samples were extremely contaminated with TBCs surpass WHO (2017a) guidelines (50 CFU/ml). The highest (average) values of TC and FC were 2400 (497.4) and 1700 (358.3) (MPN/100 ml), respectively. The highest records of total and fecal coliforms were observed in the samples close to Orabi farms. It may reflect the role of agricultural wastewater and animal wastes as significant contamination sources. On the other side, 3 water samples were free of total coliform and fecal coliform bacteria (< 1.8 MPN/100 ml) (Table S2, Fig. 5). This could be due to the soil’s role, where soil and rock filter bacteria naturally (Ikhlil, 2009; Mwabi et al., 2013).
Fecal streptococci ranged from < 1.8 to 790 (MPN/100 ml) in water samples with an average of 115.9 (MPN/100 ml) (Table 2). Like coliforms, the highest MPN/100 ml of fecal streptococci was determined in sample No. 18 located behind Orabi farms directly (Fig. 1, Table S2). The lowest fecal streptococci value was depicted in samples Nos. 1, 5, 11, 14, 17, and 28 (Fig. 5). Bacteriological results showed greater numbers of TBCs, TC, FC, and FS, reflecting the contamination of shallow groundwater with human and animal fecal content (Cabral, 2010; Stachler et al., 2017). Wastewater discharges are the predominant source of fecal microorganisms in the water samples (WHO, 2003). In general, bacterial and heavy metals results signify the extent of contamination of the shallow groundwater, making it unsafe for drinking and directly injecting into the underline Miocene aquifer. The injection of such polluted water represents a point source of the Miocene aquifer pollution. Thus, instead of water injection into the underline aquifer, it is more efficient to reuse this water after physical, chemical, and biological treatment.
Factors controlling water composition
The principal component analysis (PCA) results of the current study are presented in Table S3, comprising the factor loadings and eigenvalues of each principal cofactor. Four principal components (PCs) that explained 75.39% of the total variance were extracted. Thus, virtually the complete variance of the original data could be attributed to these 4 extracted components.
PC1 in PCA explains 39.37% of the total variance. It shows strong positive loading for TDS, EC, TH, Ca, Mg, Na, SO42–, Cl, NH4+, Cr, NO3¯, As, and Zn. These correlations explain the role of rock–water interaction in water chemistry; however, the appearance of NH4+ and NO3¯ indicated the role of wastewater seepage and agricultural practices, and so PC1 can be interpreted mixed factor. The weathering of rock-forming minerals (especially evaporates), which present in the lithology of the study area (Abdelbakey, 2014), increasing the salinity of the water. As well, the presence of As, Cr, and Zn was recorded in the rocks of the Eastern Desert (Sadek et al., 2015). Salman et al. (2019c) pointed out the contamination of orchard soil at Orabi farms within the study area with As and Cd. This PC is supported by the piper diagram results, which indicated the secondary salinity of water and the role of waterlogging and leaching of salts from the surface.
The PC2 explains 15.67% of the total data variance and mainly reflects the contributions of the anthropogenic factor on the water. Figure 6 shows that high values of NH4+, As, Fe, and Zn indicate the significant influence of the anthropogenic (leakage from industrial and domestic wastewater seepage and agricultural practices) on water chemistry. The absence of organic deposits in the study area pointed out the main source of NH4+ is the infiltration of nitrogen-rich water from surface activities (Norrman et al., 2015).
PC3 explains 12.22% of the total variance and is primarily associated with Pb, Cu, TC, FC, and FS. PC3 reflects the influence of wastewater seepage on the water because the main source of the pathogenic indicator is animal feces, whereas PC4, which is mainly chartered by pH and Cd, explains 8.14% of the total dataset variance. PC4 reflects the influence of geochemical processes, especially pH, on the concentration of Cd. At pH < 8, soluble complexes of Cd with inorganic are carbon formed and at pH ≥ 8 and precipitation of CdCO3 is favorable (Kubier et al., 2019).
Conclusively, the PCA stated the positive correlation between As and both NO3¯ and NH4+ in PC1 and PC2 that confirmed agricultural activities (fertilizers and pesticides) and leakage from industrial and domestic wastewaters could be significant sources of As in this area (El-Aassar et al., 2010).
