Abstract
Contaminations of soil and water resources with various organic and inorganic compounds are of great importance on account of the close relationship between the living organisms and their feeding. That is due to direct impact in supplying food for living organisms in terms of environmental and human health aspects. In this regard, the present study aimed to investigate the phytoremediation potential of H. strobilaceum and S. herbacea in contaminated soils. For this purpose, soil and plant samples were collected from around the sewage channel in Eshtehard industrial region of Iran. Sampling started at the edge of the channel and ended in a distance of 500 m from the channel. The distance of 1000 m from channel was considered as the control point. ICP-OES was used for the measurement of heavy metals. The obtained results showed that the highest and lowest amounts of soil lead (Pb) were 17.6 and 2.33 mg kg−1, respectively. For Cadmium (Cd), the values ranged from 0.341 to 0.11 mg kg −1 at 21–50 cm depth for the control point. For the plants, the highest and lowest amount of Pb belonged to H. strobilaceum shoot (10.38 mg kg −1) and S. herbacea root (7.54 mg kg −1), respectively. The maximum (1.64 mg kg−1) and minimum (0.36 mg kg−1) Cd concentration was observed in the root and shoot of H. strobilaceum, respectively. In both species, Translocation Factor (TF) for Pb and Cd was greater than 1 and less than 1, respectively. Cd Bio Concentration Factor (BCF) in the roots of both species was estimated to be greater than 1 while for Pb, this index was smaller. Bio Accumulation Factor (BAF) in the shoots of Pb and Cd for both plants were lower and greater than 1, respectively. In general, the results revealed that the highest concentrations of Cd and Pb are absorbed and stored by the underground organs of H. strobilaceum and S. herbacea and these plants have the ability to remove Pb and Cd from contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are the main contamination sources for soil and plants of rangelands near the industrialized areas. Heavy metals are basically not biodegradable and therefore accumulate in the ecosystem [32]. The accumulation of these metals reduces soil quality, contaminates water, soil, and plant, and results in destruction of the entire ecosystem [10, 17]. Using municipal and industrial wastewater has become common in several parts of the world [31, 35], in which lead (Pb) and cadmium (Cd) concentrations are too high and has contaminated the soil. Cd is often found in soils, which necessitates the determination of the bioabsorption and accumulation to reduce its concentration [29].
A variety of methods have been applied to clean contaminated soils. The majority of the reported methods are bacterial [33, 39] and fungal [9] bioremediation; meanwhile, several studies have also described the plant bioremediation, alternatively called phytoremediation [26]. Phytoremediation is a multipurpose method using green plants to clean up contaminated soils, which is in fact a combination of plant physiology, soil chemistry, and soil microbiology ( [30]). The use of vegetation for contaminated soils is an attractive and cost-effective alternative [18]. Phytoremediation method and using plants as contamination indicator have become a common practice in the polluted areas [15]. This method has a high potential in soil remediation owing to its multiple advantages, such as maintaining the biological activity and physical structure of the soil, being potentially visually unobtrusive, and providing the possibility of biorecovery of metals [31]. This method does not require specialized equipment and specialist personnel. It is also easily applicable and capable of absorbing various pollutants in very large areas. Moreover, compared to the existing methods, it is much less expensive and safe [36].
Research has indicated that the cost of treating soil-contaminating heavy metals with an upper tolerance plant is less than other refining methods [7, 28, 31]. The best strategy of phytoremediation is the absorption and transfer of pollutants from soil to plant without damaging the soil structure or changing its fertility [31, 40]. Phytoremediation is a multi-purpose method for the treatment of contaminated soils, which is a combination of plant physiology, soil chemistry, and soil microbiology, as well as the use of green plants to remove contaminants from groundwater, surface water and contaminated soils. Certain researchers have suggested that plants with a TF value of more than one are suitable for phytoextraction and generally require the transfer of heavy metals to harvestable parts of plants, such as stems [41]. The ability of plants to accumulate metals is a basic parameter [11, 22]. Over the recent decades, it has become very critical to identify plants that effectively remove heavy metals from the soil [11, 38]. Chen et al. [11] reported that phytoextraction is mainly applied to soils with high levels of V contamination. Generally, for phytoextraction, on a large scale, the use of plant growth stimulants is necessary for successful remediation of metal-contaminated soil.
