Abstract
Clays and muds have been used for centuries as cosmetics or pharmaceutical products for various therapies. The suitability of muds and clays for health- and beauty-related applications depends on their physicochemical properties, mineralogical composition, particle characteristics and toxicity. In this work, the physicochemical characterization of 12 mud specimens from different natural spa resorts in Greece and one from Israel (Dead Sea) is presented. All specimens were sterilized at 121 °C for 20 min, because of their intended use. The Greek mud specimens were collected from various locations in Macedonia, Western Greece and Northeast Aegean. All muds were characterized concerning their mineralogical, chemical components as well as their morphological characteristics using appropriate methods [powder X-ray diffraction (XRD), thermogravimetric analysis (TGA), nitrogen absorption specific surface area measurements (BET), scanning electron microscopy and energy dispersive X-ray spectroscopy]. The concentrations of F−, Cl−, NO3− and SO42− anions at equilibrium with the mud specimens were measured by ion chromatography. Total calcium concentration was measured using atomic absorption spectroscopy, and the concentration of total N, C, H and S in the solids was measured using elemental analysis. Moreover, total phenolic concentration (TPC) in distilled water equilibrated with the mud specimens was measured as an index for their antioxidant properties. Several muds were found to present high TPC. Several of the examined mud specimens were found to have the potential use as pharmaceuticals or cosmetics. Based on the physicochemical characteristics of the mud specimens examined, possible improvement in their use and applicability has been suggested.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Clays have been used since antiquity for their therapeutic and healing properties. Clays from different old Armenia and Cappadocia, Greece and Turkey were famous for their properties when used as antiseptic or skin cures (Carrretero et al. 2006). From generation to generation, depending on the mineral composition of lands, depending on traditional and religion practices, clays passed from geophagy to medicine, to pelotherapy and medical hydrology and humans take advantage of their properties (Ferrell 2008; Rebelo et al. 2011) as the use of natural products becomes emergent Harvey et al. 2015).
Clays are often used without prior tests of their properties. Spanish clays such as sepiolite, palygorskite and bentonite when compared to fibrous clays and bentonite used as pharmaceuticals were found suitable for pharmaceutical use (Viseras and Lopez-Galindo 1999). Smectites from Tunisia exhibit high specific surface area values and high Ca and Mg cations exchange capacity (CEC). Their high abrasion power however was considered a disadvantage (Fakhfakh et al. 2005). For matured clays, the term “peloids” has been in use, defined as “maturated mud or muddy dispersion with healing and/or cosmetic properties, composed of a complex mixture of fine-grained natural materials of geologic and/or biologic origins, mineral water or seawater, and commonly organic compounds from biological metabolic activity” (Gomes et al. 2013). Several clay samples found at the Tunisian market were found to contain illite, kaolinite and magnesium-smectite and were classified as suitable for use. In several cases, the inorganic components were predominant in mud specimens, and they contained high concentrations of crystalline silica (Khiari et al. 2014).
The wide use of peloids from Mongolian lakes in balneology has been attributed to their content of bioactive organic compounds, such as humic acids, lipids, carbohydrates, proteins and hydrogen sulfide which usually is product of the prokaryotic breakdown of organic matters (Dolmaa et al. 2009; Tserenpil et al. 2010). Investigation of Turkish thermal muds showed that they contained P, Si, Mg, F and Sr, metals found in bones as well, besides Ca. The presence of Sr has been suggested to play a stabilizing role of calcium phosphate in bones. The high CEC of known thermal muds has been attributed to the presence of Ca2+ found in smectites (Çelik Karakaya et al. 2010). However, in cases in which the origin of Ca2+ ions is from calcite, CEC is lower and may not cross the skin barrier. Nevertheless, even when thermal muds are not suitable for therapeutic use, most of them may help in health problems such as osteoporosis and arthritis (Çelik Karakaya et al. 2010; Setnescu et al. 2013). The exothermicity of therapeutic muds collected from various sites in Romania has been correlated with their treatment efficiency of various diseases. The high therapeutic efficiency of muds has been attributed to elevated organics concentration (Setnescu et al. 2013). Peloid therapy is renowned for its organic constituents which have attracted considerable research interest. Another property of peat is its “power of sorption or of ion exchange of the peloid” (Hattori 1963; Hill 1997). The sorption of metals on humic acid (HA), of heavy metals in particular, including Hg, Pb and Cd, has clinical implications contributing to body detoxification (Kerndorff and Schnitzer 1980). The organic constituents of muds used for therapeutic and cosmetic applications include humic compounds, carboxylic acids, terpenoids, steroids, phenol antioxidants (ferulic, vanillic and gallic acid) and fatty acids (Odabaşı 2014). Polyphenols have been known to be present in natural products (fruits, wine, tea, etc.), and their important antioxidant properties have been acknowledged (Rice-Evans et al. 1997). A great number of researches have focused on the positive effects of phenolic compounds extracted from plants on skin diseases (Działo et al. 2016). Chitosan and chitin are two polysaccharides which differ chemically from cellulose, because of the presence of nitrogen in their molecular structure (Bautista-Banos et al. 2006). Commercial chitosan is used in agriculture as biopesticide, in food industry as preservative, or in medicine to stop bleeding (Sarbon et al. 2015). Chitosan may be extracted from shrimp shells, mud of crab or other shellfish and has antioxidant properties (Sarbon et al. 2015). Since most of researchers ascribe the antioxidant, therapeutic and healing properties to the organic compounds of clays (Sarbon et al. 2015, Centini et al. 2015), the investigation of the organic composition of muds from natural spa resorts is important.
