Abstract
The mining and municipal wastewaters in the study area are located around Elazig, Turkey. This study investigated the translocation and accumulation of Sr into 9 terrestrial–aquatic plants from the Elazig municipal wastewater, Keban Pb–Zn and Maden Cu wastewaters. Plants and their soil samples were collected from the stream/rivers on the municipal and mining areas, and Sr values in both plant parts and their soils were analyzed by ICP-MS. The mean Sr concentrations in the soil, root and shoot of the terrestrial–aquatic plants were 101, 48.2 and 80.5 ppm, respectively (on the dried weight basis). The enrichment coefficients of root (ECR) and shoots (ECS) and translocation factors of studied plants were calculated and, then, divided into several groups as a candidate, bioaccumulator and hyperaccumulator plants according to their ECR and ECSs. These groups indicated the candidate plants: Salix sp. and Tamarix tetrandra; bioaccumulator plants: Pragmites sp. and Xanthium, and hyperaccumulator plants: Typha latifolia, Bolboscholnus ascbersus and Lythnium salicaria for Sr. These results showed that both bioaccumulator and hyperaccumulator plant groups had very high ability to accumulate strontium to plant parts from their soil. Therefore, these studied plants may be helpful/useful for the rehabilitation studies of municipal and mining soils contaminated by Sr.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Strontium (Sr) is often observed in association with felsic magmatic and carbonate rocks, and it is one of the common trace elements changing between 260 and 370 ppm in lithosphere. Sr is mobilized as easily soluble strontianite (SrCO3) in the carbonate rocks and then precipitated as celestite (SrSO4). These minerals cause the environmental problems for the plants, human and animals in terrestrial environments (Kabata-Pendias 2011). Sr in different soils is mostly controlled by parent rocks and climate conditions. Strontium might cause environmental toxicity in such mining areas and felsic magmatic rocks rich in Sr. Strontium observes as an accessory element within other mineral deposits and may occur near or in sedimentary rocks together with lenses or layers of gypsum and halite as well as in veins associated with dolomite and limestone (Burger and Lichtscheidl 2019). The highest Sr levels were observed in soils of the heavy loam. The Sr values in unpolluted soils change between 450 and 300 ppm in clays, 140 and 20 ppm in sandstones, 3100 and 20 ppm in cambisol soil, 500 and 70 in histosol and 1000 and 20 ppm in podzols (Kabata-Pendias 2011). The heavy metal toxicity is one of the major problems for the natural environments due to non-biodegradable character and its persistent in nature and gather in the organs of living plant and animals (Khan et al. 2014; Iqbal 2016; Iqbal et al. 2016, 2017; Mani et al. 2016; Sasmaz and Sasmaz 2017). The heavy metals such as Sr, As, Sb, Tl, Hg, Co, Cr, Ni, Pb, Zn, U, Th and Se in municipal and mining areas pollute the stream/rivers and surface soils (Wong 2003; US EPA 2000; Rađenović and Medunić 2015; Rađenović et al. 2016; Fiket et al. 2016; Thakur et al. 2015; Pervaiz et al. 2015; Majolagbe et al. 2016; Benabdallah et al. 2017; Fait et al. 2017; Radić et al. 2018; Siddique et al. 2018; Ivanić et al. 2019), and they can be removed by using different terrestrial–aquatic plants from the water and soils (Censi et al. 2014; Sasmaz et al. 2015, 2016a, b, c, 2018, 2019; Yildirim and Sasmaz 2017; Palutoglu and Sasmaz 2017; Palutoglu et al. 2018; Fiket et al. 2019; Dvoršćak et al. 2019; Medunić et al. 2020; Iqbal et al. 2019; Padmasubashini et al. 2020). Sr is not a micronutrient for the plant as Ca. It is absorbed for the metabolic needs of the plants and depends on both exchange diffusion and mass flow mechanism. There are a limited number of studies on Sr accumulation and toxicity in plants, and the toxic value for plants has been measured as 30 ppm by Shacklette et al. (1978). Both radioactive and stable strontium enter to the body using the same way; if they breathe dust or vapor with Sr, it will dissolve in the lung surface and quickly mix with blood. If the Sr does not mix easily with blood, then, for long time, it can stay in the lung (ATSDR 2004). So, it may also cause to unwelcome biochemical and physiological problems in animal and human due to different harmful damages on lung. Different techniques are used to remove heavy metals from water and soil (Nematollahi et al. 2014; Sasmaz et al. 2015; Nematollahi et al. 2016a, b, c; Ebrahimi and Barbieri 2019). Phytoremediation shows on the metal removal performance of each plant according to their physiological, genetic, anatomic and morphologic characters (Liu et al. 2008; Yoon et al. 2006). Although there are many studies on radioactive Sr accumulation in terrestrial plants, there are little data about stable Sr accumulation in aquatic/terrestrial plants. That’s why, the main objective of this study was (1) to explore Sr transport and uptake to plant parts from soil by investigating the accumulation and distribution of Sr in the shoots and roots of 9 terrestrial/quatic plants growing naturally in Sr-polluted water and soil/sediments of the Elazig municipal plant, Keban Pb–Zn and Maden Cu mining areas around Elazig and (2) lastly to investigate whether these aquatic/terrestrial plants can be useful for the restoration or rehabilitation of sediment or water contaminated by Sr or not.
Materials and methods
Apparatus
Sr concentrations in plant and soil samples in the study area were analyzed by using ICP-MS (PerkinElmer ELAN 9000) technique. The operation conditions of ICP-MS for Sr are given in Table 1.
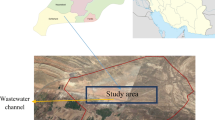
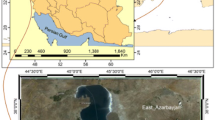
The study area
Sampling operations were carried out in the waters of the Keban Pb–Zn mining, Maden Cu mining and Elazig municipality wastewater treatment plant (Fig. 1).