Photocatalytic treatment of water
Selected representative samples were treated with nitrogen-doped titania suspension in the presence of visible light irradiation. As shown in Table 3, the bacteria were degraded completely within 60 min irradiation, as well as more than 82% of dissolved organic content was mineralized. Doping of nitrogen into nanocrystalline titania results in an extension of its light absorption properties into the visible region. Highly enhancement of the photocatalytic activity of nitrogen-doped titania may be attributed to red-shifted optical absorption edge, and lower optical bandgap compared to undoped titania. The excellent photoactivity of nitrogen-doped titania compared to the undoped one could be attributed to the contribution of nitrogen species on the decrement of titania band gab resulting in increment of titania photosensitivity in the visible region (Darwish et al., 2015). Thus, nitrogen-doped titania is a cheap as well as promising photocatalyst for many photocatalytic applications. Unfortunately, the used technique has a little effect on heavy metals and is considered ineffective for heavy metals removal (Table 3).
This technique has advantages over chlorine, the applied reagent for disinfection in the Egyptian drinking water stations due to its low cost. The chlorination process can produce many harmful disinfection by-products (DBPs) as a result of interaction with organic compounds in the water (Zhang et al., 2018b). The most widespread DBPs are trihalomethanes (THMs), such as chloroform that is carcinogenic (Murray et al., 2012). Furthermore, chlorine has limited efficacy against protozoan pathogens, which significantly causes diarrhea in children (Crider et al., 2018; Liu et al., 2016).
Conclusions
Groundwater at the study area contains acceptable levels of pH, Mg2+, K+, Cr, Cu, Mn, Fe, Zn, for drinking; nonetheless, As, Pb, Cd, TBC, TC, FC, and FS were above the permissible level. The studied samples were suitable for irrigation except for their arsenic contents and some samples (14%) with high SAR and MR. The PCA and cluster analyses pointed out mixed factors (rock–water interaction and anthropogenic activities) controlling groundwater quality in the study area. Wastewater seepage, industrial, and agricultural effluents runoff are the main anthropogenic sources of water pollution in the study area. The shallow groundwater origin is meteoric and of three main water types Na–Cl, Ca–Mg–Cl, and Ca(HCO3)2. The photocatalytic treatment of the polluted groundwater has shown high efficiency in removing approximately 95% and 82% of microbial pollutants and organic content. Ultimately, shallow groundwater injection into the underline Miocene aquifer is greatly hazardous and threatens the aquifer quality and must be ceased. It is recommended to reuse shallow groundwater for different purposes after the proposed photocatalytic treatment.
Data availability
The data are provided in the article and the Supplementary Material.
Code availability
This research uses the following software: Microsoft Word (Office365), Microsoft Excel (Office 365), ArcGIS (v.10.4), Surfer, SPSS 21, XLSTAT (USA), FastStone (v.9.0), Sigma Plot, Edraw Max (v.10).
References
Aamer, H. A., Hassan, D. M., & Kotb, S. (2016). Assessment of heavy metals pollution in groundwater and cow’s milk in Upper Egypt. Assiut Veterinary Medical Journal, 62(149), 1–12
Abdelhafiz, M. A., Elnazer, A. A., Seleem, E.-M. M., Mostafa, A., Al-Gamal, A. G., Salman, S. A., et al. (2021). Chemical and bacterial quality monitoring of the Nile River water and associated health risks in Qena-Sohag sector, Egypt. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-021-00893-3.
Abdel-Shafy, H. I., & Kamel, A. H. (2016). Groundwater in Egypt issue: Resources, location, amount, contamination, protection, renewal, future overview. Egyptian Journal of Chemistry, 59(3), 321–362. https://doi.org/10.21608/ejchem.2016.1085
Abdel Latif, A., & El Kashouty, M. (2010). Groundwater investigation in Awlad Salameh, southern Sohag, Upper Egypt. Earth Sciences Research Journal, 14(1), 63–75
Abdelbakey, S. (2014). Geo-Environmental assessment of El-Obour area, northeast Cairo, Egypt. Ph.D. Thesis, Ain Shams University, Faculty of science.