Even though Greek muds are famous and they are used since antiquity, the work done concerning their mineralogical and physicochemical properties is extremely limited. The aim of this work was the characterization of muds selected from natural spa resorts for potential use in cosmetics and pharmaceutical products. In the present work, 12 samples, collected from various natural spa resorts in Greece and one from Israel (Dead Sea) were characterized by physicochemical methods. Because of their intended use, all muds were sterilized prior to their characterization, at 121 °C for 20 min. The Greek muds used in this work, were investigated thoroughly in a previous work for their anti-inflammatory, antioxidant and anti-aging properties (Spilioti et al. 2016). In Spilioti et al. (2016), most of the mud extracts were shown to have anti-inflammatory properties, whereas the mean particle size of muds was correlated with cell adhesion inhibition. The highest total phenolic content of mud extracts was correlated with antimicrobial properties and the most stable mud was found to present anti-aging properties by favoring the increase of keratinocytes. The most stable mud was also found to exhibit moisturizing antioxidant properties by boosting the conversion of hydrogen peroxidase to oxygen and water. Clearly, the physicochemical characteristics of the particles of the composite systems like muds are very important in determining their properties (cosmetic and therapeutic). In the present work, we have attempted not only to focus on the detailed characterization of a series of Greek muds but also to classify them according to their physicochemical characteristics. For comparison reasons, a sample of mud from Israel was also characterized. More specifically, XRD was used for the mineralogical characterization of the specimens and TGA for the assessment of their thermal behavior. The morphology examination and a preliminary particle size distribution assessment were done by SEM, while EDX microanalysis was used for the elemental analysis of the solid specimens. A number of the most important inorganic anions (F−, Cl−, NO3− and SO42−) at equilibrium of the solid phase with the aqueous supernatant were measured with ion chromatography using the appropriate standard solutions. The investigated muds were classified in groups according to their composition. Because of their documented antioxidative effect, total phenolic content (TPC) of the mud specimens was measured in liquid extracts using different extraction methods.
Materials and methods
Preparation of muds
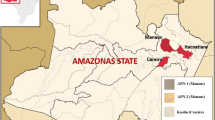
Twelve of the mud specimens, used in this work, were selected from Greek natural spa resorts and one from Israel (Dead Sea). Mud specimens #1, 2, 5, 6, 7 and 11 come from Messolonghi area, mud specimen #8 from Kyllini (Peloponesse) and mud specimen #10 from Corfu (Ionian Island). All three locations belong to Western Greece region. Mud specimens #3, 4 and 9 were selected from Kilkis, Kavala and Pieria correspondingly which belong to Macedonia (Northern Greece). Mud specimen #12 was selected from the island of Lemnos (Northeast Aegean) and mud specimen #13 from Israel (Dead Sea). The sampling domain from the specimens from Greece is shown in the map of Fig. 1. The collected samples were in the form of concentrated suspensions in water, except for mud specimens #10, 11 and #12 which were obtained as dried solids. It should be noted that mud specimens showed an apparent stability as a uniformly distributed solid suspended in the aqueous phase (Table 1). Prerequisite for the use of mud specimens in cosmetics or pharmaceutical products is lack of microbiological activity. The mud specimens were thus sterilized, prior to any characterization, in a steam sterilizer (Trayde Raypa) for 20 min at 121 °C and under pressure of ca. 1.5 atm. Preliminary examination showed that this treatment did not induce any mineralogical changes to the specimens. Past the sterilization process, the solvent was removed from the suspended solids by lyophilization (LyoQuest, Telstar, Beijer Electronics). The sterilization process was necessary because of the intended use of the mud specimens examined in pharmaceutical and cosmetic products, despite the fact that in general it is possible to have alterations through this treatment (Jager et al. 1969, Trevors 1996, Lotrario et al. 1995).