According to the increase in environmental pollution around the world, there is a vast need of various plants, particularly the native ones, to clean up the environment in the polluted areas. Utilizing native plants for phytoremediation is of considerable importance since these plants are often better in terms of survival, growth, and reproduction under environmental stress compared to exotic species as naturally selected [37].
The wastewater of Eshtehard industrial town in Alborz province of Iran passes through the rangelands around this town. Following field investigation, it was found that two species of H. strobilaceum and S. herbacea were the dominant plants around the wastewater channel and have distributed with different densities beside the channel and rangeland. It has been previously suggested that these species are resistant against contamination and are able to act as remediator. Therefore, the present research was conducted in order to investigate the phytoremediation potential of two native species, namely H. strobilaceum and S. herbacea, in the rangelands around the Eshtehard industrial town in Alborz province, Iran. Although there have been studies on the evaluation of phytoremediation potentials in some members of the Salicornioideae sub-family [15], no information has been reported on the phytoremediation potential of H. strobilaceum and S. herbacea prior to the current research.
Material and methods
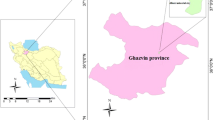
The study area is located in Eshtehard Industrial Estate, in Alborz province, Iran (Fig. 1), which is located in the geographical position of 08 72 35 North 50 37 22 East. The region’s climate, on the basis of Dumarten’s climate classification, is semi-arid with relatively hot summers and cold winters.
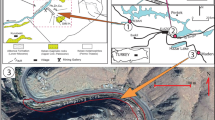
A channel starts from the Industrial Estate and transports industrial sewage through the rangeland. Regarding the kind of productions in the industrial area, Pb and Cd are released to the channel.
According to the presence of H. strobilaceum Pall. and S. herbacea L. species around the sewage channel and rangeland (Fig. 2), we decided to test the capability of these species for phytoremediation. To this end, plant and soil sampling was performed to find Pb and Cd amount in soil and plant. In addition, phytoremediation indices of Translocation Factor (TF) and Bio Concentration Factor (BCF) were evaluated for each plant species.
S. herbacea is an annual plant in the Chenopodiaceae family that appears as a herbaceous plant with succulent and fleshy stems and grows naturally on beaches and saline wetlands. H. strobilaceum is a perennial plant, woody shrub, or short-leaved shrub belonging to the Chenopodiaceae family.
Soil sampling started at the edge of the channel and ended in a distance of 500 m away from the channel. In order to find whether the soil around the channel is contaminated by Pb and Cd, the distance of 1000 m from the channel was considered as the control point. At each of the points of edge to the channel, 500 and 1000 m from the channel, five soil samples were taken from depths of 0–20 and 21–50 cm (a total number of 30 soil samples were collected from depths of 0–20 and 21–50 cm). After preparation of the soil samples in laboratory, concentration of available Pb and Cd was extracted by DTPA [21] and measured using ICP device.
To determine the amount of heavy metals in plants, 0.5 g of the plant samples (aerial or underground organs) was primarily powdered. Subsequently, 10 cc of concentrated sulfuric acid was added. In the next step, the obtained solution was put in the oven at 95 °C (before boiling) for 15 min. After cooling slightly, 5 cc of 30% oxygenated water was added and heated for 1 min to colorize the samples. Finally, after cooling, the samples were flushed with Whatman paper to a volume of 100 cc [13]. Afterwards, the samples were passed through cellulose acetate filter paper and the concentration of the desired metals was measured using the GBC Avanta model made in Australia. Primarily, 2 g of the dried and sifted soil was weighed, 15 cc of 4 N nitric acid was then added, and the mixture was placed at 60 °C for 20 h. It was then distilled twice in a 50-cc balloon with water and double distilled water [5]. In the next step, the samples were passed through 0.23 cellulose acetate paper to be prepared for reading with ICP.
