Abstract
Preliminary investigation reveals that fluorosis is reported due to the continuous intake of fluoride-rich groundwater in Vattamalikarai River basin, Tamil Nadu, India. A detailed study was attempted for evaluating the health risks associated with the intake of fluoride-rich groundwater supplied to the rural community. Groundwater samples were collected from 60 and 58 dug and tube wells during winter and southwest (SW) monsoon seasons respectively. The samples were analyzed for the determination of fluoride and other chemical parameters to examine the fitness for drinking water. Spatio-temporal variation maps reveal that fluoride concentration is high during SW monsoon season when compared with the winter season in this region. The fluoride bearing minerals present in hornblende-biotite gneiss and charnockite rock formations leached into the groundwater during rock–water interaction. To understand the subsurface hydrogeochemical reactions, inverse mass balance model was developed using NETPATH code. The model output indicates that calcite dilution, silicate (hornblende and biotite) weathering, ion exchange (Ca/Na and Mg/Na) and illite precipitation are the dominant processes controlling the groundwater chemistry along the flow paths. Non-carcinogenic risks to children and adults (women and men) were evaluated by working out intake exposure of groundwater. Hazard quotient (HQ) based on fluoride intake was calculated for children and adults. It varied from 0.08 to 2.21 with an average of 1.07 for adults. For children, it varied from 0.01 to 2.99 with the mean of 1.44. About 78%, 69% and 61% of the samples fall under the risk category for children, women and men during winter season. However, more number of samples possessed health risks (83% of samples for children, 73% of samples for women and 64% of samples for men) during SW monsoon season.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Geogenic or anthropogenic activities highly influence the groundwater quality of an area. Globally, groundwater is a major source for human consumption; hence, quality variation in it can have serious consequences. Groundwater quality is an important part considered for drinking and agricultural practices (Arya and Subramani 2013). The fitness of groundwater for various needs is measured with respect to its chemical composition. Groundwater quality also influences ecosystem, health and function of human, so it is important to detect both the natural and anthropogenic activities resulting from pollution. In the initial period of water quality deterioration, the damage may be low, but if it is not controlled at the right time, this water will not be suitable for any purpose (Rao 1997). Freshwater scarcity is a very big emerging issue in several parts of the world due to various reasons such as urbanization, industrialization, afforestation, climate change, pollution and other natural impacts. Since freshwater is essential for drinking and irrigation purposes, it is necessary to do proper management. Studies prove that nearly 200 million populace across 28 nations are at risk due to excess fluoride concentration in groundwater (Ayoob and Gupta 2006; Karunanidhi et al. 2019b). During the last three decades, ‘fluorosis’ seems to be a major emerging health issue widely spread throughout the world due to high fluoride concentration in groundwater. Nowadays, high fluoride concentration in groundwater is an emerging global issue. Researchers have reported about the high fluoride concentration in groundwater globally, viz., Korea (Chae et al. 2006), Mexico (Vasquez et al. 2006), Brazil (Kern et al. 2008), China (Zang et al. 2008), Pakistan (Rafique et al. 2008), Sri Lanka (Chandrajith et al. 2012) and India (Saxena and Sewak 2015). In India, nearly 90% of the rural populace depends on groundwater for domestic purposes. Seventeen states of India have excess fluoride in drinking water (Duraiswami and Patankar 2011). Fluoride incidence and its health risks were reported across several states of India such as Andhra Pradesh (Sharma and Rao 1997), Orissa (Kundu et al. 2001), Gujarat (Salve et al. 2008), Uttar Pradesh (Raju et al. 2009), Tamil Nadu (Sivasankar and Gomathi 2009) and Assam (Saikia and Sarma 2011).
