Abstract
This study investigates the mercury (Hg) contaminations in soil and foodstuffs along the artisanal gold mining areas, Gilgit-Baltistan Province, Pakistan. For this purpose, soils were analyzed for Hg concentrations and evaluated for the enrichment/contamination using enrichment factor or contamination factors (CF). The CF values ranged from 18.9 to 153 showed multifold higher levels of Hg contamination as compared to background or reference site. Foodstuffs including vegetables, seeds or grains and fish muscles showed Hg accumulation. Results revealed that Hg concentrations in foodstuffs were higher than the critical human health value set by European Union. The Hg in foodstuffs was consumed and, therefore, evaluated for the risk assessment indices using the daily intake (DI) and health risk index (HRI) for the exposed human population both children and adults. Results of this study revealed that cumulative HRI values through foodstuffs consumption were <1 (within safe limit), but if the current practices continued, then the Hg contamination could pose potential threat to exposed population in near future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) is a global toxin with great health concerns, which is released to ecosystem from both natural and anthropogenic sources (Dai et al. 2013; Niane et al. 2015). Natural sources are weathering and erosion of metallic Hg and sulfide ores like cinnabar HgS and anthropogenic sources include mining, industries and agriculture activities (Pinedo-Hernández et al. 2015; Yang et al. 2015). However, various studies documented that inadequate processing, e.g., artisanal gold mining, is the largest (37%) source of global mercury emission (Biber 2012; Gibb and O’Leary 2014; UNEP 2013). During processes, the Hg forms amalgam with gold. Then, amalgam is heated that could escape the Hg to the atmospheric environment and deposited on various compartments like surrounding soils and aquatic ecosystems (Biber et al. 2015; Rodrigues et al. 2014).
Human populations living in close vicinity along the artisanal gold mining areas are more susceptible to Hg exposure (Rodrigues et al. 2014). Inorganic Hg might be mobilized in the environmental compartments like water and air and could be transformed by anaerobic organisms into methyl mercury (MeHg) including monomethylmercury (MMeHg; CH3Hg+) and dimethylmercury (DMeHg; (CH3)2Hg) under favorable conditions (Leopold et al. 2010). The MeHg in water is absorbed by phytoplankton and then ingested by zooplankton and fish, thereby contaminating aquatic food chain (Gibb and O’Leary 2014; WHO 2007). The Hg could be deposited into soil from the air directly or irrigation of Hg-contaminated water. Plants have the ability to extract heavy metal(loid)s and accumulate them in their tissues. Green plants and leafy vegetables have higher ability for heavy metal(loid)s accumulation and may contaminate the terrestrial food chain (Brindha et al. 2016; Rodrigues et al. 2014; Zhong et al. 2017). Both Hg and MeHg are harmful to the central and peripheral nervous system. Harmful effects resulting from the inhalation of Hg include digestive, lungs and kidneys problems, immune systems and nervous system problem and may be fatal (WHO 2007). Children and developing fetus are more susceptible and may be exposed to MeHg causing neurodevelopmental problems. The symptoms of neurodevelopmental problems include mental retardation, delayed development, vision and hearing loss, language disorders, seizures and memory loss. Acrodynia a condition of red and painful extremities in children has been reported the chronic Hg exposure (Gibb and O’Leary 2014; WHO 2007, 2008).
Recently, a number of studies have focused on the Hg contaminations and its potential risk assessment resulted from the artisanal gold mining and extraction areas (Dai et al. 2013; Niane et al. 2015). Small-scale and artisanal gold mining and extraction practices are one of the leading causes of Hg release to aquatic ecosystems along the Hunza and Gilgit rivers, Northern Pakistan, during last few decades (Biber 2012; Riaz et al. 2016). River water is commonly used for irrigation of agriculture soils. Thus, it is necessary to investigate the Hg concentrations in soils and growing foodstuffs and evaluate the potential health risk through consumption of contaminated foodstuffs. Therefore, this study was aimed to investigate (1) Hg contaminations in agricultural soil and its enrichment/contamination using enrichment/contamination factors (EF)/(CF) and transfer factors (TFs); (2) Hg contaminations in foodstuffs including fish muscles, vegetables and cereal crops; and (3) evaluation of potential health risk of the local people in Gilgit-Baltistan Province.