For plant sampling, the average number of the species per hectare was initially estimated using 15 one m2 quadrates. Five quadrates were used around each of 0, 500, and 1000 m points. Afterwards, in points of 0 and 500 m from the channel, five individuals of each of H. strobilaceum and S. herbacea species were randomly harvested (totally 20 plant samples for both species). Aerial and underground parts of the plants were cut in order to analyze their Pb and Cd content separately. To measure Pb and Cd content in different parts of the plants, Acid Digestion method was applied. Firstly, 1 g of each plant sample was weighed and then the samples were placed in an oven at 500 °C. Before boiling the samples, the first steam of the samples was removed from the oven. Ultimately, after cooling, the samples were passed through filter paper and reached a volume of 50 cc. To evaluate the phytoremediation potential in H. strobilaceum and S. herbacea, TF and BCF were assessed. In order to evaluate the phytoextraction potential of the plants, the bioconcentration factor (BCF) and the translocation factor (TF) were calculated as follows [12]:
where Croots is the metal mass fraction in the root (mg kg−1), Csoil is the available metal level in the rhizosphere (mg kg−1), and Cshoots is the metal level in the above-ground parts of the plants (plant shoots) (mg kg−1). In addition, another good indicator is the Bioaccumulation Factor (BAF) which is the metal content in the plant shoots compared to metal concentration in the substrate [16].
Data analysis
Data analysis was performed on 20 plant and 30 soil samples. The Kolmogorov-Smirnov test was first used to check the normality of the data. Once significant differences were found with Anova, a Duncan’s Test was used to determine significant treatments among experimental groups. Statistical analyses were performed employing SPSS20 and Excel.
Results
Soil properties of the study area
Some soil properties of the study area (between the channel and control point) are represented in Table 1. With the increase in the distance from the channel, pH increased whereas EC and organic matter decreased.
Metal evaluation in the soil
The results of Pb and Cd concentration near the channel (which is shown with 0 m), in 500 m and 1000 m (control point) from the channel, are depicted in Figs. 3 and 4. According to the figures, there were significant differences among the control point and 0 and 500 m distances from the channel concerning soil Pb and Cd concentration at both depths of 0–20 cm (d1) and 21–50 cm (d2). According to these figures, in all the cases, except for soil Cd of d2, the highest amounts of Pb and Cd belonged to the nearest distance to the channel while the least amount was obtained in the control point. At depths of 0–20 and 21–50 cm, Pb concentrations near the channel were about seven times bigger than those in the control point (15.8 against 2.33 and 17.6 against 2.47, respectively). In other words, Pb concentration decreased significantly with the increase in the distance from the channel. This was also true for Cd.
Metal evaluation in the plants
The average numbers of individuals per hectare for H. strobilaceum and S. herbacea were obtained 8000 and 9000, respectively.
Figures 5 and 6 demonstrate the amounts of Pb and Cd in the plants tissues, based on their distance from the channel. Based on Fig. 5, Pb amount significantly decreased with the increase in the distance from the channel in both underground and aboveground biomasses. This was true for both species of H. strobilaceum and S. herbacea. In H. strobilaceum, Pb concentration in the aerial parts was higher compared to that in the root (13.48 against 11.18 mg kg−1 beside channel and 7.28 against 4.26 mg kg −1 with 500 m of distance from the channel). There were insignificant differences between S. herbacea root and shoot Pb in similar distances.
The comparison of Pb content in different tissues of H. strobilaceum and S. herbacea demonstrated that H. strobilaceum included greater amounts compared to S. herbacea.
In terms of Cd content in aerial and underground tissues (Fig. 6), the behavior of the plants, compared to Pb content, was completely different. In both species, the root contained significantly higher amount of Cd. Adjacent to the channel, H. strobilaceum root Cd content was 2.7 times bigger than that of its shoot (1.7 against 0.64 mg kg−1) and this ratio was 1.8. for S. herbacea.
Translocation factor, bioconcentration factor, and bioaccumulation factor
The results of TF, BCF in roots, and BAF in shoots of H. strobilaceum and S. herbacea are presented in Table 2. As could be seen, TF for Pb was bigger than 1 while for Cd, TF was obtained less than 1. The values of BCF in roots and BAF in shoots showed that the concentration of Cd in the root and shoot of H. strobilaceum and S. herbacea were bigger than that in the soil (BCF and BAF > 1). Meanwhile, for Pb, the BCF in roots and BAF in shoots showed values smaller than 1.
According to the number of plants per hectare of the studied plants, the amount of heavy metal uptake in different organs per hectare was mentioned. For pb, In relation to species S.herbacea, this species is able to absorb 67,860 and 68,130 mg kg−1 in shoots and roots, respectively. H. strobilaceum is able to absorb 83,040 and 62,960 mg kg−1 in shoots and roots, respectively. Also, for S.herbacea, the amount of Cd uptake in the roots and shoots is 8010 and 4410 mg kg−1 respectively. H. strobilaceum is able to absorb Cd 2880 and 13,120 mg kg−1 in shoots and roots, respectively.