The Keban region (E38° 72′ and N38° 78′) (Fig. 1) was one of the largest skarn type Pb–Zn deposits of Turkey, which is related to the syenomonzonitic and syenitic rocks outcropped in the south of Keban town (Akgul 2015). This mineralization observes both magmatic rock–marble contacts and magmatic intrusion, and it contains Zn–Pb–Ag, W, Fe–Cu, Fe–Mo and Mn–Ag reserves with high degree. The main economic source in this area is argentiferous Pb–Zn ores, which have been mined about 6000 years (Seeliger et al. 1985). The galleries were used to produce Pb–Zn–Ag ores for many years. (The mine was closed due to the ore is over in 1985.) For security reasons, the galleries have been closed, but the gallery has common water effluent feeding from the mining site during all year and, then, this gallery water is directly drained into Karakaya Dam Lake (Sasmaz et al. 2015).
The Maden copper deposits have been used as main copper source by the different civilizations about 4000 years in this region (Fig. 1). The deposits are underlining by gently dipping mudstones interlayered with basic volcanic rocks and mainly Eocene aged limestone. The copper deposits are in an east trending belt about 20 km long, near the Maden town. The Ana yatak has the largest ore mass in this region, and its ore dimension is observed in the mudstone layers in the form of a canoe in a depth of approximately 0.17 km, 1 km long and 0.5 km wide. The maximum dimension of the main ore body is about 50 × 300 × 550 m. It consists mainly of chalcopyrite and pyrite and locally pyrrhotite, arsenopyrite, magnetite, barite, quartz and calcite. The bornite, covellite and chalcocite minerals in supergene zone observe between massive sulfide zone and gossans. This region is commonly contaminated with activities both natural mines and ancient mining operations (Griffits et al. 1972).
Elazig municipality wastewater treatment plant is located on the 17th km on the Elazig-Bingol road, and its drainage area is between 38° 36′–39° 07′ longitudes and 38°17′–38°43′ latitudes (Fig. 1). Kehli Stream has a drainage area of 500 km2, which reaches Keban Dam Lake from the southeast of Elazig City and a length about 10 km. The stream transports effluent of the treatment plant to Keban Dam Lake and sewage of many small villages. The plant parts and their sediments and water samples were carried out on the stream of Kehli and throughout 2 months (Sasmaz et al. 2008). This facility was built in 1994 to prevent pollution of the Keban Dam Lake water basin. Wastewater Treatment Plant has been designed according to the capacity of 2020, and the construction, mechanical and electrical parts have been completed to meet the capacity of 2000. The first stage of the treatment plant is designed for 300,000 equivalent populations and 820 l/s wastewater flow, and the second stage is designed for 550,000 equivalent population and 1671 l/s wastewater flow. This facility consists of pretreatment, biological treatment and sludge removal units (Obek et al. 2007; Topal 2016; Topal and Arslan Topal 2011).
Sampling and analytical details
During the sampling, Keban Pb–Zn and Maden Cu deposit and Elazig municipality wastewater treatment plant waters were collected in plastic bottles to make both physicochemical parameters and anion–cation analysis. In addition, pH, temperature and electrical conductivity (EC) of these waters were measured on site (Table 2). Also, the water samples were filled in clean and sterilized plastic bottles to send to Acme (Canada) Laboratory for various trace element analyses. Water samples for physical and chemical parameters were analyzed in DSI laboratory (Elazig) accredited by the ISO 17025 quality system. The monitored water quality parameters were analyzed in the laboratory according to standard methods, as shown in Table 2 (APHA 1999). pH, temperature, turbidity DO, EC, HCO3−, NO3−, SO −24 , Cl−, F−, orthophosphate, COD, total N and Kjeldahl N were chosen as water quality parameters.
The soil and plant samples were collected randomly in August and September of 2019 from three different areas in the study area. The soils were taken from 31 several places of the study area in depths from 0.20 to 0.60 m together with plant root and shoots. The studied plants mostly live a few years and grown as a terrestrial/aquatic plant on the stream and river. Sr values were investigated in 9 terrestrial/aquatic and dominant species: Eupatorium cannabium (EC), Jungus (JG), Pragmites (PG), Tamarix tetrandra (TM), Xanthium (XM), Salix (SX), Bolboscholnus ascbersus (BL), Lythnium salicaria (LY) and Typha latifolia (TP). Before, the soil samples are dried in an oven at 100 °C. After that, it was digested in a mixture of HCl; H2O; HNO3 at 95 °C for 1 h. This digest was analyzed for Sr in ICP-MS. The plant shoot and root were washed in tap water and dried at 60 °C and then ashed for 24 h at 300 °C. The ash samples were digested in HNO3 for 1 h, mixed with HNO3, HCl, H2O, and then analyzed for Sr in ICP-MS. Strontium analyses were performed in a certified laboratory according to ISO/IEC 17025 General Requirements for the Competence of Testing and Calibration Laboratories and accredited by International Standards Organization (ISO) 9001 Model for Quality Assurance (www.acmelab.com/services/quality-control/). Sr was analyzed as 88Sr, using 115In by using an ICP-MS PerkinElmer Elan 9000. Laboratory for Sr analysis used the certified reference material V16 for plants and CDV-1 for soils.
The enrichment coefficients for root (ECR)
The ECR was found by dividing to the soil value of the plant root value for each sample. This value is accepted as a value showing metal ratio transported from soil to plant root. The ECR values of the hyperaccumulator plants are greater than 1, but the ECR values lower than 1 are accepted as metal excluder plants (Wei et al. 2002; Chen et al. 2005).
The enrichment coefficients for shoot (ECS)
The ECS was calculated by dividing to soil values of plant shoot values for each sample in the study area. This value indicates the accumulation performance, which the metals transported from the soil to the shoot for each sample. That’s why, the ECS shows the storing and absorbing capability of a plant shoot. The ECS values for the hyperaccumulator plants are more than 1, whereas the ECS value for non-accumulator plants is lower than 1 (Zhao et al. 2003; Wei et al. 2002). If the ECS value is close to 1 (as 0.90 or 0.80 values), then, this plant defines as candidate plants in the phytoremediation studies (Sasmaz, Akgul, et al. 2016,c).