Materials and methods
Study area
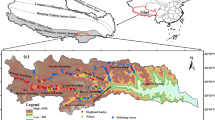
The study area is located in Gilgit-Baltistan Province formerly known as Northern Areas of Pakistan, having a total area of 72,971 km2 and population of 1.44 million. This area is famous for rivers including Indus, Gilgit and Hunza, high mountain peaks such as K-2 (above 8000 m), Deosai plain (second highest plateau after Tibetan Plateau, 4115 m), largest glaciers. Last and the most important from geological point of view is meeting point of the world’s three large mountainous ranges such as Karakorum, Hindu Kush and Himalayas. This region is rich in natural resources, rare plants and animals. Mineral resources including gold, copper and iron are also present in this area. Local population has various occupations including agriculture, government jobs, mining and gold extraction along the Hunza and Gilgit rivers (Khan et al. 2010).
Gold placer deposits along Indus River in the Gilgit-Baltistan (formally known Northern Areas) have been reported by Austro mineral in 1976 and 1978. Gold extraction is carried out by simple and basic methods of panning and amalgamation using the mercury as scavenger (Shah and Khan 2004).
Sample collection
Food plants including vegetables (n = 40), cereals (n = 19), beans (n = 6) and soil samples (n = 65) were randomly collected from the agriculture lands in 5–12 replicates sites to cover most of the study area along Hunza and Gilgit rivers having latitude 35°31′–36°09′N and longitude 73°24′–74°54′E (Table 1; Fig. 1). From agriculture fields, each food and soil sample was composite of three samples within 20 m radius that constitute one sample along a studied site. Plants along with replicates were uprooted, and the loose soil particles were removed from the roots by shaking. Each plant sample was properly numbered, stored in kraft paper and the location was marked using Global Positioning System (GPS), and transported to laboratory. In the study area, food plants are routinely washed before cooking or eating. The Hg attached with plants surface or dust particles could result in wrong interpretation of results for exposure risk assessment through food plants consumption. Therefore, plants samples were washed with tap water and finally with deionized water (DIW) and then separated into roots, stems, shoots, leaves, flowers and seeds. Each part of plant samples were oven-dried at 60 °C for 24 h. Plants samples were powdered using mechanical stainless steel grinder and preserved in polyethylene zip bags for further analyses.
Surface soils (0–20 cm depth, 1 kg) were picked from the base of each collected plant. Stones and twigs were removed in field, and then, they were stored in polyethylene zip bags, labeled and transported to laboratory. Soil samples were dried and sieved through 200 µ mesh before chemical analyses. Reference site samples were collected from agriculture fields in rural environment located along Chilas site having latitude N 35º25′ and longitude E 72º21′ about 83 km away from the gold extraction areas (contaminated sites) and used for Hg quantification and to assess the CF.
Fish trout (Salmo trutta) species (n = 12) were collected and stored in plastic bags and kept in liquid nitrogen during transportation to laboratory. In the study area, eating whole fish is not a practice and local people eat only muscles; therefore, fish muscles were separated, kept in clean, sterilized and properly capped bottles at −20 °C before extraction and analysis.
To avoid contamination, the sample collection tools including cutter, scissor, hoe and auger were washed with tap water and DIW and cleaned with tissue paper along with new disposable gloves and polyethylene zip bags before collection of each sample.