Groundwater chemistry is a significant parameter that determines its use for various needs (Subramani et al. 2005; Hema et al. 2010). Rock–water interaction along the flow path manages the chemistry of groundwater (Subramani et al. 2009). The lithological formation of charnockite, gneiss and schist contributes to the fluoride concentration of 0.51–4.0 mg/l (Madhavan and Subramanian 2006; Krishnamachari 1976). The fluoride bearing minerals in hornblende and biotite rocks easily contribute fluoride into the aquifer system (Subba et al. 2016). According to the WHO 1996, especially among children, less fluoride consumption (< 0.5 mg/l) causes health problems such as teeth and bone mineralization deficiency. On contrary, intake of excess fluoride (> 1.5 mg/l) can also affect human health. Daily intake of water is temperature-dependent; generally children consume about one liter, and adults consume two liters.
In Tamil Nadu state, 8 districts (Salem, Erode, Dharmapuri, Coimbatore, Tiruchirapalli, Vellore, Madurai and Virudhunagar) have been affected by fluorosis (CGWB 2007). The groundwater quality variation is highly erratic in Vattamalakarai River basin (Vennila et al. 2008; Arya and Subramani 2013). Excess fluoride in groundwater of Vattamalaikarai River basin, Tamil Nadu, India, may have great impacts on people. Therefore, the current paper depicts the fluoride distribution in groundwater of the basin. The health risk impacts over children and adults due to the continuous intake of fluoride-rich groundwater will be helpful for taking suitable remedial measures. In addition, NETPATH code was used in this work to understand the subsurface geochemical processes, which mainly control the chemistry of groundwater in this basin.
Study area
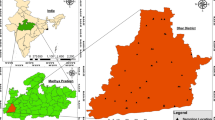
Vattamalaikarai River basin is located in the southern part of Tamil Nadu, India. The basin extents from latitudes 10° 53′ N–11° 01′ N and longitudes 77° 15′ E–77° 45′ E (Fig. 1), and it has an aerial extent of 435 km2. Vattamalaikarai River is a tributary of Amaravathi River, and it is one of the major tributaries of Cauvery River. This river is a non-perennial one. The basin area comes under the rainfall shadow region of the Western Ghats (CGWB 1979; Anandakumar et al. 2008). The average annual rainfall is 571 mm with medium humidity and temperature (Vennila et al. 2007). The area exhibits a semiarid climatic condition.
The local people depend on groundwater resources due to the non-perennial nature of river and less availability of rainfall. The basin has the highest elevation of 427 m above mean sea level (MSL) with the regional slope toward east. Geologically, the basin comprises hornblende-biotite gneiss and charnockite rocks. Red calcareous soil is widely distributed over the basin, and black soil is found on the western portion. Rainfall is the major source of groundwater recharge. The existence of weathered zone in the basin is not uniform. Weathering thickness is maximum along the north-eastern and north-western portions of the basin. Long-term water level fluctuation study shows that groundwater level of the basin ranges from 2 to 30 m (Anand et al. 2019; Arya et al. 2018). Tube wells and dug wells are used for extraction of groundwater from the aquifers. Weathered and fractured zones in the crystalline rocks of this basin mostly act as aquifers for groundwater extraction.
Methods and materials
GPS was used to record the groundwater sampling locations in the field. Fifty-eight and sixty samples were collected from the monitoring dug wells and tube wells spread over the entire basin during southwest (SW) monsoon and winter seasons respectively. The parameters such as pH, TDS and EC were analyzed in the field using portable water quality meters. The samples were transported to the laboratory for chemical testing. The standard procedures mentioned in the American Public Health Association (APHA 2005) were used to obtain the concentrations of various chemical constituents such as sodium, potassium, calcium, magnesium, chloride, bicarbonate, carbonate, sulfate and fluoride in the geochemical laboratory. Spatial maps pertaining to fluoride concentration were prepared using Arc-GIS (version 10.1) software. Health risk assessment based on fluoride concentration was performed by using Eq. 1 (USEPA 1991, Karunanidhi et al. 2019a). Hazard coefficient (HQ) was determined using Eq. 2 (Karunanidhi et al. 2019a)
In the above equations, ADD denotes the fluoride intake (mg/kg/day), CPW indicates pollutant level in groundwater (mg/L), IR implies intake rate per unit time (l/day), ED represents exposure time span in years, EF is the exposure frequency (days/year), ABW characterizes the mean weight of the individual (kg), AET signifies the mean exposure time (years) and Rfd indicates the reference dosage of fluoride.