The translocation factor (TLF)
The TLF is defined as the metal ratio transported from the plant root to plant shoot. It has greater ratios than 1 in the hyperaccumulator plant. This value shows transfer capacity and ability of strontium to plant shoot from plant root, not accumulation (Zu et al. 2005).
Statistical analysis
The ANOVA variance analyses by using SAS (SAS Institute, Cary, NC) with a critical p value of 0.05 were tested for all data. The Sr concentrations of the studied soils were correlated by using the Spearman rank correlation with other metals (Sasmaz et al. 2019). Both the statistical studies of both plant parts and soils are also given in the text.
Quality assurance
Quality assurance through the process of external auditing by recognized organizations; all facilities maintain ISO registrations and accreditations. These accreditations and registrations meet the requirements of the ISO standards and provide independent verification that the management systems have been implemented. All BVM facilities are registered to ISO 9001, and they are pending to the Bureau Veritas corporate registration. Additionally, a number of analytical hubs have received ISO/IEC 17025 accreditation for specific laboratory procedures.
Results and discussion
Sr in water
Physicochemical characteristics of the Keban Pb–Zn, Maden Cu deposit and Elazig municipal waste treatment facility waters, and their anion–cation analysis results are given in Table 3. The average Sr concentrations in waters of the Keban Pb–Zn, Maden Cu mining and Elazig municipal facility were detected to be 2159 ± 62 μg L−1, 163 ± 12 μg L−1 and 431 ± 18 μg L−1, respectively, as shown in Table 3. The measured water temperature in the study area ranged from 20.5 to 20.8 °C. The average pH and EC values of the studied waters are 7.57 and 2.38 μS cm−1 for Keban Pb–Zn, 8.36 and 1.51 μS cm−1 for Maden Cu deposit and 7.6 and 1.05 μS cm−1 for Elazig municipal wastewater treatment plant. While the Keban Pb–Zn waters have high Ca, Na, Sr, SO4 and HCO3− contents, Maden Cu deposit has high Mg, Cu, B and low HCO3− concentrations. Elazig municipal wastewater has high K, Na, P and Cl− values. As shown in Table 3, As values of the waters in the Keban, Maden and municipal areas were detected to be 93 μg L−1, 170 μg L−1 and 4,6 μg L−1, respectively. The values are higher than the limit values established for drinking water by the WHO (2006), except for municipal wastewater. The TN and orthophosphate values ranged from 6.32 to 14.46 mg/L in the Keban Pb–Zn, 0.69 to – mg/L in the Maden Cu deposit and from 1.25 to 9.78 mg/L in the Elazig municipal wastewater, respectively. This also causes heavy metal pollution in the soils and water along both the Firat and Dicle Rivers. These results showed that the studied waters contaminated by both mining activities and anthropogenic sources. The Sr concentrations of river water in the terrestrial environments change between 6 and 800 ppb (Burger and Lichtscheidl 2019. Capo et al. (1998) reported to be that the main source of dissolved strontium is ocean and the ocean contains 8 ppm Sr concentration on average. Considering that the ocean mixing period is a thousand years, Sr can reside along the several million years by mixing the ocean water in the world ocean, and after that, it transports to the atmosphere by evaporating from sea and ocean; then, it returns to the terrestrial environment as a precipitation.
Sr in soil
The soil samples were taken from around the plant roots at a depth between 30 and 40 cm within river and stream. The colors of the soils change generally from dark brown and black to light, with loamy and peaty clay structure (28.4% silt, 18.5% clay and 45.3% sand), with an organic matter content of 7.3–10.2% and with a pH of 7.2–8.4. The type of clay minerals was not worked on the X-ray diffraction. Particularly, Elazig municipal wastewater soil had higher organic matter content in compared to Keban and Maden mining soils. The average Sr value of soils in the study area was 100.2 ppm (Table 4). The Sr concentrations were found 180 ppm in the soils of Keban mining area, 87.1 ppm in soils of municipal wastewater facility and 43.5 ppm in the soils of Maden copper deposit. The highest Sr contents in the study area were observed around Keban mining area (Table 4; Fig. 2), especially in sample PG-01, PG-02 and TM-01. These results in the study area showed that high Sr content in Keban region can be related to Pb–Zn mineralization or alkaline rocks in this region. Sr has a very high positive correlation values with Mn (0.91), As (0.75), U (0.70), Cs (0.48), Rb (0.37), K (0.46) and Cd (0.36), whereas strong negative correlation was not observed between Sr and other elements (Table 5).
The Sr concentrations in the surface soils of the mining areas were observed to be between112 and 717 ppm for Keban (Elazig) Pb–Zn mining area (Sasmaz and Sasmaz 2009) and 22.6 and 691.8 ppm for Gümuskoy (Kutahya) mining areas (Sasmaz and Sasmaz 2017). The Sr content in the mineral soils of the carbonatite and pyroxenite bedrock on Stjernoy Alkaline Complex, Norway, was found high variations varying from 320 to 1300 ppm (Myrvang et al. 2016). Kabata-Pendias (2011) indicated that the main indicator showing Sr abundance in soils was related to the wall rock composition and Sr content increases linearly from basic rocks (diorite) to alkaline rocks (syenite). Sr contents of soils in different countries vary widely between 14.7 and 675 ppm, for example, Sweden (112–258 ppm), Japan (32–130 ppm), Great Britain (261 ppm), China (26–150 ppm), Russian (715–1000 ppm), Venezuela (13–39 ppm), Canada (210 ppm) and USA (305 ppm) (Kabata-Pendias 2011).