Sample preparation and chemical analysis
Food plant samples along with certified reference materials were extracted according to the method adopted from the Rasmussen et al. (1991) and Zheng et al. (2007). Briefly, dry powdered samples (2 g of each plant part) were mixed with 10 ml of concentrated nitric acid (HNO3) in glass beakers and left overnight. Next morning, the mixture of sample and HNO3 in beakers were heated on hot plate at 100 °C until get transparent solution and allowed for cooling. After cooling, add 4 ml of perchloric acid (HClO4) and heat for few minutes. Then, add 10 ml of HNO3 and heat till small portion of solution left behind. Solutions were cooled and filtered through glass microfiber filter paper and diluted up to 50 ml with DIW. For soil, we used aqua regia including hydrochloric acid (HCl) and HNO3 having ratio (3:1) as adopted by Dai et al. (2013) with some modification for analysis of Hg. Briefly, dry powdered soil sample (1 g) in a Teflon beaker was mixed with 10 ml aqua regia and heated at 100–120 °C until small portion left behind. Soil solutions were cooled, and then, add 20 ml of 2 N HCl and heat for few minutes. After filtration through glass microfiber filter paper, it was diluted up to 30 ml with DIW (Khan et al. 2010).
Fish samples were prepared and extracted according to the method adopted by Voegborlo and Akagi (2007). Briefly, 0.5 g sample was mixed with 1 ml H2O in a beaker, then with mixture of 1 ml HNO3, 1 ml HClO4 and 5 ml sulfuric acid (H2SO4) and heated at 100 °C. Extract was cooled and filtered through glass microfiber filter paper and diluted up to 50 ml with DIW.
Prepared samples of foodstuffs and soil were analyzed for Hg by the atomic absorption spectrophotometer (AAS, PerkinElmer-700) equipped with mercury hydride system (MHS-15) using sodium tetrahydrobromate (NaBH4) as a reducing agent with HCl. These analyses were performed in the Geochemistry laboratory of National Center of Excellence in Geology, University of Peshawar, Pakistan.
Quality assurance and control
For quality assurance and control, the certified reference materials for vegetables, cabbage GBW10014 (GSB-5), spinach GBW10015 (GSB-6), rice GBW10010 (GSB-1) and soil GBW07404 (GSS-4), and blanks were used in triplicates. The recoveries of Hg in certified reference materials were in good agreements and ranged from 83.4 ± 7.5 to 103.5 ± 6.2% for vegetable and soil, respectively. During analyses, the precision and bias were less than 20%. For AAS calibration, the standard solutions were prepared from Fluka Kamica (Buchs, Switzerland) in acidic medium (5.0% v/v nitric acid with a few drops of KMnO4) and used for calibration curve. Standards and blanks were measured with regular interval of every ten samples to check results accuracy and precision. These analyses were performed under standard operating conditions having r > 0.999 and detection limit of 0.009 µg kg−1. All chemicals and regents used in the analyses were of analytical grade, and glasswares were washed with 2% HNO3 and dried in oven before use.
Transfer factors (TFs) calculation
The concentrations of Hg in extracts of soil and plant parts including roots, stems, leaves and seeds/fruit were calculated on dry weight basis. Plant TFs were adopted from elsewhere (Ávila et al. 2017; Khan et al. 2014, 2016a) and calculated using following equation:
where C plant and C soil represent the concentrations of Hg in extracts of plant parts and soil, respectively, while TFs showed the uptake from soil to roots, stems, leaves and seeds/fruit.
Contamination factors (CFs)
Soil pollution was measured for Hg using the CFs technique depending on Hg concentration. The following equation was used to determine the CFs as adopted by (Muhammad et al. 2013).
where C soil represents Hg concentration in the field soil of contaminated area, while C reference represents the concentration in reference or control soil (Feng et al. 2007).
Risk assessment
Basic information about age, body weight, smoking, food habits and health problems of local people was collected during field visit (Table 2). In this region, around twelve hundred (1200) families are involved in artisanal gold mining and extraction. Basic information together with the Hg concentrations in foodstuffs was used for the risk assessment via average daily intake (DI) and health risk index (HRI) (Cheng et al. 2015; Islam et al. 2017).
Daily intake
The following equation was used to determine the values of DI for Hg:
where C Hg, C factor, D food intake and BWaverage represent the Hg concentrations in edible parts of foodstuffs (mg kg−1), conversion factor, daily intake of vegetables and average body weight, respectively. Conversion factor 0.085 was used to convert the fresh vegetable into dry weight (Khan et al. 2010, 2013).