XRD and TGA methods
The mineralogical composition of sterilized and freeze-dried mud specimens was done using powder X-ray diffraction (XRD, D5000, Siemens). The positions of the XRD reflections obtained were used for the identification of the mineral components with reference to ICCD standard files. In the case of XRD measurements, in order to increase the accuracy of measurements, the samples were washed several times with deionized water and dried, for the removal of halite (NaCl) which was a product of the of seawater drying. TGA analysis was done with a thermal analysis analyzer (Q50) in nitrogen atmosphere, at a heating rate of 10 °C/min. The remaining percentage mass for the test specimens was defined as:
where Μi, Mo are the mass values at time ti and before specimen heating, respectively. The rate of mass loss M *i was calculated from the first derivative of M *i -t profiles:
The first derivative identified the temperature interval values (T1–T2) at which mass loss was completed and the temperature at which the rate of mass loss was maximized (inflection point) (Ti). The specific surface area (SSA) of the specimens was estimated from nitrogen absorption measurements (BET) for the muds before and after the removal of NaCl.
Preparation of samples for ion chromatography
The measurement of suspensions of the test mud specimens for the equilibrium concentration of F−, Cl−, NO3− and SO42− anions was done by ion chromatography (Dionex D-120) equipped with a IonPac analytical column AS16 with guard column AG16, and the eluent used was NaOH solution (5 mM). The fluid samples in which the concentration of the anions was measured, depended on the physical state of the specimens on collection. For the specimens in which the volume of supernatant liquid was insufficient (very dense suspensions), 6 g of lyophilized mud were suspended in 200 mL of water for 48 h under stirring, and past this period, the suspensions were vacuum filtered. For specimens in which the supernatant liquid of the collected samples was sufficient for the measurements, the mud suspensions were vacuum filtered, and analysis of the filtrate was done following appropriate dilution. Elemental analysis of the freeze-dried mud specimens for carbon, hydrogen, nitrogen and sulfur was done with an elemental analyzer (Carlo Erba CHNS 1108).
Samples preparation for the measurement of acid-soluble calcium
Calcite content was measured by two methods. In method #1, AAS was used for the measurement of the acid-soluble calcium concentration in four of the mud specimens studied. It was assumed that acid-soluble calcium corresponds to carbonate minerals (calcite). More specifically, 1 g of sterilized and lyophilized mud was suspended in 50 mL of 1 N nitric acid solution for 24 h under stirring. Next, the suspensions were vacuum filtered, and the total calcium concentration was measured with AAS. In method #2, the remainder of the solids of the suspensions of the specimens equilibrated with 1 N HNO3 solution was dried in an oven for 48 h at 37 °C. The weight difference from the initial sample mass yielded an estimate of the content of the solid in CaCO3 assuming that it was the only acid-soluble mineral. The assumption was supported from the mineralogical analysis data.
TPC measurements
TPC measurements were done in the supernatant aqueous phase of the as-received mud specimens, i.e., in the form of concentrated suspensions past the sterilization of the specimens, which was done shortly after samples delivery at the laboratory (within 3–4 days). The supernatant fluids from each specimen were separated from the respective solids by filtration (membrane filters, Sartorius 0.22 μm). The Folin–Ciocalteu method was used for TPC measurements. Briefly, in a glass test tube, 1 mL from the fluid was mixed with 6 mL of distilled water, and 0.5 mL of the Folin–Ciocalteu phenol’s reagent (Merck) were added. Next, 1.5 mL of Na2CO3 solution (1.89 M) and 1 mL of distilled water were added. The samples were thoroughly mixed in a vortex apparatus, and they were left to rest for 2 h in a dark place. Past this waiting period, light absorbance was measured at 760 nm in a UV/VIS spectrophotometer (Rayleigh UV 1601). Gallic acid standard solutions (Gallic acid monohydrate, Sigma-Aldrich) were used for the calibration in the concentration range between 7.5 and 250 mg/L (linear range) (Waterman and Mole 1994, Zagklis et al. 2015).
In order to investigate the effect of treatment of the mud specimens on the measurement of TPC, TPC was measured for three selected samples following the following procedures:
-
1.
Measurement directly in the supernatant of the sterilized specimens following separation of the liquid from the solid phase by filtration.
-
2.
Measurement in the filtrate of sterilized freeze-dried muds suspended in distilled water and equilibrated under stirring for 48 h.
-
3.
Measurement in the filtrate of sterilized and freeze-dried muds suspended in distilled water and equilibrated under stirring for 124 h.