Discussion
The amount of heavy metals mainly followed a decreasing trend with the increase in the distance from the wastewater channel of the studied industrial estate. Regarding the two investigated depths, there was no significant difference between the amount of Pb and Cd in the soil, but there was almost the highest amount of Pb and Cd in the sampling points near the sewer. In this regard, one reason can be interpreted due to changes in terms of soil acidity.
In this regard, Shen et al. [34] reported that the availability of heavy metals could depend on the soil properties, which was strongly affected by the soil acidity, such that acidity was recognized as the main factor affecting the availability of metals in the soil for the plant to absorb the heavy metals. Here, it is worthwhile to mention that in the soil environment, the solution concentration of the metal contaminants increases by reducing the amount of the acidity, as their displacements mainly occur from exchange points to solid surfaces by increasing the activity of hydrogen ions, represented as the soil acidity reducer.
Although this issue may increase the availability of contaminants for abortion by plants, it may also increase the concentrations of metal available to plants and cause toxicity in them [17]. In this research, the soil acidity is lower at closer distances to the channel. Accordingly, this issue can be a reason for the greater number of heavy elements in the soil around the channel over longer distances.
Findings revealed that industrial wastewater has affected heavy metals accumulation in soil near the channel. A relative difference of 7 for Pb between soil sample adjacent to the channel and control point (1000 m from channel) confirms this claim. For Cd this ratio obtained about 2.5. Alloway [2] announced that the natural range of Cd in soil fluctuates between 0.1 and 2 ppm. According to this, Cd amount was more than critical content in soil. Concentration of soil Cd beside channel was more than control point. Also the first depth of soil contained higher level of Cd compared to the second depth. No regular trend was observed for Pb. According to the previous works, this may be due to changes in the environment, such as changes in soil acidity [23] which explained that the amount of Cd with pH has a photographic relationship. Similarly, in the study soil pH was more than 7.8 which may Pb to more Cd accumulation. Pb significant difference at two depths can be related to the very slow movement of Pb in the soil due to some processes such as ion exchange, trap and complex with organic matter.
Based on different studies, the amount of TF is more than one for both investigated plant species for Pb, indicating that these two species have the necessary ability to absorb Pb from soil to root, and then transferring the Pb from the root to the leave. In other words, these plants absorb the Pb metal from the soil and then transfer it to the leaves through the phytoremediation process. Note that the normal range of the Pb concentrations was reported 2–6 ppm for the plant species [19]. This paper revealed that S. herbacea species can absorb 7.57 mg kg−1of the Pb in the root in the study area, whereas it is 7.54 mg kg−1in its leaves.
Meanwhile, the H. strobilaceum can absorb 7.87 mg kg−1of Pb in the root while it is 10.38 mg kg−1in its leaves. In this way, Kabata Pendias [19] described that although Pb might exist naturally in all plants, it had no essential role in plant metabolism. As such, plants that could grow in the presence of high concentrations of this element in the soil and did not react negatively were resistant to this metal. In the phytoremediation process and contaminated soil treatment, some factors such as a strong root system as well as the transferring element factor from the roots to the leaves are very important. Considering these factors, H. strobilaceum is a plant via high power for the remediation of Pb -contaminated soils.
Moreover, Addy et al. [1] proposed that the normal range of Cd in soil was 0.1–2 ppm. Meanwhile, they mentioned that those soils via more Cd levels from this range were contaminated with cadmium. Cadmium can be toxic to the plant even in very small amounts of 0.003 ppm. Therefore, those plants that could continue their metabolism at this concentration without any problems can be resistant and suitable for the cadmium remediation from the soil [3].
The obtained results from the plant species indicated that the amount of Cd in the root of S. herbacea was 0.89 mg kg−1, while it was 0.49 mg kg−1in its leave. Moreover, it was 1.64 mg kg−1in the root of H. strobilaceum, while it was 0.36 mg kg−1in its leaves, indicating that the amount of this metal in the soil of the study area was higher than its normal level. It should also be mentioned that in the case of Cd in H. strobilaceum, the accumulation of this element in the roots of the plant was higher than their leaves (i.e. TF was less than 1). Furthermore, in the case of S. herbacea, although the accumulation of Cd in the leave was higher than in the root, the difference between this element in the leave and root was very small, so that TF was calculated less than one.