Health risk index
Health risk indices (HRI) were calculated for consumption of Hg-contaminated foodstuffs, and the average DI of Hg was divided by the oral reference dose (RfD) of total mercury (THg), i.e., 5 µg kg−1 per week (Ávila et al. 2017; Rodrigues et al. 2014). The values of HRI < 1 mean that Hg intake is within safe limit, but if it is >1, then it shows high risk of the exposed population.
Data analyses
Analytical data were treated to calculate their mean and standard deviation values using the Microsoft Office Excel (Microsoft office, 2007). Pearson correlations between the Hg concentrations in soils and their standing food plants were measured by SPSS (version 21) (SPSS Inc., Chicago, IL, USA).
Results
Mercury concentrations in soil and foodstuffs
The concentrations of Hg in foodstuffs and soil are summarized in Table 1. The highest Hg mean concentration (271 µg kg−1) was observed in the onion field soil and the lowest in tomato (34.2 µg kg−1). Results revealed multifold higher CF values in the study area as compared to reference or non-contaminated soil limit (average = 2 µg kg−1). Plant parts showed great variation in the uptake of Hg. Parts of plant that showed the highest Hg mean concentrations were wheat root (64.2 µg kg−1), onion stem (31.0 µg kg−1), marijuana leaves (80.7 µg kg−1) and lettuce fruit (79.9 µg kg−1), while the lowest for lettuce root, (3.63 µg kg−1), marijuana stem (0.93 µg kg−1), cabbage leaves (0.45 µg kg−1) and pea seeds (6.81 µg kg−1). Fish muscle showed the Hg mean concentration as 13.5 µg kg−1 (Table 1). This concentration of Hg in fish muscles was found lower than that reported by Castilhos et al. (2015) for the Creporizinho fish (0.36 ± 0.33 μg g−1) in Brazil.
Transfer factors
Transfer factors (TFs) values for soil to root were the highest (0.62) for wheat and the lowest (0.03) for lettuce. Similarly, the highest TFs values for stem, leaves and fruits/seeds were in the cabbage (0.31), pea (0.67) and peppermint (0.86), while the lowest in lettuce (0.03), cabbage (0.03) and maize (0.08), respectively (Table 3). Results showed that in plant the Hg accumulation was higher in roots of wheat, maize, cabbage and tomato, stem of onion, leaves of marijuana and pea, seeds/fruits of peppermint and lettuce as compared to other parts and revealed their hazardous portion.
Risk assessment
Mercury concentrations in the foodstuffs were used to evaluate the potential risk assessment of local population (adults and children) in the study area, and the results are summarized (Figs. 2, 3). The highest DI values were observed for lettuce and the lowest for cabbage and tomato (Fig. 2). Furthermore, the children intakes of Hg though food consumption were higher than those of adults. The DI values of Hg were evaluated for the chronic or non-carcinogenic risk using HRI. In the study area, results revealed that HRI values were found <1 (safe limit) for individual food; however, collectively the HRI values through all selected food consumption were found close to 1 (Fig. 3).
Mercury concentrations in the plants and respective soil samples were analyzed for correlation matrix using the Pearson correlation, and results are summarized in Table 4. Significant correlations were observed between the seeds or fruit of wheat (r = 0.855) and maize (r = 0.740) and their respective soils. Similarly, significantly positive correlations were existed between the cabbage leaves (0.961) and respective soil (Table 4).
Discussion
Mercury concentrations showed higher variation in soils of the study area. Average Hg concentrations in soils were multifold (18.9–153) higher than the reference or control values. Higher Hg concentrations in soil could be attributed to the local artisanal gold mining practices. In artisanal gold mining practices, the Hg is used to extract gold from ore to form amalgam. The amalgam is heated, evaporating the Hg from mixture. The vaporized Hg sooner or later settles in soil and the lakes and rivers causing water and soil contamination (AMAP/UNEP 2013; Biber et al. 2015; Gibb and O’Leary 2014). Besides that, litter fall is another source of Hg contaminations into soil (Dai et al. 2013). Moreover, elevated Hg concentrations in agriculture soil could be attributed to long-term Hg-contaminated water irrigation practices (Qiu et al. 2008).