-
4.
Measurement in the filtrate of sterilized freeze-dried mud specimens suspended in distilled water, sonicated for 10 min in an ultrasonic bath, followed by equilibration in water for 124 h.
-
5.
Measurement in the filtrate of sterilized muds separated from the initial liquid phase by centrifugation, followed by suspension in distilled water, sonication for 10 min in ultrasonic bath and equilibration for 48 h.
-
6.
Treatment as in 5 but in non-sterilized mud specimens.
Results and discussion
Mineralogical characterization
Mud specimens locations of origin, original physical state and SSA values are summarized in Table 1. Except for mud specimens #3 and #4, the rest of the mud specimens showed low SSA values (lower than 6 m2/g); however, when halite was removed, the SSA values were higher mainly in the case of specimens #3, 9 and 10. The measured SSA values are of the same order of magnitude with mud specimens measured in the area of Adriatic coast (Mihelcic et al. 2012) except for specimens #3, 4, 9 originated from Northern Greece and #10 originated from Corfu. The components identified by XRD are summarized in Table 2. Quartz, calcite and aluminosilicates were present in most of the muds. Calcite presents several properties and usually it is used as antacid, antidiarrheal, abrasive or as supplement, in pharmaceutical products or in cosmetics (Carretero and Pozo 2010). A possible use of muds containing calcite could be in products for skin peeling. TGA analysis profiles revealed several identical characteristics for the mud specimens (Figs. 2, 3 and Table 2), and the muds were classified into four groups. Concerning the inorganic component, XRD analysis showed that, mud specimens of group#1 (muds #2, 5, 6, 7, 11, 13) consisted of quartz, calcite, albite, muscovite, kaolinite (aluminosilicates) (Földvári 2011). Kaolinite is known in cosmetology for its anti-inflammatory properties, whereas muscovite presents moisturizing properties (Carretero and Pozo 2010). In Spilioti et al. (2016), special attention was given to the most stable mud (#6) which experimentally was found to present anti-inflammatory and moisturizing properties which now could be attributed to the presence of kaolinite and muscovite. This finding is consistent with the fact that TGA curves showed, among others, early mass losses between 400 and 700 °C which corresponds to mass loss due to the presence of organic compounds before the inflection corresponding to calcite decomposition to CaO + CO2 at 700 °C. According to XRD analysis, quartz, calcite, albite, muscovite, zeolite, nacrite, etc. (Table 2), were detected in mud specimens of group #2 (muds #3, 4, 12) again in agreement with respective TGA analysis showing mass loss in the temperature interval (300–700) °C. No further mass loss was found past 700 °C, suggesting very low concentrations or complete absence of calcite. Mud specimens of group #3 (muds #8, 10) yielded rather large mass loss at 701 °C for mud specimen #8, and 689 °C for mud specimen #10) which correspond to albite, muscovite and anorthite. Calcite detected by XRD analysis for the specimens of group #3 may be at low concentrations. Group #4 (mud specimens #9 and 1) were found to consist of gypsum, apart from quartz, calcite, albite, muscovite, etc., and the TGA analysis showed mass changes at 736 °C corresponding to the decomposition of CaSO4 to CaO + SO2.
Information for the relative concentration of organic and inorganic components of the mud specimens was obtained from TGA analysis of the specimens. Mass loss due to adsorbed humidity was neglected, ΔM *a defines the mass loss after the removal of crystalline water and before the inflection point of the TGA curves corresponding to calcite, and ΔM *b defines the mass loss corresponding to calcite decomposition. Mass changes during heating of the mud specimens (ΔM *a and ΔM *b ) are shown in Fig. 4. Calcite (Fig. 4) was absent or at very low concentrations in mud specimens of specimens groups #2, 3. The fraction of mass loss due to organic content, ΔM *o , corresponding to the temperature range before calcite decomposition, to the total mass loss (neglecting humidity and crystalline water) was calculated according to Eq. (3).
The calculated results obtained from Eq. (3) are depicted in Fig. 5, in which as may be seen the organic content fraction was higher for specimen groups #2 and #3. Thus, for these groups (mud specimens #3, 4, 8, 10, 12) the organic content is attributed to other components and not to the presence of CaCO3. In the case of group #1, the organic fraction was higher for specimens #2, 13, 5 and 11. It should be noted that the high organic content fraction was related to the high SiO2 fraction according to the mineralogical characterization, which showed that the mineral component of the mud specimens consisted of quartz and calcite. Concerning the sterilization process, it has been reported that it is possible that organics are released (mainly humics), and therefore, the reported values may be underestimated for the raw materials (Razavi Darbar and Lakzian 2007).