In general, H. strobilaceum had a higher potential for soil remediation and absorb more Pb and Cd than S. herbacea. Of course, in a number of previous studies, the ability of Salicornia plants species to absorb material from the reported environment which is more salt-related [15, 20].
The TF value of Pb for H. strobilaceum and S. herbacea was greater than 1. This indicating that these two species have the ability to absorb and transfer Pb from soil to their vegetation parts. Other studies have reported similar results for heavy metal uptake capacity for this plant. Bobtana et al. [6] reported that H. strobilaceum is a moderate extractor which incline to phytoextraction process in case of Pb and Cd. They also, disclosed that H. strobilaceum might be promising phytoextraction or phytostabilization species due to superior in terms of growth and survival, reproduction and accumulate metals in their tissues in contaminated soils. In previous studied, Anum et al. [4] stated that C. ambigua and C. officinalis L. ornamental plants can safely be grown for phytoremediation aims to decrease soil pollution in term of heavy metal accumulation.
In the present study, each individual of S. herbacea was able to accumulate 6.65 and 7. 54 ppm of Pb in its root and shoot, respectively. Also, each individual of H. strobilaceum was able to accumulate 7.85 and 9.14 mg kg−1of Pb in the root and shoot. This means that they have accumulated excessive amounts of Pb. The natural range of Pb concentration for plants is reported to be 2–6 ppm [19].
Also, TF value of Cd for H. strobilaceum and S. herbacea was lesser than 1. This factor was about half amount for the Pb (0.55) and shows the lower ability of both species to transfer the metal and therefore H. strobilaceum has the lowest TF (0.22). Migaszewski and Paslawski [24] reported that plants with TF more than 1 are suitable for heavy metal remediation. In others studies in phytoremediation, Burd et al. [8] and Muddarisna et al. [25] disclosed that in the plants that they have studies, the BCF was smaller than one, while the TF was more than one, indicating that the plants were suitable for phytoremediation.
Moreover, other factors such as BCF and BAF of H. strobilaceum and S. herbacea was noticeable. BCF is the ratio of heavy metal accumulated by plants to that dissolved in the environment and is important assets to evaluate the feasibility of any plant species for the phytoremediation [27]. BCF of Pb was more than Cd in roots was in the range of but in previous study showed low-moderate and high accumulation for Pb and Cd, respectively. This factor varies depending on the type of plant and the area has been reported. For example, a BCF = 23.51 was reported for Cd in Anthemis stiparum which shows a high potential of phytoremediation near the phosphate treatment industry, Tunisia [14]. Also Retamal-Salgado et al. (2019) reported a high potential for other studied plant to absorb Cd in aerials parts (TF > 2 and BAF > 1). In this research the species showed BCF less than 1 for Pb, which means that these plants store the metal in their underground tissues. It should be noted that the absorption and transfer of metals and their distribution in plant organs are different, which may be due to different growth conditions of plants which in turn is affected by the interaction between root-soil, root-bacteria and root-microcephaly.
In this research, two investigated plant species can be utilized for the remediation of the soils contaminated by industrial wastewater in the area of Eshtehard industrial estate. Actually, the current research was conducted to investigate the potential of these two species. According to the obtained results, it is suggested that the proposed species that were recognized as the resistant ones, can be exploited for phytoremediation, which is a relatively simple and low-cost method.
Overall, according to the obtained results, the species of H. strobilaceum and S. herbacea can be capable to perform the proper phytoremediation of Pb from the soil, as both species can absorb, transport, and accumulate Pb in their organs. However, this issue was not true in the case of Cd. On the other hand, H. strobilaceum has a higher phytoremediation capacity for Pb metal than S. herbacea, based on both TF and BCF factors, but the opposite is true for Cd.
Conclusion
The present research shed light on a high, but different ability of H. strobilaceum and S. herbacea species for absorption, transport, and accumulation of heavy metals (Pb and Cd) in their organs. In general, the highest concentrations of these two metals are absorbed and stored by the underground organs of H. strobilaceum and S. herbacea. Both species were found to have a high potential to absorb, transfer, and accumulate Pb and Cd. H. strobilaceum had further potential than S. herbacea for Cd absorption. Given the presence of native H. strobilaceum and S. herbacea plants in this area and considering ecological principles, they could be suggested for planting for phytoremediation around the industrial towns that are polluted with Pb.