Sr in plants
The average Sr concentrations of the studied plant roots and shoots are 48.2 and 80.5 ppm, respectively. The minimum and maximum levels of Sr in these plants are 25.4 and 274 ppm, in the shoots and 9.6 and 104 ppm, respectively, in the roots (on the dried weight basis).
The average Eupatorium cannabium (EC)’s soil, root and shoot concentrations for Sr are, respectively, 132.9, 84.9 and 67.8 ppm (Table 4; Fig. 2). The Sr concentrations in EC’s soils are higher than the root and shoot concentrations, with the minimum and maximum Sr concentrations of EC changing from 62.9 to 72.7 ppm for the shoots and from 69.1 to 100.7 ppm for the roots. These values showed that they are greater Sr concentrations than in Pais and Jones (2000)’s the reference plant (50 ppm). The ECR and ECS values of Sr for EC’s roots and shoots are given, as 0.63 and 0.51, respectively, and are lower than in their soil. The TLF values of EC for Sr vary from 0.72 to 0.91 (mean 0.82). The values showed that EC is not good plant to bioaccumulate Sr because of lower ECSs and ECRs than 1.
The average Sr levels of Jungus (JG)’ soil, roots and shoots are, 122.4, 65.5 and 58.1 ppm, respectively (Table 4; Fig. 2). The average ECR, ECS and TLFs for Sr are, 0.55, 0.50 and 0.99, respectively. These concentrations indicate that Jungus is not excellent plant for the bioaccumulation Sr due to the ECR and ECS < 1.
The average Sr concentrations in the soil, root and shoot levels of Pragmites (PG) are, respectively, 140.7, 26.3, and 119.2 ppm (Table 4; Fig. 2). The highest Sr levels in both soils and shoots were observed in Keban’s Pragmites samples (Samples PGK-1, PGK-2 and PGK-3) (p < 0.05). The mean ECR, ECS and TLFs of Pragmites samples are 0.30, 0.97 and 4.54, respectively. As seen from Pragmites’ TLF values for Sr, these values are very high and also indicate that this is rapidly transported to the shoot parts without waiting for the root. This shows that Pragmites’ shoot can be advantageous in Sr bioaccumulation studies because of the average ECS values close to 1.
The Sr levels in Tamarix tetrandra (TM)’s soil, roots and shoots are, respectively, 120.1, 40.8 and 54.2 ppm. The average Sr values of TM’s shoot and root are lower than in TM’s soil. The average ECR and ECS values in TM for Sr are 0.37 and 0.71, respectively (Table 4; Fig. 2) and lower than 1. These values show that TM is not very good plant for the bioaccumulation of Sr because of lower ECS and ECRs than 1.
The Sr concentrations in Xanthium (XM)’s soil, root and shoots of Xanthium (XM) are 77.9, 54.5 and 88.0 ppm, respectively. The samples (Samples XMB-2 and XMB-3) taken in Elazig municipal area are high Sr contents in their shoots, except for XMM-01’s shoot sample collected from Maden region. The ECR, ECS and TLFs of XM are, respectively, 0.70, 1.12 and 1.79 (Table 4; Fig. 2). These values show that EM’s shoot, not root, can be useful for Sr bioaccumulation studies.
The Sr concentrations in Salix (SX)’s soil, root and shoots are 84.1, 68.8 and 61.8 ppm, respectively. The average Salix’s ECR, ECS and TLFs for Sr are, respectively, 0.84, 0.76 and 0.91 (Table 4; Fig. 2). These parameters indicate that Salix is not an ideal plant for the Sr bioaccumulation, but it can be the candidate plant to accumulate Sr because of ECS and ECRs close to 1 (Table 4; Fig. 2).
The average Sr levels of the Bolboscholnus ascbersus (BL)’s soil, root and shoots are 26.5, 27.3, and 44.7 ppm, respectively (Table 4; Fig. 2). The Sr levels of the B. ascbersus’s shoot and root have higher levels than those in soils. The average ECR, ECS and TLF values B. ascbersus are, respectively, 1.07, 1.70 and 1.97 (Table 4; Fig. 2). Therefore, these values indicate that B. ascbersus’s roots and shoots due to higher ECR and ECSs than 1 could be an excellent plant for cleaning or rehabilitating of areas contaminated by Sr (Table 4; Fig. 2).
The average Lythnium salicaria’s (LY) soil, root and shoot levels are 42.9, 54.6 and 80.9 ppm, respectively (Table 4; Fig. 2). The Sr concentrations of L. salicaria’s root and shoots are higher than in L. salicaria’s soils. The mean ECR, ECS and TLF values for Sr are 1.24, 1.99 and 1.88, respectively (Table 4; Fig. 2). These values point out that L. salicaria’s root and shoots for Sr could be very good hyperaccumulator plant because of more ECS and ECRs than 1.
The Sr concentrations in Typha latifolia’s (TP) soils, roots and shoots are 58.2, 48.8 and 85.4 ppm, respectively. The average ECR, ECS and TLFs of T. latifolia’s for Sr are 0.90, 1.68 and 1.79, respectively (Table 4; Fig. 2). These parameters are higher than 1 (except for TPM-2 sample), which indicates that the T. latifolia’s shoot and root could use as an useful plant in the rehabilitating studies of areas contaminated by Sr.
Gjengedal et al. (2015) reported that the changeability in the element transfer to plants from soil is associated with their needs to certain nutrients, the biogeochemical differences among plant species and binding strengths of elements to soil. Other variables related to plant nutrients contain, for example, cation exchange capacity, soil organic matter, pH, rhizosphere and microbial activities (Myrvang et al. 2016). Strontium is mainly taken up by plant roots from soil and water in aquatic environments under the natural conditions, but it is in addition absorbed by leaves in varying degrees after Sr fallout from air (Burger and Lichtscheidl 2019). Alternative crops plants were grown to accumulate Sr from agricultural areas polluted by the radionuclides in the northern and middle of Sweden. Among these plants, Von Fircks et al. (2002) reported mainly that Sr accumulations in Salix species are higher in shoots in compared to other plant parts. Myrvang et al. (2016) detected that it was accumulated 340–180 ppm Sr in the shoot and stems of Salix sp and 640–190 ppm Sr in the shoot and stems of Salix reticulate grown on the carbonatite soils of alkaline complex, Northern Norway.