Abdelhafiz, M. A. (2017). Hydrochemical and environmental assessment of waterlogging and soil for different purposes at El Obour City, East Cairo, Egypt. MSc, Al-Azhar University, Faculty of Science, Assiut.
Adachi, K., & Tainosho, Y. (2004). Characterization of heavy metal particles embedded in tire dust. Environment International, 30(8), 1009–1017. https://doi.org/10.1016/j.envint.2004.04.004
Ahmed, M. F., Mokhtar, M. B., & Alam, L. (2021). Carcinogenic and non-carcinogenic health risk of arsenic ingestion via drinking water in Langat River Basin, Malaysia. Environmental Geochemistry and Health, 43(2), 897–914. https://doi.org/10.1007/s10653-020-00571-w
Ajami, H. (2021). Geohydrology: Groundwater. In D. Alderton & S. A. Elias (Eds.), Encyclopedia of geology. (2nd ed., pp. 408–415). Academic Press.
APHA. (1998). Standard methods for the examination of water and wastewater: 19th edition supplement. American Public Health Association.
APHA. (2012). Standard methods for the examination of water and wastewater (22.th ed.). Washington, DC: American Public Health Association (APHA), American Water Works Association (AWWA) and Water Environment Federation (WEF).
Awad, M., Nada, A. A., Hamza, M. S., & Froehlich, K. (1995). Chemical and isotopic investigation of groundwater in Tahta region, Sohag-Egypt. Environmental Geochemistry and Health, 17(3), 147–153. https://doi.org/10.1007/BF00126083
Awad, S., El Fakharany, M. A., & Hagran, N. M. (2015). Environmental impact on water resources at the Northwestern Part of the Nile Delta, Egypt. The Journal of American Science, 11(11), 1–11. https://doi.org/10.7537/marsjas111115.01
Bahrami, M., Zarei, A. R., & Rostami, F. (2020). Temporal and spatial assessment of groundwater contamination with nitrate by nitrate pollution index (NPI) and GIS (case study: Fasarud Plain, southern Iran). Environmental Geochemistry and Health, 42(10), 3119–3130. https://doi.org/10.1007/s10653-020-00546-x
Barseem, M., Lateef, T., & Deen, H. (2015a). Geoelectrical contribution for solving water logging in selected sites, kilometer 35, Cairo - Ismailia Desert Rode, Egypt. Egyptian Geophysical Society EGS Journal, 13(1), 67–82
Barseem, M., Lateef, T., & Deen, H. (2015b). Geoelectrical contribution for solving water logging in selected sites, kilometer 35, Cairo - Ismailia Desert Rode, Egypt. Egyptian Geophysical Society EGS Journal, 13, 67–82
Batzill, M., Morales, E. H., & Diebold, U. (2006). Influence of nitrogen doping on the defect formation and surface properties of TiO2 rutile and anatase. Physical Review Letters, 96(2), 026103. https://doi.org/10.1103/PhysRevLett.96.026103
Boonkaewwan, S., Sonthiphand, P., & Chotpantarat, S. (2021). Mechanisms of arsenic contamination associated with hydrochemical characteristics in coastal alluvial aquifers using multivariate statistical technique and hydrogeochemical modeling: A case study in Rayong province, eastern Thailand. Environmental Geochemistry and Health, 43(1), 537–566. https://doi.org/10.1007/s10653-020-00728-7
Cabral, J. P. S. (2010). Water microbiology. Bacterial pathogens and water. International Journal of Environmental Research and Public Health, 7(10), 3657–3703
Chaney, R. L. (2010). Cadmium and zinc. In P. S. Hooda (Ed.), Trace elements in soils. (pp. 409–439). Wiley Online Library.