References
Addy M, Losey B, Mohseni R, Zlotnikov E, Vasiliev E. Adsorption of heavy metal ions on mesoporous silica-modified montmorillonite containing a grafted chelate ligand. Appl Clay Sci. 2012;59–60:115–20.
Alloway BJ. Heavy metal in soil. Dordretch: Springer+ Busoness Media; 2013. 104p.
Amoie A, Mahvi A. Evaluation of optimal operational conditions in phytoremediation of soil contaminated with lead and cadmium by native plants of Iran. Sci J Kurdistan Univ Med Sci. 2014;17:231–44.
Anum S, Khan SM, Chaudhary HJ, Ahmad Z, Afza R. Phytoremediation of nickel polluted ecosystem through selected ornamental plant species in the presence of bacterium Kocuria rhizophila. Bioremediation J. 2019;23(3):215–26.
APHA, AWWA, WEF. 1998. Standard methods for the examination of water and wastewater. Washington, 19 Pp.
Bobtana F, Elabbar F, Bader N. Evaluation of Halocnemum strobilaceum and Hammada scoparia plants performance for contaminated soil phytoremediation. J Med Chem Sci. 2019;2(4):126–9.
Brunner I, Luster J, Günthardt-Goerg MS, Frey B. Heavy metal accumulation and phytostabilisation potential of tree fine roots in a contaminated soil. Environ Pollut. 2008;152:559–68. https://doi.org/10.1016/j.envpol.2007.07.006.
Burd GI, Dixon DG, Glick BR. Plant growth – promoting bacteria that decrease heavy metal toxicity in plants. Can J Microbiol. 2000;46:237–45.
Cerniglia CE. Fungal metabolism of polycyclic aromatic hdrocarbons: past, present and future applications in bioremediation. J Ind Microbiol Biotechnol. 1997;19(5–6):324–33.
Chang JH, Dong CD, Shen SY. The lead contaminated land treated by the circulation-enhanced electrokinetics and phytoremediation in field scale. J Hazard Mater. 2019;368:894–8.
Chen L, Liu JR, Gao J, Hu WF, Yang JY. Vanadium in soil-plant system: source, fate, toxicity, and bioremediation, vol. 405: J Hazard Mater.; 2020. p. 124200.
Čudić V, Stojiljković D, Jovović A. Phytoremediation potential of wild plants growing on soil contaminated with heavy metals. Arh Hig Rada Toksikol. 2016;67:229–39.
Du Laing G, Tack FMG, Verloo MG. Performance of selected destruction methods for the determination of heavy metals in reed plants Phragmites australis. Jour Analyt Chim Acta. 2003;49:191–8.
Galfati I, Bilal E, Sassi AB, Abdallah H, Zaïer A. Accumulation of heavy metals in native plants growing near the phosphate treatment industry, Tunisia. Carpathian J Earth Environ Sci. 2011;6(2):85–100.
Ghazisaeedi F, Zamani MM, Ghadbeigi S, Mortazavi SH, Fallahpour M, Ghazisaidi H, Zamani M, Bakhtiarian A, Khoshkholgh Sima NA, Amini M. Bioremediation of the crude oil contamination of soil by the indigenous, herbaceous plant Salicorniaeuropea in Iran. Thrita. 2014;3(2):e17409. https://doi.org/10.5812/thrita.17409.
Goswami R, Thakur R, Sarma KP. Uptake of lead from aqueous solution using Eichhornaia crussipes: effect on chlorophyll content and photosynthetic rate. Int J Chem Tech Res. 2010;283:1702–5.
Gupta DK, Cropas FJ, Palma JM. Heavy metal stress in plants. Heidelberg: Springer; 2013. Library of control number: 2013944774. IBSN978–3–642-38468-4 DOI10.10071978–3–642-38468-1.245p
Jahantab E, Jafari M, Motesharezadeh B, Tavili A, Zargham N. Remediation of petroleum-contaminated soils using Stipagrostis plumosa, Calotropis procera L., and Medicago sativa under different organic amendment treatments. ECOPERSIA. 2018;6(2):101–9.
Kabata-Pendias, A. 2011. Trace elements in soils and plants. Taylor & Francis Group.
Kannan PR, Deepa S, Yasothai A, Kanth SV, Rao JR, Chandrasekaran B. Phytoremediation of tannery wastewater treated lands. Part II: using harvested salicornia brachiata plants for the preservation of sheep skins. J Soc Leather Technol Chem. 2009;93(6):240–4.