Shahraki et al. (2008) investigated Sr contents of the terrestrial plants grown on the soils Sarcheshmeh copper mine, and they found that Sr concentrations of the root and shoot samples contained 47.4 and 132 ppm for the Phragmites australis and 98.8 and 188 ppm for the Tamarix ramossima (Shahraki et al. 2008).
Sasmaz and Sasmaz (2017) studied the accumulation and translocations of Sr into 11 native plants in the Gümüşköy mining soils. The plants were divided to different groups such as the candidate, good and the best plants according to ECRs and ECSs. Among these plants, Centaurea cyanus Anchusa arvensis. Alyssum saxatile, Onosma sp. and Carduus nutans plants were accepted as the best plants for Sr accumulations. The Glaucium flavum, Verbascum thapsus, Cynoglossum officinale. Isatis and Phlomis sp. were accepted as good plants to accumulate Sr from the soils contaminated by Sr.
Petrescu and Bilal (2006) detected that the highest Sr values in conifers are accumulated in the Picea excelsa and Abies alba grown on the mining areas. Aerial parts of the Picea excelsa and Abies alba were observed to accumulate more Sr in compared to root parts of the plants.
Qi et al. (2015) worked the accumulation and distribution of Sr in husk oat, wheat, barley and naked oat on their phytoremediation potentials. They reported that average Sr values in the shoots of oat, naked oat and barley cultivar have high Sr concentrations. The leaves had the highest Sr values, and the lowest values were observed in grains. Mean ECS values changed from 0.521 to 1.34; the Sr removal ratio from the soil to the shoots detected to be higher than 1.4% in the following days to 120 days. These results showed that these plants can use effectively to rehabilitee the soils polluted by Sr.
Zu et al. (2005) described the hyperaccumulator plant as a plant that accumulates several times more metal than in uncontaminated soils. Among these plants, the Euphorbia macroclada grown on the Keban mining soils was observed to accumulate a few times higher Sr in compared to the soil samples (Sasmaz and Sasmaz 2009).
The Energy Department of USA tested the possible productivity of Salix exigua for phytoextraction of the soil Sr into above-ground shoots and through the plant roots to protect the environment polluted with Sr. This study indicated that the Sr content of the soil decreases after the plants take Sr from the soil. In addition, the study showed that the transfer of Sr together with the food chain of herbivorous insects from Salix grown in contaminated soil by Sr is nonexistent or minor (Fellows et al. 2009). Wang et al. (2017) investigated Sr accumulations in soil contaminated by Sorghum bicolor. The study investigated the physiological profiles in the soil microbial level and it was seen that Sr-spiked soil has increased both the stem biomass of the plant and soil microbial diversity.
Among the aquatic plants, Van Hoeck et al. (2015) examined that the Sr accumulation by using Lemna minor fronds and root during a 7-day time. In conclusion, these results indicated for the first time that β-radiation procures a negative growth in L. minor.
Sasmaz et al. (2015) investigated the removal efficiencies of different metals (Cu-Pb–Zn-As, U-Th, La-Ce-Y, Cr-Ni-Co) in gallery water in Keban mining area by using Lemna gibba and Lemna minor. According to the accumulations, the obtained efficiencies in L. minor and L. gibba are: 1259% at day 2 and 1015% at day 2 for Pb; 87% at day 2 and 36% at day 3 for Cu; 7070% at day 3 and 19,709% at day 2 for As and 628% at day 3 and 382% at day 3 for Zn, respectively. The present study unveiled that both L. gibba and L. minor had very high capacity to accumulate Cu, Pb, Zn and As in gallery water polluted by different elements (Sasmaz et al. 2016a, b, c, d, 2018, 2019).
Among numerous remediation tools (Gupta and Voronina 2018), the phytoremediation is an inexpensive, environmentally and friendly method to remove toxic materials from the environment. Many scientific reports recommend that phytoremediation is an multipurpose, economic, effective and green method to clean the polluted areas. These techniques also have some disadvantages as the need to the rather high time for the cleaning of toxic materials in compared to removal method or chemical treatments. Particularly in the nuclear usages, phytoremediation can only use applicable with low activities. It produces a large amount of contaminated phytoma, which is still a major environmental problem. In this way, it also increases the likelihood of toxin entering the food chain. Nevertheless, it is a powerful option for improvement due to its environmental friendliness and low costs. Further detailed studies need to understand the Sr uptake mechanism, fate inside plants, to be done at genetic level and feasibility of the technique for Sr accumulation in any nuclear power plant leak or decommissioning (Gupta et al. 2018a, b).
Conclusions
The aquatic and terrestrial plants collected from municipal and mining areas around Elazig were separated into several groups such as the candidate, bioaccumulator and hyperaccumulator plants with regard to Sr phytoremediation by considering the enrichment coefficients (ECR, ECS and TLFs) (Fig. 3). The candidate plants have lower ECR and ECSs than 1: Salix sp. and Tamarix tetrandra; bioaccumulator plants with ECR and ECS are close to 1: Pragmites sp. and Xanthium; and hyperaccumulator plants are higher ECR and ECS than 1: Typha latifolia, Bolboscholnus ascbersus and Lythnium salicaria for Sr. Therefore, the Typha latifolia, Bolboscholnus ascbersus and Lythnium salicaria plants would be beneficial and advantageous for the cleaning and/or rehabilitating of water and soils contaminated by Sr (Fig. 3). Plantings of the best bioaccumulation plants can be used in the bioaccumulation studies to remove environmental contaminants and the rehabilitation/cleaning of the contaminated areas by Sr. Among the studied plants, especially Pragmites sp. and Xanthium’s shoots can use in biomonitoring practices because the ECR and ECS have close values to 1. The TLF values for the studied plants indicate the transport ability from root to shoot of Sr. According to their TLFs, Pragmites, Bolboscholnus ascbersus, Lythnium salicaria, Typha latifolia, Tamarix tetrandra and Xanthium plants have been shown to be very effective in carrying Sr from root to shoot.