SEM images obtained for the 13 muds are presented in Fig. 6. In Spilioti et al. (2016), minimum, maximum and mean particle sizes are summarized. Differences in particles morphology are attributed to their origin and components. According to EDX microanalysis (Table 3), Si was found to be present in higher concentrations in mud specimens #3, 4 and 12 as already shown, whereas it was found in lower concentrations in mud specimens #1 and 13. Calcium concentration was higher for mud specimens #10, 5 and 1. High concentrations of F were found in mud specimens #2, 5, 6, 13. Mg concentration was significant in mud specimens #1 and 11. Al concentration was significant for mud specimens #3, 4, 8, 11 and 12. Fe was found in mud specimens #3, 4, 7, 8, 9, 10, 11 and 12. Ti was detected in mud specimen #8 collected near the beach of Kyllini a frequented bathing center. As a rule, when clays are present, the concentrations of Fe and Al are relatively high (Singh and Gilkes 1991, Healy and Wilson 1971).
Total calcium concentration
Since the main components of the test muds were SiO2 and CaCO3, in order to estimate CaCO3 concentration, the total calcium concentration (Cat) was measured using AAS in two samples from group #1 of mud specimens, in one sample from group #3 and in sample #13 (Israel). Specimens #2 and 6 contained low calcium concentrations, whereas mud specimen #10 yielded the highest calcium concentration (Table 4). The results obtained from the dissolution of the solids and AAS analysis were consistent with the results obtained from TGA, XRD and EDX analyses.
The estimate of the relative amount of CaCO3 using method #2 (i.e., the weight difference of the initial equilibrated with 1 N HNO3 solid phase and the dried remainder of the solids of the suspensions), showed that sample #13 contained the highest CaCO3 concentration (~ 82.6 wt%), for sample #6 CaCO3 concentration was 69 wt%, and for samples #2 and 6 it was 40 wt%.
The difference in the trends of concentrations between the two methods may be ascribed to the different content of soluble calcium (originating from the contact of the mud specimens with natural waters).
Inorganic anions and organic components of the mud specimens
Ion chromatographic analysis was done in the supernatant liquid of the original mud specimens #1–9, whereas the only treatment of the as-collected specimens was sterilization. For mud specimens #10, 11, 12 and 13 because of the very limited quantity of the supernatant fluid, suspensions of the sterilized and freeze-dried muds in water were used following the procedure discussed in 2.3. F− ions were found in samples #6, 10 and 11 (Table 5). However, EDX analysis showed that F was high in mud specimens #2, 5 and 6. For specimens #1–7 and #9–11, the high concentrations of Cl− ions found were attributed to the maturation fluid. Low concentration values of Cl− ions were found for specimens #4, 8, 12, 13. The EDX analysis (Table 3) gave medium to very low Cl concentration values for samples #2, 3, 4, 5, 10, 11, 12, 13. Samples #10 and #11 showed high concentration values of NO3− ions. In samples #9, 10 and 11, high concentration values of SO42− were found (Table 5). The differences between ion concentrations obtained by EDX and by IC are due to the fact they correspond to different material domains. EDX microanalysis is done in the solid and the concentrations of the elements reported refer to the solid. With IC, ions at equilibrium with the solid were measured. It is most likely that the ions are bound to the surface either in the form of minerals (at too low concentrations to be detected by XRD) or they are bound by adsorption. Since muds are used in suspensions, it is very interesting to know the composition of the aqueous fluid at equilibrium with mud. Several studies relate balneotherapy with sulfur water with the improvement in osteoarthritis (Errasfa and Harzy 2012, Bellometti et al. 1997, Benedetti et al. 2007). SO42− ion concentrations measured at equilibrium of water with most of the muds examined are much higher than those measured in thermal water samples from Saturnia spa (Centini et al. 2015, Benedetti et al. 2007). The increased SO42− content corresponded apparently to higher sulfur content of the muds, which upon equilibration in the presence of oxygen was oxidized. SO42− ion concentration therefore in equilibrium with the respective mud specimens should be considered as an indicator of the corresponding beneficial effect of the mud specimens. It should also be noted that PSD is an important parameter of the test mud specimens as it determines their rheological properties, applicability on surfaces, absorbability of beneficial agents and the stability of the respective suspensions.
The elemental analysis results for Ν, C, Η and S are summarized in Table 6. Organic carbon is present at high concentrations in all mud specimens except for samples #2, 9 and 12. The highest nitrogen concentration values were found for samples #2, 11 and 13, and the highest sulfur concentration values were found for samples #2 and 9. Sulfur is often used in cosmetics (creams, lotions, etc.) due to the antiseptic and disinfectant properties it presents, whereas it is known as a keratolytic reducer (Carretero and Pozo 2010).