Finally, hydrogeochemical inverse mass balance model (NETPATH) was used to understand the major subsurface hydrogeochemical processes in the groundwater system of the Vattamalaikarai River basin.
Results and discussion
Evaluation of geochemical characteristics of groundwater for consumption
The analytical results of various physicochemical parameters are depicted in Table 1. The examination of chemical parameters indicates that pH values ranges from 6.8 to 8.2, which shows slightly alkaline nature of water. The groundwater in the basin is moderately mineralized. The chemical constituents were evaluated with the World Health Organization (WHO 2017) standards for testing the suitability for drinking and public health purposes. High concentrations of sodium, calcium and magnesium ions in groundwater are due to weathering of silicate minerals present in the country rocks of the study area (Karunanidhi et al. 2013; Duraisamy et al. 2018). Comparatively, the groundwater quality is better in the tube wells than the open wells. In most of the open wells and tube wells K+, Ca2+ and Mg2+ concentrations are within the maximum allowable limits for drinking. Fluoride concentration is found to be high in the groundwater samples and exceeds the limit given by the WHO (> 1.5 mg/l) for human intake. Due to high fluoride content in the drinking water, the local people of this area got affected with fluorosis.
Spatio-temporal variation of fluoride in groundwater
Fluoride is one of the naturally occurring major trace elements in groundwater. Fluoride constituent in drinking water is having both useful and harmful effects on human health. Fluoride content has an inevitable role in mineralization of bones and dental enamel formation in smaller quantity (Chouhan and Flora 2008). Fluoride intake exceeds the permissible limit, becomes toxic to human health system, and causes clinical and metabolic disturbances in the form of dental and skeletal fluorosis (Aravinthasamy et al. 2019b). Naturally occurring fluoride in groundwater is governed largely by climate and geology (Aravinthasamy et al. 2019a). Naturally occurring fluoride is released into water during rock water interaction (Wenzel and Blum 1992; Bardsen et al. 1996). Many parts of India, including Tamil Nadu, Kerala, Andhra Pradesh and Karnataka, possess the fluoride mineral bearing bed rock, which match with the distribution of fluoride in the aquifer system. Jacks et al. (2005) reported that during the weathering process stability of fluor-apatite is relatively high, which is responsible for the release of fluoride minerals into groundwater from mica, hornblende and pyroxene. Chemical weathering of hornblende-biotite gneiss together with other parameters is favorable for the fluoride incidence into groundwater. Studies illustrate that fluorite and apatite in the host rocks of the study area are the major sources for fluoride in groundwater (CGWB 1979).
To understand the trend of fluoride concentration in the river basin, both the SW monsoon and winter season water samples from dug wells and tube wells were analyzed and compared with the WHO standards. In order to predict the pattern and distribution of fluoride in the groundwater of the Vattamalaikarai River basin, the fluoride levels were categorized as 1.0 mg/l, between 1.0 and 1.5 mg/l, and above 1.5 mg/l, together with the maximum fluoride concentration found. The results are shown in Table 2, which state that during SW monsoon, out of 40 dug wells 19 dug wells (48%) exceed the permissible limit, and during winter season out of 42 dug wells 11 (26%) samples exceed the permissible limit.
In the study area, tube wells possess less fluoride concentration when compared with the dug/open wells. It is also observed that the fluoride concentration is less during winter season due to the dilution mechanism. The spatio-temporal occurrence of fluoride in open and tube wells during SW monsoon and winter season is illustrated in Fig. 2.
Health risks due to fluoride intake
Non-carcinogenic risks to children and adults (women and men) were assessed by working out intake exposures of fluoride-rich groundwater. Hazard quotient (HQ) was computed from the SW monsoon and winter season data for children, women and men separately. As far as possible, the non-carcinogenic risk for human wellbeing ought not to surpass 1. From the computed HQ values, safe and risk samples for children and adults were calculated. The results indicate that 49 (83%) samples of SW monsoon and 46 (78%) samples of winter season possess health risk for children. For women, 43 (73%) samples of SW monsoon and 41 (69%) samples of winter season are under risk category. Similarly for men, 38 (64%) samples of SW monsoon and 36 (61%) samples of winter season are under risk (Table 3).