Soil ecosystem acts as a potential source and sinks in the biogeochemical cycle of Hg, owing to their dynamic interaction with other environmental compartments (Kim and Lindberg 1995). Most of the continental Hg deposition occurs in soils and could be bounded to soil organic matters (Fitzgerald Jr 1995; Lindqvist 1991). Microbial activity and soil characteristics make the inorganic Hg to more mobile and toxic forms (Lindqvist 1991; Ullrich et al. 2001). Elevated level of Hg contaminations in soil could be resulted in higher uptake of growing plant tissue such as roots and shoots (Dai et al. 2013; Meng et al. 2012), and higher accumulation (Table 1). The studied plants showed great variation of Hg concentrations among the species and their various tissues. Variation in Hg concentrations among plants and their tissues was attributed to the variation in species nature, Hg contaminations and soil characteristics (Brioschi et al. 2013; Delgado et al. 2012).
Previously, several studies have been focused on Hg contamination in rice which is the main food item particularly in the country like China (Feng et al. 2007; Li et al. 2015), but in the selected study area rice is rarely cultivated as compared to other foodstuffs. Due to this reason, the rice was not included in this study. Furthermore, methyl Hg (MeHg) is more toxic than inorganic Hg, and the human beings could be exposed to it through rice and fish. In the present study, total Hg concentration was quantified in the selected foodstuffs which can provide a baseline study for further research work, particularly related to organic Hg such as MeHg in the fish of Gilgit.
The Pearson correlation values between the soils and grown plants were greatly varied (high to low and positive to negative) in the study area (Table 4). Significant positive correlations were observed between the seeds of wheat (r = 0.855) and maize (r = 0.740), cabbage leaves (0.961) and their respective soils. Positive correlation of plants and respective soil means that the accumulation of Hg could be positively enhanced if the concentration increased in soil and vice versa. Majority of various plant parts showed weak correlations that could be attributed to the plant types and their physiologies and soil different properties (Shah et al. 2014). Being a highly volatile element, Hg escapes to air after uptake and bioaccumulation in plant tissues, particularly from leaves which could be another reason of weak correlation (Bash et al. 2007; Hussein et al. 2007).
Mercury concentrations in foodstuffs are the primary pathway of local population exposure (Hussain et al. 2014; Zhang et al. 2010). In the study area, the children revealed higher average DI of Hg concentration as compared to adults (Fig. 2). Higher intake of Hg concentrations in children could be resulted in higher HRI values than in adults (Fig. 3). Children are considered as one of the most vulnerable population to environmental threats. However, children exposure is often higher due to childhood behaviors and body weight that make them more susceptible to exposures (Bose-O’Reilly et al. 2010; Weiss 2000). Results revealed that HRI values for individual food are <1; however, local population use these foods collectively. That is why, collective food HRI values were calculated and found close to 1 for children and could surpass this limit in near future if the current practices continued. HRI values >1 (exceeded the safe limit) could produce the chronic risk in the exposed population (Khan et al. 2016b). In this study, the HRI values were found higher than those reported by Cheng et al. (2015) for the exposed population via fish consumption. Higher HRI values for children as compared to adults of this study were consistent with those reported by Yang et al. (2015). Higher HRI values in the study area were attributed to the local artisanal gold mining practices. This higher HRI values could be resulted in various chronic and acute toxicity problems in the exposed human population.
Conclusion
Artisanal gold mining practices released Hg into the local environment of Gilgit-Baltistan Province. Soil acts as a potential sink; therefore, the Hg is released into air deposits on the surrounding land. This deposition could be resulted in higher contamination of farm land soils and subsequently uptake by the growing food plants. Once Hg is accumulated in foodstuffs, then it becomes part of food chain and poses potential threat to human beings. Therefore, DI and HRI values through consumption of foodstuffs were calculated in the study area. This study concluded that HRI values for children were higher than for adults and could be a matter of great concern to the local exposed population in the near future, if the current Hg practices continued in such un-environmental friendly way.