TPC measurements
The antioxidant properties of phenols could be one more criterion for the selection of the mud specimen which could possibly be beneficial in potential use in cosmetics or in pharmaceutical products (Rice-Evans et al. 1997; Sarbon et al. 2015; Spilioti et al. 2016). The results of TPC measurements in the supernatant aqueous phase of the sterilized mud specimens are summarized in Table 7 for samples #1–9. For samples #10, 11 and 12, measurements of TPC were not possible since the specimens were received in the form of dry solids. In the supernatant fluid of samples #1 and 9, TPC was below detection limit. Samples #5, 6, 7 contained higher TPC amounts in comparison with samples #2, 3, 4, 8. Worthy to note that in Spilioti et al. (2016), TPC was measured in the muds extracts obtained from the solid phase, and the highest value was found for specimen #3 (40.5 mg/L), whereas median TPC values were also found for specimens #1, 6, 9, 11, 13 (5.5–15 mg/L). Mud #6 presents the higher TPC value in the supernatant fluid (98.4 mg/L) in comparison with the other muds and low value (5.5 mg/L) in the mud extract (Spilioti et al. 2016). In Centini et al. (2015), the organic matter of thermal muds was investigated in relation with maturation time; however, to our knowledge, there are no TPC measurements in natural spa muds in the literature. The differences between the TPC concentration values measured in the specimens were attributed to their origin. The mud specimens were selected from different regions, and consequently phenolic compounds contained were of variable molecular weight. The identification of phenolic compounds of the most suitable muds with the higher TPC concentration should be planned in future works.
The specimen pre-treatment methods, such as use of high temperature or sonication, may affect the measured TPC in different ways depending on the molecular weight of the contained phenols (Annegowda et al. 2012; Réblová 2012; Sharma et al. 2015). Thus, it was further attempted to investigate the effect of the specimens pre-treatment on the measured TPC. The TPC values obtained for the different methods are summarized in Table 8. As may be seen from the results for TPC analysis following pre-treatment methods 5 and 6 (“TPC measurements” section), sterilization at 121 °C resulted in TPC reduction possibly because of thermal decomposition of phenolic compounds at the temperatures employed in the sterilization of the specimens. Comparison of the results of TPC analysis for the specimens treated according to methods 3 and 4, the sonication treatment of the specimens did not contribute to any significant extent to the desorption of phenols from the suspended solids. The comparison of results from methods 2 and 5 showed that lyophilization affected TPC only in the case of mud specimen #6.
Conclusions
Muds from Greek natural spa resorts and from Israel (Dead Sea) were characterized in detail with respect to their mineralogical and chemical composition. The characterization aimed at their classification based on their principal components. The mineralogical characterization was done with XRD. TGA analysis completed the information of the extent of inorganic–organic components of the mud specimens. EDX and elemental analyses were performed on the solids and the concentration of F−, Cl−, NO3− and SO42− ions in equilibrium with water was measured. This analysis helped in the classification of the mud specimens. According to their mineral content, the muds could be possibly used for the development of novel cosmetics and/or pharmaceutical products. Muds components (calcite, kaolinite, muscovite, sulfur) give them antacid, abrasive, anti-inflammatory, antiseptic and disinfectant attributes. The antioxidant properties of muds could be also related with high TPC values. In this aspect, samples #5, 6 and 7 from Messolonghi area are the most promising. According to the TPC measurements on differently pre-treated samples, steam sterilization of muds seems to reduce significantly the TPC concentration. The use of radiation as a sterilization method is recommended for the preservation of the high TPC of the mud specimens. It should be noted that the highest TPC levels were measured in the aqueous medium in equilibrium with the mud specimens (not in the solid phase), suggesting that phenolic compounds, do leach out of the solid matrix, a parameter most important for therapeutic/cosmetic application of muds.
References
Annegowda, H. V., Bhat, R., Min-Tze, L., Karim, A. A., & Mansor, S. M. (2012). Influence of sonication treatments and extraction solvents on the phenolics and antioxidants in star fruits. Journal of Food Science and Technology, 49(4), 510–514.
Bautista-Banos, S., Hernandez-Lauzardo, A. N., Velazquez-del Valle, M. G., Hernandez-Lopez, M., Ait Barka, E., Bosquez-Molina, E., et al. (2006). Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Protection, 25(2), 108–118.
Bellometti, S., Giannini, S., Sartori, L., & Crepaldi, G. (1997). Cytokine levels in osteoarthrosis patients undergoing mud bath therapy. International Journal of Clinical Pharmacology Research, 17(4), 149–153.