Chen, J., Qian, H., Gao, Y., & Li, X. (2018). Human health risk assessment of contaminants in drinking water based on triangular fuzzy numbers approach in Yinchuan City, Northwest China. Exposure and Health, 10, 155–166. https://doi.org/10.1007/s12403-017-0252-z
Chobba, B. M., Messaoud, M., Weththimuni, M. L., Bouaziz, J., Licchelli, M., De Leo, F., et al. (2019). Preparation and characterization of photocatalytic Gd-doped TiO2 nanoparticles for water treatment. Environmental Science and Pollution Research, 26(32), 32734–32745. https://doi.org/10.1007/s11356-019-04680-7
Crider, Y., Sultana, S., Unicomb, L., Davis, J., Luby, S. P., & Pickering, A. J. (2018). Can you taste it? Taste detection and acceptability thresholds for chlorine residual in drinking water in Dhaka, Bangladesh. Science of The Total Environment, 613–614, 840–846. https://doi.org/10.1016/j.scitotenv.2017.09.135
Darwish, W., Bieh, M., Abdel-Mottaleb, M., Bieh, H., Nada, A., Elnazer, H., et al. (2015). Spectrophotometric evaluation of photocatalytic activity of nanostructured N-TiO2 and N, F-TiO2 by oxidative degradation of phthalocyanine star polymer. Advances in Natural and Applied Sciences, 9(12), 105–115
Das, S., Misra, A. J., Habeeb Rahman, A. P., Basu, A., Mishra, A., Tamhankar, A. J., et al. (2020). Designing novel photocatalysts for disinfection of multidrug-resistant waterborne bacteria. In L. Ledwani & J. S. Sangwai (Eds.), Nanotechnology for energy and environmental engineering. (pp. 441–476). Springer International Publishing.
Divya, A. H., & Solomon, P. A. (2016). Effects of some water quality parameters especially total coliform and fecal coliform in surface water of Chalakudy River. Procedia Technology, 24, 631–638. https://doi.org/10.1016/j.protcy.2016.05.151
El-Aassar, A. H. M., Abo-El-Fadl, M. M., & Shawky, H. A. (2010). Environmental impacts on groundwater quality in El-Obour city and its vicinities-east Cairo-Egypt. Egyptian Journal of Desert Research, 60(1), 31–50.
El-Fakharany, M. A., Mansour, N. M., Yehia, M. M., & Monem, M. (2017). Evaluation of groundwater quality of the Quaternary aquifer through multivariate statistical techniques at the southeastern part of the Nile Delta, Egypt. Sustainable Water Resources Management, 3(1), 71–81. https://doi.org/10.1007/s40899-017-0087-6
El Nazer, H. E. D., Salman, S., & Elnazer, A. (2017). Irrigation water quality assessment and a new approach to its treatment using photocatalytic technique: Case study Yaakob village, SW Sohag, Egypt. Journal of Materials and Environmental Science, 8, 310–317
Elnazer, A. A., Salman, S. A., Seleem, E. M., & Abu El Ella, E. M. (2015). Assessment of some heavy metals pollution and bioavailability in roadside soil of Alexandria-Marsa Matruh Highway, Egypt. International Journal of Ecology, 2015, 689420. https://doi.org/10.1155/2015/689420
Elnazer, A. A., Seleem, E.-M.M., Zeid, S. A. M., Ismail, I. S. A., Bahlol, H. A., & Salman, S. A. (2021). Hydrochemical evaluation of the quaternary aquifer and its suitability for different purposes at south Al Waqf city, Qena, Upper Egypt. Groundwater for Sustainable Development, 12, 100517. https://doi.org/10.1016/j.gsd.2020.100517
Gole, J. L., Stout, J. D., Burda, C., Lou, Y., & Chen, X. (2004). Highly Efficient formation of visible light tunable TiO2-xNx photocatalysts and their transformation at the nanoscale. The Journal of Physical Chemistry B, 108(4), 1230–1240. https://doi.org/10.1021/jp030843n
He, X., Li, P., Wu, J., Wei, M., Ren, X., & Wang, D. (2021). Poor groundwater quality and high potential health risks in the Datong Basin, northern China: Research from published data. Environmental Geochemistry and Health, 43(2), 791–812. https://doi.org/10.1007/s10653-020-00520-7
Howard, K., & Gerber, R. (2018). Impacts of urban areas and urban growth on groundwater in the Great Lakes Basin of North America. Journal of Great Lakes Research, 44(1), 1–13. https://doi.org/10.1016/j.jglr.2017.11.012
Ikhlil, A. I. H. (2009). Groundwater quality of springs and dug wells in Dura Area. M.Sc., College of Graduate Studies and Academic Research, Hebron University, Palestine.