This investigation shows that children are more prone to risk when compared with women and men. Therefore, continuous intake of fluoride-rich groundwater may lead to serious health effects on children. The spatial representation of HQ values (Figs. 3, 4) indicate that about 389.94 km2 area during SW monsoon and 340.79 km2 area during winter season are under risk for children. Groundwater existing over 292.76 km2 area during SW monsoon and 266.47 km2 area during winter season are under risk for women. Similarly about 194.66 km2 basin area during SW monsoon and 119.11 km2 basin area during winter season are under risk for men. This also suggests monsoon precipitation dilutes the concentration of fluoride.
Similar health risk evaluation studies were attempted in various regions of the India, viz., Shanmuganadhi River basin (Karunanidhi et al. 2019a), Haryana state (Garg et al. 2009), Siddipet (Narsimha and Rajitha 2018) and Punjab (Ahada and Suthar 2017). They have also pointed out that compared to adults, children are under more risk for fluorosis. High concentration of fluoride could also cause stomach cancer, dental and skeletal fluorosis and loss of memory in children (Lu et al. 2000; Ayoob and Gupta 2006).
Geochemical inverse mass balance model
Groundwater quality is mainly governed by the reaction between groundwater and minerals in the aquifer system, which helps in identifying the groundwater genesis (Cederstorm 1946; Subramani et al. 2009). Based on lithology, pump test results and groundwater flow well pairs were selected for NETPATH modeling to understand the hydrogeochemical processes in the basin (Plummer 1991; Subramani et al. 2013). NETPATH model helps to evaluate several geochemical processes such as silicate weathering, ion exchange, dilution and dissolution (Subramani et al. 2013). These processes are mainly controlled by the geochemistry of aquifer matrix and mineral distribution in the sample phases (Khadra and Stuyfzand 2016). The regional groundwater flow in the basin is toward southeast (Arya et al. 2018). With respect to the flow direction, net geochemical mass balance reactions between initial and final water of selected well pairs, D5 and D4; D16 and D18; D22 and D23; D36 and D37, were identified (Fig. 5). The flow rate depends on the gradient (slope) in the groundwater table and the permeability of the soil (Arya and Subramani 2015). The analytical results of groundwater samples of SW monsoon and winter season were used in this model. The model outputs are presented in Table 4.
For mass balance modeling, calcium, magnesium, carbonate, sodium, sulfur, potassium and chloride concentrations were considered. Mass balance model shows that dilution, illite precipitation and ion exchange are the dominant activities that govern the groundwater chemistry along the flow paths. As a result of these reactions, concentration of magnesium ions was dominant in the basin. Moreover, dissolution of NaCl and illite was also involved in the process. High concentrations of Na and Cl in groundwater of charnockitic terrain were due to these processes. Ca and Mg minerals were absorbed by the aquifer matrix, and Na was released into the groundwater during the ion exchange process. In the two models, it is observed that Ca ion was released into groundwater and Na ion was absorbed by the aquifer matrix (i.e., reverse ion exchange). However, the return flow of irrigation water causes the increase in Na ion concentration. The ion exchange reactions taking place between Ca or Mg and Na ions are represented as follows (Eqs. 3 and 4) (Martinez and Bocanegra 2002):
X—represents matrix
It was found that in certain parts of the study area, increase in the concentrations of sodium, potassium and bicarbonate ions in groundwater is due to calcite dissolution, ion exchange and illite precipitation. The major hydrogeochemical reactions taking place in the basin are reverse ion exchange, silicate minerals (biotite and hornblende) dissolution, dilution and precipitation of illite. Higher concentrations of magnesium ions in the groundwater of this basin are due to weathering of charnockite and gneiss. It is also observed that dissolution of halite is due to irrigation return flow. Similarly, higher calcium and magnesium concentrations are due to dissolution of biotite and hornblende minerals in the bed rock of the basin.