Lindsay WL, Norvell WA. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J. 1978;42(3):421–8.
Liu S, Ali S, Yang R, Tao J, Ren B. A newly discovered cd hyperaccumulator Lantana camara L. J Hazard Mater. 2019;371:233–42.
Manousaki E, Kalogerakis N. Phytoextraction of Pb and Cd by thr Mediterranean saltbush: metaluptake relation to salinity. J Environ Sci Poll Res. 2009;16:844–54.
Migaszewski ZM, Paslawski P. Trace element and sulphur stable isotope ratios in soils and vegetation of the Holy Cross Mountains. Geol Quart. 2009;40:575.
Muddarisna N, Krisnayanti BD, Utami SR, Handayanto E. The potential of wild plants for phytoremediation of soil contaminated with mercury of gold cyanidation tailings. IOSR J Environ Sci Toxicol. 2013;4(1):15–9.
Newman LA, Reynolds CM. Phytodegradation of organic compounds. Curr Opin Biotechnol. 2004;15(3):225–30.
Pandey VC. Phytoremediation of heavy metals from fly ash pond by Azolla caroliniana. Ecotoxicol Environ Saf. 2012;82:8–12.
Pulford ID, Watson C. Phytoremediation of heavy metal contaminated land by trees – a review. Environ Int. 2003;29:529–40. https://doi.org/10.1016/S0160-4120(02)00152-6.
Retamal-Salgado J, Hirzel J, Walter I, Matus I. Bioabsorption and bioaccumulation of cadmium in the straw and grain of maize (Zea mays L.) in growing soils contaminated with cadmium in different environment. Int J Environ Res Public Health. 2017;14(11):1399.
Roohi R, Jafari M, Jahantab E, Aman MS, Moameri M, Zare S. Application of artificial neural network model for the identification the effect of municipal waste compost and biochar on phytoremediation of contaminated soils. J Geochem Explor. 2020;208:106399.
Saffari Aman M, Jafari M, Karimpour Reihan M, Motesharezadeh B. Assessing some shrub species for phytoremediation of soils. Environ Earth Sci. 2018;77:82. https://doi.org/10.1007/s12665-018-7256-2.
Sajad MA, Khan MS, Ali H. 42. Lead phytoremediation potential of sixty-one plant species: an open field survey. Pure Appl Biol (PAB). 2019;8(1):405–19.
Samanta SK, Singh OV, Jain RK. Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends Biotechnol. 2002;20(6):243–8.
Shen ZG, Li XD, Wang CC, Chen HM, Chua H. Lead phytoextraction from contaminated soil with high biomass plant species. J Environ Qual. 2002;31:1893–900.
Singh RP, Agrawal M. Potential benefits and risks of land application of sewage sludge. Waste Manag. 2008;28(2):347–58.
Tavili A, Jahantab E, Jafari M, Motesharezadeh B, Zargham N, Aman MS. Assessment of TPH and nickel contents associated with tolerant native plants in petroleum-polluted area of Gachsaran, Iran. Arab J Geosci. 2019;12(10):325.
Vimal Chandra P. Assisted phytoremediation of fly ash dumps through naturally colonized plants. Ecol Eng. 2015;82:1–5.
Wang L, Hou D, Shen Z, Zhu J, Jia X, Ok YS, Tack F, Rinklebe J. Field trials of phytomining and phytoremediation: a critical review of influencing factors and effects of additives. Crit Rev Env Sci Technol. 2019;50:2724–74. https://doi.org/10.1080/10643389.2019.1705724.
Watanabe K. Microorganisms relevant to bioremediation. Curr Opin Biotechnol. 2001;12(3):237–41.
Yongpisanphopa J, Sandhya B, Kruatrachueb M, Pokethitiyookb P. Phytoremediation potential of plants growing on the Pb-contaminated soil at the song Tho Pb mine, Thailand. Soil Sediment Contam. 2017;26(4):426–37.
Yoon J, Cao X, Zhou Q, Ma LQ. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ. 2006;368(2–3):456–64.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tavili, A., Hassanabadi, F., Jafari, M. et al. Phytoremediation ability of H. strobilaceum and S. herbacea around an industrial town. J Environ Health Sci Engineer 19, 1713–1721 (2021). https://doi.org/10.1007/s40201-021-00725-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-021-00725-7