Jadhav, S. V., Bringas, E., Yadav, G. D., Rathod, V. K., Ortiz, I., & Marathe, K. V. (2015). Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal. Journal of Environmental Management, 162, 306–325. https://doi.org/10.1016/j.jenvman.2015.07.020
Kabata-Pendias, A., & Mukherjee, A. B. (2007). Trace elements from soil to human. (1st ed.). Berlin: Springer.
Karunanidhi, D., Aravinthasamy, P., Subramani, T., & Muthusankar, G. (2021a). Revealing drinking water quality issues and possible health risks based on water quality index (WQI) method in the Shanmuganadhi River basin of South India. Environmental Geochemistry and Health, 43(2), 931–948. https://doi.org/10.1007/s10653-020-00613-3
Karunanidhi, D., Subramani, T., Roy, P. D., & Li, H. (2021b). Impact of groundwater contamination on human health. Environmental Geochemistry and Health, 43(2), 643–647. https://doi.org/10.1007/s10653-021-00824-2
Krishan, G. (2019). Groundwater salinity . Current World Environment, 14, 186–188. https://doi.org/10.12944/CWE.14.2.02
Kubier, A., Wilkin, R. T., & Pichler, T. (2019). Cadmium in soils and groundwater: A review. Applied Geochemistry, 108, 104388. https://doi.org/10.1016/j.apgeochem.2019.104388
Li, B., Xia, M., Zorec, R., Parpura, V., & Verkhratsky, A. (2021). Astrocytes in heavy metal neurotoxicity and neurodegeneration. Brain Research, 1752, 147234. https://doi.org/10.1016/j.brainres.2020.147234
Li, P., & Wu, J. (2019). Drinking water quality and public health. Exposure and Health, 11(2), 73–79. https://doi.org/10.1007/s12403-019-00299-8
Linsebigler, A. L., Lu, G., & Yates, J. T. (1995). Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chemical Reviews, 95(3), 735–758. https://doi.org/10.1021/cr00035a013
Liu, J., Platts-Mills, J. A., Juma, J., Kabir, F., Nkeze, J., Okoi, C., et al. (2016). Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: A reanalysis of the GEMS case-control study. Lancet (London, England), 388(10051), 1291–1301. https://doi.org/10.1016/S0140-6736(16)31529-X
Machiwal, D., Jha, M. K., Singh, V. P., & Mohan, C. (2018). Assessment and mapping of groundwater vulnerability to pollution: Current status and challenges. Earth-Science Reviews, 185, 901–927. https://doi.org/10.1016/j.earscirev.2018.08.009
Mandour, R. A., & Azab, Y. A. (2011). Toxic levels of some heavy metals in drinking groundwater in Dakahlyia Governorate, Egypt in the year 2010. International Journal of Occupational and Environmental Medicine, 2(2), 112–117
Mansour, N. M. (2020). Hydrochemical studies and evaluation of groundwater quality of the Quaternary aquifer at Faquss, Al Sharqiya Governorate, Egypt. Sustainable Water Resources Management, 6(2), 19. https://doi.org/10.1007/s40899-020-00374-y
Masoud, A. A., Abdel-Wahab Arafa, N. A., & El-Bouraie, M. (2018). Patterns and trends of the pesticide pollution of the Shallow Nile Delta Aquifer (Egypt). Water, Air, & Soil Pollution, 229(5), 148. https://doi.org/10.1007/s11270-018-3802-5
Melegy, A. A., Shaban, A. M., Hassaan, M. M., & Salman, S. A. (2014). Geochemical mobilization of some heavy metals in water resources and their impact on human health in Sohag Governorate, Egypt. Arabian Journal of Geosciences, 7(11), 4541–4552. https://doi.org/10.1007/s12517-013-1095-y
Mishra, S., Dwivedi, S., & Singh, R. (2010). A review on epigenetic effect of heavy metal carcinogens on human health. The Open Nutraceuticals Journal, 3, 188–193
Mthembu, P. P., Elumalai, V., Brindha, K., & Li, P. (2020). Hydrogeochemical processes and trace metal contamination in groundwater: Impact on human health in the Maputaland Coastal Aquifer, South Africa. Exposure and Health, 12(3), 403–426. https://doi.org/10.1007/s12403-020-00369-2
Murray, C. J. L., Vos, T., Lozano, R., Naghavi, M., Flaxman, A. D., Michaud, C., et al. (2012). Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet, 380(9859), 2197–2223. https://doi.org/10.1016/S0140-6736(12)61689-4
Mwabi, J. K., Mamba, B. B., & Momba, M. (2013). Removal of waterborne bacteria from surface water and groundwater by cost-effective household water treatment systems (HWTS): A sustainable solution for improving water quality in rural communities of Africa. Water SA, 39(4), 445–456
MWRI. (2014). Water scarcity in Egypt: The urgent need for regional cooperation among the Nile Basin Countries. Ministry of Water Resources and Irrigation.