Conclusions
As Vattamalaikarai River is a non-perennial one and rainfall scarcity in the area results in increasing demand of water for various needs, groundwater is considered to be the main source by the local people. Various chemical properties of groundwater samples were analyzed and compared with the drinking standards proposed by the WHO with special reference to fluoride concentration. Seasonal and spatial variations of fluoride occurrence in open/dug wells and tube wells were examined. In the open wells, about 48% of samples exceeded the permissible limit for consumption purpose (> 1.5 mg/l) during SW monsoon, and 26% of samples during winter season exceeded the limit. Fluoride concentration was less in bore wells, particularly during winter season.
Geological formations are responsible for the high fluoride concentration in most of the sampling locations. Using NETPATH code, four models were prepared to understand the subsurface hydrogeochemical processes in different geological formations. Dissolution of silicate minerals such as hornblende and biotite was common in the models. Similarly, dissolution of calcite and NaCl was also observed. One model indicated illite dissolution, and two models indicated illite precipitation. Exchange between Na/Ca and Na/Mg was also noticed in the model outputs. Dissolution of fluoride bearing minerals present in hornblende-biotite gneiss and charnockite rocks into groundwater during rock–water interaction is responsible for high fluoride concentration.
With regard to human health risk, non-carcinogenic risks based on hazard quotient (HQ) were computed for adults (men and women) and children. The results signify that 83% of SW monsoon samples and 78% of winter season samples are under risk for children. About 73% of SW monsoon samples and 69% of winter season samples possess risk for women. Similarly, 64% of SW monsoon samples and 61% of winter season samples possess risk for men category. HQ values highlight that children are at more risk than adults in Vattamalaikarai basin. The excess fluoride content in the groundwater of the study area implies that effective remedial measures should be adopted to reduce the risk in future. This study recommends the construction of artificial recharge structures to reduce fluoride concentration in groundwater.
References
Ahada, C. P. S., & Suthar, S. (2017). Assessment of human health risk associated with high groundwater fluoride intake in southern districts of Punjab. India: Expo Health. https://doi.org/10.1007/s12403-017-0268-4.
Anand, B., Karunanidhi, D., Subramani, T., Srinivasamoorthy, K., & Suresh, M. (2019). Long-term trend detection and spatiotemporal analysis of groundwater levels using GIS techniques in Lower Bhavani River basin, Tamil Nadu, India. Environment, Development and Sustainability. https://doi.org/10.1007/s10668-019-00318-3.
Anandakumar, S., Subramani, T., & Elango, L. (2008). Spatial variation and seasonal behaviour of rainfall pattern in Lower Bhavani River basin, Tamil Nadu, India. The Ecoscan, 2(1), 17–24.
APHA. (2005). Standard methods for the examination of water and wastewater (21st ed.). Washington, DC: American Public Health Association.
Aravinthasamy, P., Karunanidhi, D., Subramani, T., Anand, B., Roy, P. D., & Srinivasamoorthy, K. (2019a). Fluoride contamination in groundwater of the Shanmuganadhi River Basin (south India) and its association with other chemical constituents using geographical information system and multivariate statistics. Geochemistry. https://doi.org/10.1016/j.chemer.2019.125555.
Aravinthasamy, P., Karunanidhi, D., Subramani, T., Srinivasamoorthy, K., & Anand, B. (2019b). Geochemical evaluation of fluoride contamination in groundwater from Shanmuganadhi River basin, South India: implication on human health. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-019-00452-x.
Arya, S., & Subramani, T. (2013). Rapid assessment of suitability of groundwater for drinking and irrigation purposes. Pollution Research, 32(2), 321–326.
Arya, S., & Subramani, T. (2015). Groundwater flow and fluctuation using GIS in a hard rock region, South India. Indian Journal of Geo-Marine Sciences, 44(09), 1422–1427.