References
Akgul, B. (2015). Geochemical associations between fluorite mineralization and A-type shoshonitic magmatism in the Keban-Elazig Area, East Anatolia, Turkey. Journal of African Earth Sciences, 111, 222–230.
APHA. (1999). Standard methods for the examination of water and wastewater. Washington: American Public Health Association.
ATSDR. (2004). Toxicological profile for Strontium. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
Benabdallah, N., Harrache, D., Mir, A., De La Guardia, M., & Benhachem, F. (2017). Bioaccumulation of trace metals by red alga Corallina elongata in the coast of Beni Saf, west coast, Algeria. Chemistry International, 3, 220–231.
Burger, A., & Lichtscheidl, I. (2019). Strontium in the environment: Review about reactions of plants towards stable and radioactive strontium isotopes. Science of the Total Environment, 653, 1458–1512.
Capo, R., Stewart, B., & Chadwick, O. (1998). Strontium isotopes as tracers of ecosystem processes: Theory and methods. Geoderma, 82, 197–225.
Censi, P., Saiano, F., Pisciotta, A., & Tuzzolino, N. (2014). Geochemical behaviour of rare earth in Vitis vinifera grafted onto different rootstocks and growing on several soils. Science of the Total Environmental, 473–474, 597–608.
Chen, S. B., Zhu, Y. G., & Hu, Q. H. (2005). Soil to plant transfer of 238U, 226Ra and 232Th on U mining-impacted soil from southeastern China. Journal of Environmental Radioactivity, 82, 223–236.
Dvoršćak, M., Stipičević, S., Mendaš, G., Drevenkar, V., Medunić, G., Stančić, Z., et al. (2019). Soil burden by persistent organochlorine compounds in the vicinity of a coal-fired power plant in Croatia: A comparison study with an urban-industrialized area. Environmental Science and Pollution Research, 26, 23707–23716.
Ebrahimi, P., & Barbieri, M. (2019). Gadolinium as an emerging micro contaminant in water resources: Threats and opportunities. Geosciences, 9(2), 93.
Fait, S., Fakhi, S., ElMzibri, M., Faiz, Z., Fougrach, H., Badri, W., et al. (2017). Distribution of metallic trace elements (ETM) in surface soils around the Mediouna discharge (southern of Casablanca). Chemistry International, 3, 378–385.
Fellows, R. J., Fruchter, J. S., & Driver, C. J. (2009). 100-N Area Strontium-90 treatability Demonstration project: food chain transfer for phytoremediation along the 100-N Columbia River Riparian Zone. Washinghton: US Department of Energy, Pacific NW National Lab.
Fiket, Ž., Medunić, G., & Kniewald, G. (2016). Rare earth element distribution in soil nearby thermal power plant. Environmental Earth Sciences, 75(7), 5981–5989.
Fiket, Ž., Vidaković-, Medunić G., Cifrek, Ž., Jezidžić, P., & Cvjetko, P. (2019). Effect of coal mining activities and related industry on composition, cytotoxicity and genotoxicity of surrounding soils. Environmental Science and Pollution Research, 27, 6613–6627.
Gjengedal, E., Martinsen, T., & Steinnes, E. (2015). Background levels of some major, trace, and rare earth elements in indigenous plant species growing in Norway and the influence of soil acidification, soil parent material, and seasonal variation on these levels. Environmental Monitoring and Assessment, 187, 386–414.
Griffits, W. R., Albers, J. P., & Oner, O. (1972). Massive sulfide copper deposits of the Ergani-Maden Area Southeastern Turkey. Economic Geology, 67, 701–716.
Gupta, D., Deb, U., Walther, C., & Chatterjee, S. (2018a). Strontium in the ecosystem: Transfer in plants via root system. In D. Gupta & C. Walther (Eds.), Behaviour of strontium in plants and the environment (pp. 1–18). Cham: Springer.
Gupta, D., Schulz, W., Steinhauser, G., & Walther, C. (2018b). Radiostrontium transport in plants and phytoremediation. Environmental Science and Pollution Research, 25, 29996–30008.
Gupta, D. K., & Voronina, A. (2018). Remediation measures for radioactively contaminated areas. New York: Springer.
Iqbal, M. (2016). Vicia faba bioassay for environmental toxicity monitoring: A review. Chemosphere, 144, 785–802.
Iqbal, M., Abbas, M., Nazir, A., & Qamar, A. Z. (2019). Bioassays based on higher plants as excellent dosimeters for ecotoxicity monitoring: A review. Chemistry International, 5, 1–80.
Iqbal, M., Iqbal, N., Bhatti, I. A., Ahmad, N., & Zahid, M. (2016). Response surface methodology application in optimization of cadmium adsorption by shoe waste: A good option of waste mitigation by waste. Ecological Engineering, 88, 265–275.
Iqbal, M., Nisar, J., Adil, M., Abbas, M., Riaz, M., Tahir, M. A., et al. (2017). Mutagenicity and cytotoxicity evaluation of photo-catalytically treated petroleum refinery wastewater using an array of bioassays. Chemosphere, 168, 590–598.
Ivanić, M., Fiket, Ž., Medunić, G., Furdek Turk, M., Marović, G., Senčar, J., et al. (2019). Multi-element composition of soil, mosses and mushrooms and assessment of natural and artificial radioactivity of a pristine temperate rainforest system (Slavonia, Croatia). Chemosphere, 215, 668–677.
Kabata-Pendias, A. (2011). Trace elements in soils and plants. Boca Raton: CRC Press.