Nadeem-ul-Haq, A., & M. A., Haque, Z., Badar, N., & Mughal, N. . (2009). Drinking water contamination by chromium and lead in industrial lands of Karachi. Journal of Pakistan Medical Association, 59(5), 270–274
Nakadaira, H., Nakamura, K., Mutoh, K., Yamamoto, M., & Katoh, K. (2000). Arsenic residues in well water 36 y after endemic arsenic poisoning. Archives of Environmental Health: An International Journal, 55(5), 364–364. https://doi.org/10.1080/00039890009604031
Nan, Z., Xu, W., & Zhao, C. (2006). Spatial distribution of selected trace metals in urban soils of Lanzhou City, Gansu Province, Northwestern of China. In 2006 IEEE international symposium on geoscience and remote sensing, July 31–August 4 2006 2006 (pp. 3397–3400). doi:https://doi.org/10.1109/IGARSS.2006.872.
Norrman, J., Sparrenbom, C. J., Berg, M., Dang, D. N., Jacks, G., Harms-Ringdahl, P., et al. (2015). Tracing sources of ammonium in reducing groundwater in a well field in Hanoi (Vietnam) by means of stable nitrogen isotope (δ15N) values. Applied Geochemistry, 61, 248–258. https://doi.org/10.1016/j.apgeochem.2015.06.009
Omar, M. E. D. M., & Moussa, A. M. A. (2016). Water management in Egypt for facing the future challenges. Journal of Advanced Research, 7(3), 403–412. https://doi.org/10.1016/j.jare.2016.02.005
Osman, G. A., Shaban, A. M., Melegy, A. A., Hassaan, M. M., & Salman, S. A. (2012). A baseline study on microbial and inorganic chemicals contaminants of health importance in groundwater and surface water of Sohag Governorate, Egypt. Journal of Applied Sciences Research, 8, 5765–5773
Pelaez, M., Nolan, N. T., Pillai, S. C., Seery, M. K., Falaras, P., Kontos, A. G., et al. (2012). A review on the visible light active titanium dioxide photocatalysts for environmental applications. Applied Catalysis B: Environmental, 125, 331–349. https://doi.org/10.1016/j.apcatb.2012.05.036
Piper, A. M. (1944). A graphic procedure in the geochemical interpretation of water analyses. EOS, Transactions American Geophysical Union, 25(6), 914–928. https://doi.org/10.1029/TR025i006p00914
Raghunath, H. M. (1987). Groundwater. Wiley Eastern Ltd.
Richards, L. A. (1954). Diagnosis and improvement of saline and alkali soils. (Vol. 78)US Dept. Agri., USDA.
Sadek, M., Ali-Bik, M. W., & Hassan, S. M. (2015). Late Neoproterozoic basement rocks of Kadabora-Suwayqat area, Central Eastern Desert, Egypt: geochemical and remote sensing characterization. Arabian Journal of Geosciences, 8, 10459–10479. https://doi.org/10.1007/s12517-015-1973-6
Salem, H. M., Eweida, E. A., & Farag, A. (2000). Heavy metals in drinking water and their environmental impact on human health. Paper presented at the ICEHM international conference for environmental hazards mitigation.