Arya, S., Vennila, G., & Subramani, T. (2018). Spatial and seasonal variation of groundwater levels in Vattamalaikarai River basin, Tamil Nadu, India—study using GIS and GPS. Indian Journal of Geo-Marine Sciences, 47(09), 1749–1753.
Ayoob, S., & Gupta, A. K. (2006). Fluoride in drinking water: A review on the status and stress effects. Critical Reviews in Environmental Science and Technology, 36, 433–487.
Bardsen, A., Bjorvatn, K., & Selvig, K. A. (1996). Variability in fluoride content of sub surface water reservoirs. Acta OdontolScand., 54, 343–347.
Brouwer, I. D., Bruin, A. D., Backer, D. O., & Hautvast, J. G. A. J. (1988). Unsuitability of world health organisation guidelines for fluoride concentrations in drinking water in Senegal. The Lancet, 331(8579), 223–225.
Cederstorm, D. J. (1946). Genesis of groundwater in the coastal plain of Virginia. Economic Geology, 41(3), 218–245.
CGWB. (1979). SIDA Assisted project for Tamil Nadu and Kerala States, Central Ground Water Board, Government of India, South Eastern Coastal Region, Chennai.
CGWB. (2007). Ground water quality features of the country (assessed every year during April/May from 1974 to 2007), Central Groundwater Board report. http://cgwb.gov.in/documents.
Chae, G. T., Yun, S. T., Kwon, M. J., Kim, Y. S., & Mayer, B. (2006). Batch dissolution of granite and biotite in water: Implication for fluorine geochemistry in groundwater. Geochemical Journal, 40, 95–102.
Chandrajith, R., Padmasiri, J. P., Dissanayake, C. B., & Prematilaka, K. M. (2012). Spatial distribution of fluoride in groundwater of Sri Lanka. Journal of the National Science Foundation of Sri Lanka, 40(4), 303–309.
Chouhan, S., & Flora, S. J. (2008). Effects of fluoride on the tissue oxidative stress and apoptosis in rats: biochemical assays supported by IR spectroscopy data. Toxicology, 254, 61–67.
Duraisamy, S., Govindhaswamy, V., Duraisamy, K., Krishinaraj, S., Balasubramanian, A., & Thirumalaisamy, S. (2018). Hydrogeochemical characterization and evaluation of groundwater quality in Kangayam taluk, Tirupur district, Tamil Nadu, India, using GIS techniques. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-018-0183-z.
Duraiswami, R. A., & Patankar, U. (2011). Occurrence of fluoride in the drinking water sources from Gad river basin, Maharashtra. Journal Geological Society of India, 77, 167–174.
Garg, V. K., Suthar, S., & Singh, S. (2009). Drinking water quality in villages of south western Haryana, India: Assessing human health risks associated with hydrochemistry. Environmental Geology, 58, 1329–1340. https://doi.org/10.1007/s00254-008-1636-y.
Hema, S., Subramani, T., & Elango, L. (2010). GIS study on vulnerability assessment of water quality in a part of Cauvery River. International Journal of Environmental Sciences, 1(1), 1–17.
Jacks, G., Bhattacharya, P., Chaudhary, V., & Singh, K. P. (2005). Controlson the genesis of some high-fluoride groundwaters in India. Applied Geochemistry, 20, 221–228.
Karunanidhi, D., Vennila, G., Suresh, M., & Subramanian, S. K. (2013). Evaluation of the groundwater quality feasibility zones for irrigational purposes through GIS in Omalur Taluk, Salem District, South India. Environmental Science and Pollution Research, 20(10), 7320–7333. https://doi.org/10.1007/s11356-013-1746-2.
Karunanidhi, D., Aravinthasamy, P., Subramani, T., Wu, J., & Srinivasamoorthy, K. (2019a). Potential health risk assessment for fluoride and nitrate contamination in hard rock aquifers of Shanmuganadhi River basin, South India. Human and Ecological Risk Assessment. An International Journal. https://doi.org/10.1080/10807039.2019.1568859.