Benedetti, S., Pagliarani, S., Benvenuti, F., Marini, D., Galli, T., Oliva, F., et al. (2007). Antioxidative effects of sulphurous water from macerata feltria thermal resort in patients with osteoarthritis. Progress in Nutrition, 9(1), 1–7.
Carretero, M. I., & Pozo, M. (2010). Clay and non-clay minerals in the pharmaceutical and cosmetic industries Part II. Active ingredients. Applied Clay Science, 47, 171–181.
Carrretero, M. I., Gomes, C. S. F., & Tateo, F. (2006). Clays and human health. Handbook of Clay Science, 1, 717–741.
Çelik Karakaya, M., Karakaya, N., Sarıoğlan, Ş., & Koral, M. (2010). Some properties of thermal muds of some spas in Turkey. Applied Clay Science, 48(3), 531–537.
Centini, M., Tredici, M. R., Biondi, N., Buonocore, A., Maffei Facino, R., & Anselmi, C. (2015). Thermal mud maturation: organic matter and biological activity. International Journal of Cosmetic Science, 37, 339–347.
Dolmaa, G., Tserenpil, Sh, Ugtakhbayar, O., Shevchenko, S. G., Kliba, L. V., & Voronkov, M. G. (2009). Characterization and organic compounds in peloids from Mongolia. Proceedings of the Mongolian Academy of Sciences, 4, 3–21.
Działo, M., Mierziak, J., Korzun, U., Preisner, M., Szopa, J., & Kulma, A. (2016). The potential of plant phenolics in prevention and therapy of skin disorders. International Journal of Molecular Sciences, 17(2), 160–201.
Errasfa, M., & Harzy, T. (2012). Sulphur thermal water improves blood lipids but not total anti-oxidant capacity in knee osteoarthritis patients. Shiraz E-Medical J., 13(2), 54–58.
Fakhfakh, E., Chakroun, I., Chaari, I., Medhioub, M., Rocha, F., Gomes, C., et al. (2005). Chemical and physical characterization of some Tunisian smectites for human healing use. Acta Geodynamics et Geomaterialia, 2(2), 138.
Ferrell, R. E. (2008). Medicinal clay and spiritual healing. Clays and Clay Minerals, 56(6), 751–760.
Földvári, M. (2011). Handbook of thermogravimetric system of minerals and its use in geological practice. Occasional Papers of the Geological Institute of Hungary, 213, 1–180.
Gomes, C., Carretero, M. I., Pozo, M., Maraver, F., Cantista, P., Armijo, F., et al. (2013). Peloids and pelotherapy: Historical evolution, classification and glossary. Applied Clay Science, 75–76, 28–38.
Harvey, A. L., Edrada-Ebel, R., & Quinn, R. J. (2015). The re-emergence of natural products for drug discovery in the genomics era. Nature Reviews Drug Discovery, 14(2), 111–129.
Hattori, I. (1963). Pelotherapy. In S. Licht (Ed.), Medical hydrology (pp. 273–290). USA: Connecticut.
Healy, W. B., & Wilson, G. F. (1971). Deposits of Rumen Epithelium associated with the ingestion of soil. New Zealand Journal of Agricultural Research, 14(1), 122–131.
Hill, M. J. (1997). Intestinal flora and endogenous vitamin synthesis. European Journal Cancer Prevention, 6(1), 43–45.
Jager, G., van der Boon, J., & Rauw, G. J. G. (1969). The influence of soil steaming on some properties of the soil on the growth and heading of winter glasshouse lettuce. I. Changes in the chemical and physical properties. Netherlands Journal of Agricultural Science, 17, 143–152.
Kerndorff, H., & Schnitzer, M. (1980). Sorption of metals on humic acid. Geochimica et Cosmochimica Acta, 44(11), 1701–1708.
Khiari, I., Mefteh, S., Sánchez-Espejo, R., Cerezo, P., Aguzzi, C., López-Galindo, A., et al. (2014). Study of traditional Tunisian medina clays used in therapeutic and cosmetic mud-packs. Applied Clay Science, 101, 141–148.
Lotrario, J. B., Stuart, B. J., Lam, T., Arands, R. R., O’Connor, O. A., & Kosson, D. S. (1995). Effects of sterilization methods on the physical characteristics of soil—implications for sorption isotherm analyses. Bulletin of Environmental Contamination and Toxicology, 54(5), 668–675.