Salman, S. A., Arauzo, M., & Elnazer, A. A. (2019a). Groundwater quality and vulnerability assessment in west Luxor Governorate, Egypt. Groundwater for Sustainable Development, 8, 271–280. https://doi.org/10.1016/j.gsd.2018.11.009
Salman, S. A., Asmoay, A., El-Gohary, A., & Sabet, H. (2019b). Evaluation of human risks of surface water and groundwater contaminated with Cd and Pb in the southern El-Minya Governorate, Egypt. Drinking Water Engineering and Science, 12, 23–30. https://doi.org/10.5194/dwes-12-23-2019
Salman, S. A., & Elnazer, A. A. (2015). Evaluation of groundwater quality and its suitability for drinking and agricultural uses in SW Qena Governorate, Egypt. Advances in Natural and Applied Sciences, 9, 16+
Salman, S. A., & Elnazer, A. A. (2020). Assessment and speciation of chromium in groundwater of south Sohag Governorate, Egypt. Groundwater for Sustainable Development, 10, 100369. https://doi.org/10.1016/j.gsd.2020.100369
Salman, S. A., Zeid, S. A. M., Seleem, E. M., & Abdel-Hafiz, M. A. (2019c). Soil characterization and heavy metal pollution assessment in Orabi farms, El Obour, Egypt. Bulletin of the National Research Centre, 43(1), 42. https://doi.org/10.1186/s42269-019-0082-1
Shaji, E., Santosh, M., Sarath, K. V., Prakash, P., Deepchand, V., & Divya, B. V. (2020). Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geoscience Frontiers. https://doi.org/10.1016/j.gsf.2020.08.015
Smithers, J., Gray, R., Johnson, S., & Still, D. (2017). Modelling and water yield assessment of Lake Sibhayi. Water SA, 43, 480–491
Stachler, E., Kelty, C., Sivaganesan, M., Li, X., Bibby, K., & Shanks, O. C. (2017). Quantitative CrAssphage PCR assays for human fecal pollution measurement. Environmental Science & Technology, 51(16), 9146–9154. https://doi.org/10.1021/acs.est.7b02703
Sulin, V. (1946). Waters of petroleum formations in the system of natural water. (pp. 35–96). Gostoptekhiz-dat.
Taha, A. A., El-Mahmoudi, A. S., & El-Haddad, I. M. (2004). Pollution sources and related environmental impacts in the new communities southeast Nile Delta, Egypt. Emirates Journal for Engineering Research, 9(1), 35–49
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., & Sutton, D. J. (2012). Heavy metal toxicity and the environment. Experientia supplementum, 2012(101), 133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Trick, J. K., Stuart, M., & Reeder, S. (2018). Contaminated groundwater sampling and quality control of water analyses. In B. De Vivo, H. E. Belkin, & A. Lima (Eds.), Environmental geochemistry. (2d ed., pp. 25–45). Amsterdam: Elsevier.
Ukah, B. U., Egbueri, J. C., Unigwe, C. O., & Ubido, O. E. (2019). Extent of heavy metals pollution and health risk assessment of groundwater in a densely populated industrial area, Lagos, Nigeria. International Journal of Energy and Water Resources, 3(4), 291–303. https://doi.org/10.1007/s42108-019-00039-3
UNESCO. (2009). Water in a changing world (WWDR-3): The 3rd United Nations World Water Development Report.
USDA. (2011). Assessing water quality for human consumption, agriculture, and aquatic life uses tom pick. United States Department of Agriculture, National Agricultural Statistics Service.