Karunanidhi, D., Aravinthasamy, P., Subramani, T., Roy, P. D., & Srinivasamoorthy, K. (2019b). Risk of fluoride-rich groundwater on human health: Remediation through managed aquifer recharge in a hard rock terrain, South India. Natural Resources Research. https://doi.org/10.1007/s11053-019-09592-4.
Kern, M. L., Vieiro, A. P., & Machado, G. (2008). The fluoride in the groundwater of Guarani aquifer system: The origin associated with black shales of Parana´ Basin. Environmental Geology, 55, 1219–1233.
Khadra, W. M., & Stuyfzand, P. J. (2016). Mass balancing to define major hydrogeochemical processes in salinizing dolomitic limestone aquifers: Example from Eastern Mediterranean (Lebanon) 24th salt water intrusion meeting and the 4th Asia-Pacific coastal aquifer management meeting, Cairns, Australia (pp. 110–116).
Krisnmachari, K. (1976). Further observations on syndrome of endemic Genu Valgum of South India. Indian Journal of Medical Research, 64, 284–291.
Kundu, N., Panigrahi, M. K., Tripathy, S., Munshi, S., Powell, M. A., & Hart, B. R. (2001). Geochemical appraisal of fluoride contamination of groundwater the Nayagarh District, Orissa. Environmental Geology, 41, 451–460.
Lu, Y., Sun, Z. R., & Wu, L. N. (2000). Effect of high fluoride water on intelligence in children. Fluoride, 33(2), 74–78.
Madhavan, N., & Subramanian, V. (2006). Environmental impact assessment including evolution of fluoride and arsenic contamination process in groundwater and remediation of contaminated groundwater system. In M. Thangarajan (Ed.), Sustainable development and management of groundwater resources (pp. 128–155). New Delhi: Capital Publishing Company. ISBN 81-85589-30-5.
Martinez, D. E., & Bocanegra, E. M. (2002). Hydrogeochemistry and cation exchange processes in the coastal aquifer of MarDelPlata, Argentina. Hydrogeology Journal, 10, 393–408.
Narsimha, A., & Rajitha, S. (2018). Spatial distribution and seasonal variation in fluoride enrichment in groundwater and its associated human health risk assessment in Telangana State, South India. Hum Ecol Risk Assess Int J. https://doi.org/10.1080/10807039.2018.1438176.
Plummer, L. N. (1991). An interactive code (NETPATH) for modeling NET geochemical reactions along a flow PATH. U. S. Geological Survey: Water—Resources Investigation Report. 91-4078, 227.
Rafique, T., Naseem, S., Bhanger, M. I., & Usmani, T. H. (2008). Fluoride ion contamination in the groundwater of Mithi sub-district, the Thar Desert, Pakistan. Environmental Geology, 56, 317–326.
Raju, N. J., Dey, S., & Das, K. (2009). Fluoride contamination in groundwaters of Sonbhadra District, Uttar Pradesh, India. Current Science, 96(7), 979–985.
Rao, Y. S., Reddy, T. V. K., & Nayudu, P. T. (1997). Groundwater quality in the Niva River basin, Chittoor district, Andhra Pradesh, India. International Journal of Environmental Geology, 32(1), 56–63.
Saikia, M. M., & Sarma, H. P. (2011). Fluoride geochemistry of Kollong river basin, Assam, India. Archives of Applied Science Research, 3(3), 367–372.
Salve, P. R., Maurya, A., Kumbhare, P. S., Ramteke, D. S., & Wate, S. R. (2008). Assessment of groundwater quality with respect to fluoride. Bulletin of Environmental Contamination and Toxicology, 81, 289–293.
Sarma, D. R. R., & Rao, S. L. N. (1997). Fluoride concentrations in ground waters of Visakhapatnam, India. Bulletin of Environmental Contamination and Toxicology, 58, 241–247.
Saxena, K. L., & Sewak, R. (2015). Fluoride consumption in endemic villages of india and its remedial measures. International Journal of Engineering Science Invention, 4(1), 58–73.