Mihelcic, G., Kniewald, G., Ivanisevic, G., Cepelak, R., Mihelcic, V., & Vdovic, N. (2012). Physico-chemical characteristics of the peloid mud from Morinje Bay (eastern Adriatic coast, Croatia): Suitability for use in balneotherapy. Environmental Geochemistry and Health, 34(2), 191–198.
Odabaşı, E. (2014). Thermal mud molecular overview. TAF Preventive Medicine Bulletin, 13(3), 257–264.
Razavi Darbar, S., & Lakzian, A. (2007). Evaluation of chemical and biological consequences of soil sterilization methods. Caspian Journal of Environmental Sciences, 5(2), 87–91.
Rebelo, M., Viseras, C., López-Galindo, A., Rocha, F., & Ferreira da Silva, E. (2011). Characterization of Portuguese geological materials to be used in medical hydrology. Applied Clay Science, 51(3), 258–266.
Réblová, Z. (2012). Effect of temperature on the antioxidant activity of phenolic acids. Czech Journal of Food Sciences, 30(2), 171–177.
Rice-Evans, C. A., Miller, N. J., & Paganga, G. (1997). Antioxidant properties of phenolic Compounds. Trends in Plant Science, 2(4), 152–159.
Sarbon, N. M., Sandanamsamy, S., Kamaruzaman, S. F. S., & Ahmad, F. (2015). Chitosan extracted from mud crab (Scylla olivicea) shells: Physicochemical and antioxidant properties. Journal of Food Science and Technology, 52(7), 4266–4275.
Setnescu, T., Bancuta, J., Setnescu, R., Bancuta, R., Chilian, A., Bumbac, M., et al. (2013). Characterization of therapeutic muds collected at different sites in Roumania. Revue Roumaine de Chimie, 58(7–8), 599–610.
Sharma, K., Ko, E. Y., Assefa, A. D., Ha, S., Nile, S. H., Lee, E. T., et al. (2015). Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. Journal of Food and Drug Analysis, 23(2), 243–252.
Singh, B., & Gilkes, R. J. (1991). Concentration of iron oxides from soil clays by 5 m NaOH treatment: the complete removal of sodalite and kaolin. Clay Minerals, 26, 463–472.
Spilioti, E., Vargiami, M., Letsiou, S., Gardikis, K., Sygouni, V., Koutsoukos, P., et al. (2016). Biological properties of mud extracts derived from various spa resorts. Environmental Geochemistry and Health, 39(4), 821–833.
Trevors, J. (1996). Sterilization and inhibition of microbial activity in soil. Journal of Microbiological Methods, 26(1–2), 53–59.
Tserenpil, Sh, Dolmaa, G., & Voronkov, M. G. (2010). Organic matters in healing muds from Mongolia. Applied Clay Science, 49(1–2), 55–63.
Viseras, C., & Lopez-Galindo, A. (1999). Pharmaceutical applications of some spanish clays sepiolite, palygorskite, bentonite: some preformulation studies. Applied Clay Science, 14(1), 69–82.
Waterman, P. G., & Mole, S. (1994). Analysis of phenolic plant metabolites. In J. H. Lawton & G. E. Likens (Eds.), Methods in ecology. Oxford: Blackwell.
Zagklis, D. P., Vavouraki, A. I., Kornaros, M. E., & Paraskeva, C. A. (2015). Purification of olive mill wastewater phenols through membrane filtration and resin adsorption/desorption. Journal of Hazardous Materials, 285, 69–76.
Acknowledgements
This research has been co-financed by the European Union (European Social Fund-ESF) and Greek Secretariat of Research and Technology (NSRF 2013–2015) under the R&T Cooperation between Greece and Israel: “Physicochemical and Biological Characterization and Improvement of natural muds to produce high-added value products (Pharmamuds).” We specially thank Mr. George Georgiadis (Physis & Ousia Co.) for providing the mud specimens from Messolonghi and Ms. Liora Chilron (Anna Lotan Co.) for providing the mud sample from Israel. We also thank Mr. Venetatos (Pikrolimni Spa Co.), Pilotherapia Co., Mr. S. Nikoloutsopoulos, the municipality of Pydna and Mr. Giarmadouros for providing the mud samples from Kilkis, Kavala, Kyllini, Pieria and Lemnos correspondingly. Finally, we thank Associate Professor Michael Kornaros and the laboratory of “Biochemical Engineering & Environmental Technology (LBEET)” Chemical Engineering Department, University of Patras, for the use of laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamitsou, M.D., Sygouni, V., Kanellopoulou, D.G. et al. Physicochemical characterization of sterilized muds for pharmaceutics/cosmetics applications. Environ Geochem Health 40, 1449–1464 (2018). https://doi.org/10.1007/s10653-017-0066-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-017-0066-8