Usman, U. A., Yusoff, I., Raoov, M., & Hodgkinson, J. (2020). Trace metals geochemistry for health assessment coupled with adsorption remediation method for the groundwater of Lorong Serai 4, Hulu Langat, west coast of Peninsular Malaysia. Environmental Geochemistry and Health, 42(10), 3079–3099. https://doi.org/10.1007/s10653-020-00543-0
Vasudevan, U., Gantayat, R. R., Chidambaram, S., Prasanna, M. V., Venkatramanan, S., Devaraj, N., et al. (2021). Microbial contamination and its associations with major ions in shallow groundwater along coastal Tamil Nadu. Environmental Geochemistry and Health, 43(2), 1069–1088. https://doi.org/10.1007/s10653-020-00712-1
Ward, J. H. (1963). Hierarchical grouping to optimize an objective function. Journal of the American Statistical Association, 58(301), 236–244. https://doi.org/10.1080/01621459.1963.10500845
Wen, D., Zhang, F., Zhang, E., Wang, C., Han, S., & Zheng, Y. (2013). Arsenic, fluoride and iodine in groundwater of China. Journal of Geochemical Exploration, 135, 1–21. https://doi.org/10.1016/j.gexplo.2013.10.012
WHO. (2003). Guidelines for safe recreational water environments. Volume 1, coastal and fresh waters. World Health Organization.
WHO. (2011). Guidelines for drinking-water quality (4th edn, Vol. 4). World Health Organization.
WHO. (2017a). Guidelines for drinking-water quality. (4th ed.). World Health Organization.
WHO. (2017b). UN-water global analysis and assessment of sanitation and drinking-water (GLAAS) report. Financing universal water, sanitation and hygiene under the Sustainable. (pp. 96).
WHO. (2019). Drinking-water fact sheets. Retrieved from World Health Organization. https://www.who.int/en/news-room/fact-sheets/detail/drinking-water.
Xiong, B., Li, R., Johnson, D., Luo, Y., Xi, Y., Ren, D., et al. (2021). Spatial distribution, risk assessment, and source identification of heavy metals in water from the Xiangxi River, Three Gorges Reservoir Region, China. Environmental Geochemistry and Health, 43(2), 915–930. https://doi.org/10.1007/s10653-020-00614-2
You, X., Liu, S., Dai, C., Guo, Y., Zhong, G., & Duan, Y. (2020). Contaminant occurrence and migration between high- and low-permeability zones in groundwater systems: A review. Science of The Total Environment, 743, 140703. https://doi.org/10.1016/j.scitotenv.2020.140703
Zeid, S., Seleem, E., Salman, S., & Abdel-Hafiz, M. (2018). Water quality index of shallow groundwater and assessment for different usages in El-Obour city, Egypt. Journal of Materials and Environmental Science, 9(7), 1957–1968
Zhang, Y., Wu, J., & Xu, B. (2018a). Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Environmental Earth Sciences, 77(7), 273. https://doi.org/10.1007/s12665-018-7456-9
Zhang, J., Balkovič, J., Azevedo, L. B., Skalský, R., Bouwman, A. F., Xu, G., et al. (2018b). Analyzing and modelling the effect of long-term fertilizer management on crop yield and soil organic carbon in China. Science of The Total Environment, 627, 361–372. https://doi.org/10.1016/j.scitotenv.2018.01.090
Zhang, X., Zhi, X., Chen, L., & Shen, Z. (2020). Spatiotemporal variability and key influencing factors of river fecal coliform within a typical complex watershed. Water Research, 178, 115835. https://doi.org/10.1016/j.watres.2020.115835
Acknowledgements
The authors would like to thank the CAS-TWAS president doctoral fellowship program. The authors are grateful to the editor and anonymous reviewers for their suggestions and comments which enhanced the paper's quality.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly toward the final makeup of the paper. MAA and SAS contributed to conceptualization, investigation, formal analysis, visualization, data curation, writing—review and editing, and validation; EMS contributed to conceptualization, data collection, visualization and validation; HAEN contributed to formal analysis, investigation, writing and editing; SAMZ conceived the idea of the study, conceptualization and validation; BM helped in supervision, conceptualization, review—revision and editing, discussed the results, and provided valuable feedbacks after proofreading. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Consent to participate
No human participants were involved in this study.
Consent to publish
No human participants were involved in this study.
Ethics approval
Not applicable.
Ethical approval for animal research
This article does not contain any studies on animals performed by any authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abdelhafiz, M.A., Seleem, EM.M., El Nazer, H.A. et al. Shallow groundwater environmental investigation at northeastern Cairo, Egypt: quality and photo-treatment evaluation. Environ Geochem Health 43, 4533–4551 (2021). https://doi.org/10.1007/s10653-021-00933-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-00933-y