References
AMAP/UNEP (Arctic Monitoring and Assessment Programme/United Nations Environment Programme). (2013). Technical background report for the global mercury assessment.
Ávila, P. F., Ferreira da Silva, E., & Candeias, C. (2017). Health risk assessment through consumption of vegetables rich in heavy metals: The case study of the surrounding villages from Panasqueira mine Central Potugal. Environmental Geochemistry and Health, 39, 565–589.
Bash, J. O., Bresnahan, P., & Miller, D. R. (2007). Dynamic surface interface exchanges of mercury: A review and compartmentalized modeling framework. Journal of Applied Meteorology and Climatology, 46, 1606–1618.
Biber, K. (2012). Investigation of the source, fate, and transport of mercury in Hunza River. Pakistan: Northern Areas.
Biber, K., Khan, S. D., & Shah, M. T. (2015). The source and fate of sediment and mercury in Hunza River basin, Northern Areas, Pakistan. Hydrological Processes, 29, 579–587.
Bose-O’Reilly, S., McCarty, K. M., Steckling, N., & Lettmeier, B. (2010). Mercury exposure and children’s health. Current Problems in Pediatric and Adolescent Health Care, 40, 186–215.
Brindha, K., Pavelic, P., Sotoukee, T., Douangsavanh, S., & Elango, L. (2016). Geochemical characteristics and groundwater quality in the Vientiane Plain Laos. Exposure and Health, 9, 1–16.
Brioschi, L., Steinmann, M., Lucot, E., Pierret, M.-C., Stille, P., Prunier, J., et al. (2013). Transfer of rare earth elements (REE) from natural soil to plant systems: Implications for the environmental availability of anthropogenic REE. Plant and Soil, 366, 143–163.
Castilhos, Z., et al. (2015). Human exposure and risk assessment associated with mercury contamination in artisanal gold mining areas in the Brazilian Amazon. Environmental Science and Pollution Research, 22, 11255–11264.
Cheng, Z., et al. (2015). Environmental mercury concentrations in cultured low-trophic-level fish using food waste-based diets. Environmental Science and Pollution Research, 22, 495–507.
Dai, Z., et al. (2013). Assessing anthropogenic sources of mercury in soil in Wanshan Hg mining area, Guizhou, China. Environmental Science and Pollution Research, 20, 7560–7569.
Delgado, J., Pérez-López, R., Galván, L., Nieto, J. M., & Boski, T. (2012). Enrichment of rare earth elements as environmental tracers of contamination by acid mine drainage in salt marshes: A new perspective. Marine Pollution Bulletin, 64, 1799–1808.
Feng, X., et al. (2007). Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou Province, China. Environmental Science & Technology, 42, 326–332.
Fite, T., & Leta, S. (2015). Determination of levels of As, Cd, Cr, Hg and Pb in soils and some vegetables taken from river mojo water irrigated farmland at Koka Village, Oromia State, East Ethiopia. International Journal of Sciences: Basic Applied Research, 21, 352–372.
Fitzgerald, R. H., Jr. (1995). Acetabular Labrum Tears: Diagnosis and Treatment. Clinical Orthopaedics and Related Research, 311, 60–68.
Gibb, H., & O’Leary, K. G. (2014). Mercury exposure and health impacts among individuals in the artisanal and small-scale gold mining community: A comprehensive review. Environmental Health Perspectives, 122, 667–672.
Hussain, M., Muhammad, S., Malik, R. N., Khan, M. U., & Farooq, U. (2014). Status of heavy metal residues in fish species of Pakistan. In D. Whitacre (Ed.), Reviews of environmental contamination and toxicology (Continuation of residue reviews) (Vol. 230, pp. 111–132). Berlin: Springer.