Khan, K., Khan, H., Lu, Y., Ihsanullah, I., Nawab, J., Khan, S., et al. (2014). Evaluation of toxicological risk of foodstuffs contaminated with heavy metals in Swat, Pakistan. Ecotoxicology and Environmental Safety, 108, 224–232.
Liu, X. H., Gao, Y. T., Sardar, K., Duan, G., Chen, A. K., Ling, L., et al. (2008). Accumulation of Pb, Cu, and Zn in terrestrial plants growing on contaminated sites and their potential accumulation capacity in Heqing, Yunnan. Journal of Environmental Sciences, 20, 1469–1474.
Majolagbe, A. O., Adeyi, A. A., & Osibanjo, O. (2016). Vulnerability assessment of groundwater pollution in the vicinity of an active dumpsite (Olusosun), Lagos, Nigeria. Chemistry International, 2, 232–241.
Mani, D., Kumar, C., & Patel, N. K. (2016). Integrated micro-biochemical approach for phytoremediation of cadmium and lead contaminated soils using Gladiolus grandiflorus L cut flower. Ecotoxicology and Environmental Safety, 124, 435–446.
Medunić, G., Grigore, M., Dai, S., Berti, D., Hochella, M. F., Mastalerz, M., et al. (2020). Characterization of superhigh-organic-sulfur Raša coal, Istria, Croatia, and its environmental implication. International Journal of Coal Geology, 217, 103344.
Myrvang, M., Hillersøy, M., Heim, M., Bleken, M., & Gjengedal, E. (2016). Uptake of macro nutrients, barium, and strontium by vegetation from mineral soils on carbonatite and pyroxenite bedrock at the Lillebukt Alkaline Complex on Stjernøy, Northern Norway. Journal of Plant Nutrition and Soil Science, 179, 705–716.
Nematollahi, M. J., Ebrahimi, P., & Ebrahimi, M. (2014). Comparison of environmental geochemistry of heavy metals in coastal and marine sediments of Chabahar and Gowatr Bays, SE Iran. Geochemistry Journal, 1(3), 1–6.
Nematollahi, M. J., Ebrahimi, P., & Ebrahimi, M. (2016a). Evaluating hydrogeochemical processes regulating groundwater quality in an unconfined aquifer. Environmental Processes, 3(4), 1021–1043.
Nematollahi, M. J., Ebrahimi, P., Razmara, M., & Ghasemi, A. (2016b). Hydrogeochemical investigations and groundwater quality assessment of Torbat-Zaveh plain, Khorasan Razavi, Iran. Environmental Monitoring and Assessment, 188(1), 1–21.
Obek, E., Yakupogullari, Y., Tepe, O., & Toraman, Z. (2007). Elazig Belediyesi atiksu aritma tesisi giris ve cikis sularinin helmintolojik riskinin arastirilmasi. Uludah Universitesi Dergisi, 12(1), 77–83.
Padmasubashini, V., Sunilkumar, B., Krishnakumar, M., & Singh, S. B. (2020). Method validation and uncertainty for the determination of rare earth elements, yttrium, thorium and phosphorus in monazite samples by ICP-OES. Chemistry International, 6, 98–109.
Pais, I., & Jones, J. B. (2000). The handbook of trace elements (pp. 222). Florida: St. Lucie Press.
Palutoglu, M., Akgul, B., Suyarko, V., Yakovenko, M., Kryuchenko, N., & Sasmaz, A. (2018). Phytoremediation of cadmium by native plants grown on mining soil. Bulletin of Environmental Contamination and Toxicology, 100, 293–297.
Palutoglu, M., & Sasmaz, A. (2017). 29 November 1795 Kahramanmaras Earthquake, Southern Turkey. Bulletin of the Mineral Research and Exploration, 155, 81–90.
Pervaiz, M., Butt, K. M., Raza, M. A., Rasheed, A., Ahmad, S., Adnan, A., et al. (2015). Extraction and applications of aluminum hydroxide from bauxite for commercial consumption. Chemistry International, 1, 99–102.
Petrescu, L., & Bilal, E. (2006). Natural actinides studies in conifers grown on uranium mining dumps (the East Carpathians, Romania). Carpathian Journal of Earth and Environmental Sciences, 1, 63–80.
Qi, L., Qin, X. L., Li, F. M., Siddique, K., Brandl, H., Xu, J. Z., et al. (2015). Uptake and distribution of stable strontium in 26 cultivars of three crop species: Oats, wheat, and barley for their potential use in phytoremediation. International Journal of Phytoremediation, 17, 264–271.
Rađenović, A., & Medunić, G. (2015). Removal of Cr(VI) from aqueous solution by a commercial carbon black. Desalination and Water Treatment, 55(1), 183–192.
Rađenović, A., Medunić, G., & Sofilić, T. (2016). The use of ladle furnace slag for the removal of hexavalent chromium from an aqueous solution. Metallurgical Research and Technology, 113, 6.
Radić, S., Medunić, G., Kuharić, Ž., Roje, V., Maldini, K., Vujčić, V., et al. (2018). The effect of hazardous pollutants from coal combustion activity: Phytotoxicity assessment of aqueous soil extracts. Chemosphere, 199(1), 191–200.
Sasmaz, M., Akgul, B., Yildirim, D., & Sasmaz, A. (2016a). Bioaccumulation of thallium by the wild plants grown in soils of mining area. International Journal of Phytoremediation, 18(11), 1164–1170.
Sasmaz, M., Akgül, B., Yıldırım, D., & Sasmaz, A. (2016b). Mercury uptake and phytotoxicity in terrestrial plants grown naturally in the Gumuskoy (Kutahya) mining area, Turkey. International Journal of Phytoremediation, 18(1), 69–76.
Sasmaz, A., Dogan, I. M., & Sasmaz, M. (2016c). Removal of Cr, Ni and Co in the water of chromium mining areas by using Lemna gibba L. and Lemna minor L. Water and Environmental Journal, 30(3–4), 235–242.