Singh, B., Gaur, S., & Garg, V. K. (2007). Fluoride in drinking water and human urine in Southern Haryana. India. Journal of Hazardous Materials, 144, 147–151.
Sivasankar, V., & Gomathi, R. (2009). Fluoride and other quality parameters in the groundwater samples of Pettaivaithalai and Kulithalai areas of Tamil Nadu, Southern India. Water Quality Expo Health, 1, 123–134.
Subba, R. N., Surya, R. P., Dinakar, A., Nageswara Rao, P. V., & Marghade, D. (2016). Fluoride occurrence in the groundwater in a coastal region of Andhra Pradesh, India. Applied water Science, 7(3), 1467–1478.
Subramani, T., Anandakumar, S., Kannan, R., & Elango, L. (2013). Identification of major hydrogeochemical processes in a hard rock terrain by NETPATH modeling. Book on Earth Resources and Environment, 29, 365–370.
Subramani, T., Elango, L., & Damodarasamy, S. R. (2005). Ground water quality and suitability for drinking and agricultural in Chithar river basin, Tamil Nadu, India. Environmental Geology, 47, 1099–1110.
Subramani, T., Rajmohan, N., & Elango, L. (2009). Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, Southern India. Environmental Monitoring and Assessment, 162(1–4), 123–137. https://doi.org/10.1007/s10661-009-0781-4.
USEPA. (1991). Risk assessment guidance for superfund, vol 1: human health evaluation manual (part B, development of risk-based preliminary remediation goals). EPA-9585.7-01B. Office of Emergency and Remedial Response, Washington, DC.
USEPA. (2014). Human health evaluation manual, supplemental guidance: Update of standard default exposure factors-OSWER Directive 9200.1-120. PP.6.
Vasquez, L. V., Hernandez, J. R., Lopez, J. R., Uribe, A. S., & Mancilla, O. L. (2006). The origin of fluoride in groundwater supply to Hermosillo City, Sonora, Mexico. Environmental Geology, 51, 17–27.
Vennila, G., Subramani, T., & Elango, L. (2007). Rainfall variation analysis of Vattamalaikarai sub-basin, Tamil Nadu, India. Applied Hydrology, 20(3), 50–59.
Vennila, G., Subramani, T., & Elango, L. (2008). GIS based groundwater quality assessment of Vattamalaikarai Basin, Tamil Nadu, India. Nature Environment Pollution Technology, 7, 585–592.
Viero, A. P., Roisenberg, C., Roisenberg, A., & Vigo, A. (2009). The origin of fluoride in the granitic aquifer of Porto Alegre, Southern Brazil. Environmental Geology, 56, 1707–1719.
Wenzel, W. W., & Blum, W. E. H. (1992). Fluoride speciation and mobility in fluoride contaminated soil and minerals. Journal of Soil Science, 153, 357–364.
WHO. (2017). Guidelines for drinking water quality: fourth edition incorporating the first addendum. Geneva: World Health Organization.
Wu, J., Li, P., & Qian, H. (2015). Hydrochemical characterization of drinking groundwater with special reference to fluoride in an arid area of China and the control of aquifer leakage on its concentrations. Environmental Earth Sciences, 73(12), 8575–8588.
Zhang, B., Hong, M., Zhang, B., Zhang, X.-l., & Zhao, Y.-s. (2008). Fluorine distribution in aquatic environment and its health effect in the Western Region of the Songnen Plain, Northeast China. Environmental Geology, 56, 1707–1719.
Acknowledgements
The authors are grateful to the Department of Science and Technology (DST), Government of India, New Delhi (File No.SR/S4/ES-541/2010 dated. 28.01.2015), for providing funds to carry out field works in Vattamalaikarai River basin, Tamil Nadu, India.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author declares that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arya, S., Subramani, T., Vennila, G. et al. Health risks associated with fluoride intake from rural drinking water supply and inverse mass balance modeling to decipher hydrogeochemical processes in Vattamalaikarai River basin, South India. Environ Geochem Health 43, 705–716 (2021). https://doi.org/10.1007/s10653-019-00489-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-019-00489-y