Hussein, H. S., Ruiz, O. N., Terry, N., & Daniell, H. (2007). Phytoremediation of mercury and organomercurials in chloroplast transgenic plants: Enhanced root uptake, translocation to shoots, and volatilization. Environmental Science and Technology, 41, 8439–8446.
Islam, M. A., Romić, D., Akber, M. A., & Romić, M. (2017). Trace metals accumulation in soil irrigated with polluted water and assessment of human health risk from vegetable consumption in Bangladesh. Environmental Geochemistry and Health. doi:10.1007/s10653-017-9907-8.
Khan, M. U., Malik, R. N., & Muhammad, S. (2013). Human health risk from Heavy metal via food crops consumption with wastewater irrigation practices in Pakistan. Chemosphere, 93, 2230–2238.
Khan, M. U., Muhammad, S., & Malik, R. N. (2014). Potential risk assessment of metal consumption in food crops irrigated with wastewater. CLEAN–Soil, Air, Water, 42, 1415–1422.
Khan, M., Muhammad, S., Malik, R., Khan, S., & Tariq, M. (2016a). Heavy metals potential health risk assessment through consumption of wastewater irrigated wild plants: A case study. Human and Ecological Risk Assessment: An International Journal, 22, 141–152.
Khan, S., Rauf, R., Muhammad, S., Qasim, M., & Din, I. (2016b). Arsenic and heavy metals health risk assessment through drinking water consumption in the Peshawar District. Pakistan Human and Ecological Risk Assessment: An International Journal, 22, 581–596.
Khan, S., Rehman, S., Khan, A. Z., Khan, M. A., & Shah, M. T. (2010). Soil and vegetables enrichment with heavy metals from geological sources in Gilgit, northern Pakistan. Ecotoxicology and Environmental Safety, 73, 1820–1827.
Kim, K.-H., & Lindberg, S. E. (1995). Design and initial tests of a dynamic enclosure chamber for measurements of vapor-phase mercury fluxes over soils. Water, Air, and Soil Pollution, 80, 1059–1068.
Leopold, K., Foulkes, M., & Worsfold, P. (2010). Methods for the determination and speciation of mercury in natural waters—A review. Analytica Chimica Acta, 663, 127–138. doi:10.1016/j.aca.2010.01.048.
Li, P., Feng, X., Chan, H.-M., Zhang, X., & Du, B. (2015). Human body burden and dietary methylmercury intake: The relationship in a rice-consuming population. Environmental Science & Technology, 49, 9682–9689.
Lindqvist, K. S. (1991). Epidemiology of traffic accidents in a Swedish municipality. Accident Analysis and Prevention, 23, 509–519.
Meng, B., Feng, X., Qiu, G., Wang, D., Liang, P., Li, P., et al. (2012). Inorganic mercury accumulation in rice (Oryza sativa L.). Environmental Toxicology and Chemistry, 31, 2093–2098.
Muhammad, S., et al. (2013). Wild plant assessment for heavy metal phytoremediation potential along the mafic and ultramafic terrain in northern Pakistan. BioMed Research International, 2013, 9.
Niane, B., et al. (2015). Human exposure to mercury in artisanal small-scale gold mining areas of Kedougou region, Senegal, as a function of occupational activity and fish consumption. Environmental Science and Pollution Research, 22, 7101–7111.
Pinedo-Hernández, J., Marrugo-Negrete, J., & Díez, S. (2015). Speciation and bioavailability of mercury in sediments impacted by gold mining in Colombia. Chemosphere, 119, 1289–1295.
Qiu, G., Feng, X., Li, P., Wang, S., Li, G., Shang, L., et al. (2008). Methylmercury accumulation in rice (Oryza sativa L.) grown at abandoned mercury mines in Guizhou. China Journal of Agricultural and Food Chemistry, 56, 2465–2468.
Rasmussen, P. E., Mierle, G., & Nriagu, J. O. (1991). The analysis of vegetation for total mercury. Water Air & Soil Pollution, 56, 379–390.