Sasmaz, A., Obek, E., & Hasar, H. (2008). The accumulation of heavy metals in Typha latifolia L. grown in stream carrying secondary effluent. Ecological Engineering, 33, 278–284.
Sasmaz, M., Obek, E., & Sasmaz, A. (2018). The accumulation of La, Ce and Y by Lemnaminor and Lemna gibba in the Keban gallery water, Elazig Turkey. Water and Environment Journal, 32(1), 75–83.
Sasmaz, M., Obek, E., & Sasmaz, A. (2019). Bioaccumulation of cadmium and thallium in Pb-Zn tailing waste water by Lemna minorand Lemna gibba. Applied Geochemistry, 100, 287–292.
Sasmaz, M., Öbek, E., & Sasmaz, A. (2016d). Bioaccumulation of uranium and thorium by Lemna minor and Lemna gibba in Pb-Zn-Ag tailing water. Bulletin of Environmental Contamination and Toxicology, 97(6), 832–837.
Sasmaz, A., & Sasmaz, M. (2009). The phytoremediation potential for strontium of indigenous plants growing in a mining area. Environmental and Experimental Botany, 67(1), 139–144.
Sasmaz, M., & Sasmaz, A. (2017). The accumulation of strontium by native plants grown on Gumuskoy mining soils. Journal of Geochemical Exploration, 181, 236–242.
Sasmaz, M., Topal, E. I. A., Obek, E., & Sasmaz, A. (2015). The potential of Lemna gibba L. and Lemna minor L. to remove Cu, Pb, Zn, and As in gallery water in a mining area in Keban, Turkey. Journal of Environmental Management, 163, 246–253.
Seeliger, T. C., Pernicka, E., Wagner, G. A., Begemann, F., Schmitt-Strecker, S., Eibner, C., et al. (1985). Arch€aometallurgische Untersuchungen in Nord- und Ostanatolien. Jahrbuch Romisch-German Zentralmuseum, 32, 597–659.
Shacklette, H. T., Erdman, J. A., & Harms, T. F. (1978). Trace elements in plant foodstuffs in toxicity of heavy metals in the environments: Part I. New York (p. 25).
Shahraki, S. A., Ahmadimoghadam, A., Naseri, F., & Esmailzade, E. (2008). Study the accumulation of strontium in plant growing around Sarcheshmeh Copper Mine, Iran (pp. 239–242). Ostrava: VSB Technical University of Ostrava.
Siddique, A., Hassan, A., Khan, S. R., Inayat, A., Nazir, A., & Iqbal, M. (2018). Appraisal of heavy metals and nutrients from phosphate rocks, Khyber Pakhtunkhwa, Pakistan. Chemistry International, 4, 1–6.
Thakur, R., Tarafder, P. K., & Jha, R. R. (2015). Micelle-mediated extraction of cobalt and its spectrophotometric determination in rocks, soils, sediments and sea-bed polymetallic nodules. Chemistry International, 5, 109–116.
Topal, M. (2016). Elazig Belediyesi Atiksu Tesisi Cikis sularının verildiği Kehli Deresinin sulama suyu olarak kullanılabilirliğinin değerlendirilmesi. Igdir Universitesi Fen Bil. Dergisi, 6(2), 139–149.
Topal, M., & Arslan Topal, E. I. (2011). Evaluation of the Elazığ municipal wastewater treatment plant with some parameters in 2010–2011 winter season. Cumhuriyet Science Journal, 32, 1–12.
US EPA. (2000). Introduction to phytoremediation. National Risk Management Research Laboratory, Office of Research and Development, EPA/600/R-99/107.
Van Hoeck, A., Horemans, N., Van Hees, M., Nauts, R., Knapen, D., Vandenhove, H., et al. (2015). β-Radiation stress responses on growth and antioxidative defense system in plants: A study with strontium-90 in Lemna minor. International Journal of Molecular Sciences, 16, 15309–15327.
Von Fircks, Y., Rosen, K., & Sennerby-Forsse, L. (2002). Uptake and distribution of 137Cs and 90Sr in Salix viminalis plants. Journal of Environmental Radioactivity, 63, 1–14.
Wang, H., Chen, C., & Wang, J. (2017). Phytoremediation of strontium contaminated soil by Sorghum bicolor (L) Moench and soil microbial community-level physiological profiles (CLPPs). Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-017-8432-8.
Wei, C. Y., Chen, T. B., & Huang, Z. C. (2002). Cretan bake (Pteris cretica L.): An arsenic-accumulating plant. Acta Ecologica Sinica, 22, 777–782.
WHO. (2006). Guidelines for drinking-water quality. Geneva: World Health Organization.
Wong, M. H. (2003). Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere, 50, 775–780.
Yildirim, D., & Sasmaz, A. (2017). Phytoremediation of As, Ag, and Pb in contaminated soils using terrestrial plants grown on Gumuskoy mining area (Kütahya Turkey). Journal of Geochemical Exploration, 182, 228–234.
Yoon, J., Cao, X., Zhou, Q., & Ma, L. Q. (2006). Accumulation of Pb, Cu, and Zn in terrestrial plants growing on a contaminated Florida site. Science of the Total Environment, 368, 456–464.
Zhao, F. J., Lombi, E., & Mc Grath, S. P. (2003). Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant and Soil, 249, 37–43.
Zu, Y. Q., Li, Y., Chen, J. J., Chen, H. Y., Qin, L., & Schvartz, C. (2005). Hyperaccumulation of Pb, Zn and Cd in herbaceous grown on lead-zinc mining area in Yunnan, China. Environment International, 31, 755–762.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sasmaz, M., Uslu Senel, G. & Obek, E. Strontium accumulation by the terrestrial and aquatic plants affected by mining and municipal wastewaters (Elazig, Turkey). Environ Geochem Health 43, 2257–2270 (2021). https://doi.org/10.1007/s10653-020-00629-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00629-9