Riaz, A., Khan, S., Shah, M. T., Li, G., Gul, N., & Shamshad, I. (2016). Mercury contamination in the blood, urine, hair and nails of the gold washers and its human health risk during extraction of placer gold along Gilgit, Hunza and Indus rivers in Gilgit-Baltistan, Pakistan. Environmental Technology & Innovation, 5, 22–29.
Rodrigues, S., et al. (2014). Oral bioaccessibility and human exposure to anthropogenic and geogenic mercury in urban, industrial and mining areas. Science of the Total Environment, 496, 649–661.
Shah, M. T., Ara, J., Muhammad, S., Khan, S., Asad, S. A., & Ali, L. (2014). Potential heavy metals accumulation of indigenous plant species along the mafic and ultramafic terrain in the Mohmand Agency, Pakistan. CLEAN–Soil, Air, Water, 42, 339–346.
Shah, M. T., & Khan, H. (2004). Exploration and extraction of placer gold in the terraces of Bagrot valley, Gilgit, northern Pakistan. Geological Bulletin, University of Peshawar, 37, 27–40.
Ullrich, S. M., Tanton, T. W., & Abdrashitova, S. A. (2001). Mercury in the aquatic environment: A review of factors affecting methylation. Critical Reviews in Environmental Science and Technology, 31, 241–293.
UNEP (United Nations Environment Programme). (2013). Mercury—Time to act.
USEPA (US Environmental Protection Agency). (2002). Supplemental guidance for developing soil screening levels for superfund sites. Washington: Environmental Protection Agency, Office of Emergency and Remedial Response.
Voegborlo, R., & Akagi, H. (2007). Determination of mercury in fish by cold vapour atomic absorption spectrometry using an automatic mercury analyzer. Food Chemistry, 100, 853–858.
Weiss, B. (2000). Vulnerability of children and the developing brain to neurotoxic hazards. Environmental Health Perspectives, 108, 375–381.
WHO (World Health Organization). (2007). Exposure to mercury: A major public health concern. Geneva: WHO.
WHO (World Health Organization). (2008). Mercury: Assessing the burden of disease at national and local levels. Environmental Burden of Disease Series, No. 16. Geneva: WHO.
Yang, J., Chen, L., Shi, W.-L., Liu, L.-Z., Li, Y., & Meng, X.-Z. (2015). Mercury distribution in sediment along urban–rural gradient around Shanghai (China): Implication for pollution history. Environmental Science and Pollution Research, 22, 1697–1704.
Yanyu, W., Junliang, T., & Qixing, Z. (1992). Study on the proposed environmental guidelines for Cd, Hg, Pb and As in soil of China. Journal of Environmental Sciences, 4, 66–73.
Zhang, H., Feng, X., Larssen, T., Qiu, G., & Vogt, R. D. (2010). In inland China, rice, rather than fish, is the major pathway for methylmercury exposure. Environmental Health Perspectives, 118, 1183.
Zheng, N., Wang, Q., & Zheng, D. (2007). Health risk of Hg, Pb, Cd, Zn, and Cu to the inhabitants around Huludao Zinc plant in China via consumption of vegetables. Science of the Total Environment, 383, 81–89.
Zhong, T., Xue, D., Zhao, L., & Zhang, X. (2017). Concentration of heavy metals in vegetables and potential health risk assessment in China. Environmental Geochemistry and Health. doi:10.1007/s10653-017-9909-6.
Acknowledgements
We are thankful for the financial support of Higher Education Commission, Pakistan, PAK–US Science and Technology cooperation program Phase IV and Chinese Academy of Sciences President’s International Fellowship for Visiting Scientists (2015VEB055). We also acknowledge the reviewers for their time and detailed comments that have improved quality of this paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We all authors declared that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Riaz, A., Khan, S., Muhammad, S. et al. Mercury contamination in selected foodstuffs and potential health risk assessment along the artisanal gold mining, Gilgit-Baltistan, Pakistan. Environ Geochem Health 40, 625–635 (2018). https://doi.org/10.1007/s10653-017-0007-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-017-0007-6