Abstract
Background and aims
Rare Earth Elements (REE) are widely used to trace natural geochemical processes. They are also increasingly used by man (electronics industry, medicine, agriculture) and therefore considered as emerging pollutants. The present study documents REE mobility in non-polluted natural soil-plant systems in order to characterize their environmental availability for future anthropogenic pollution.
Methods
The study is based on a field approach in non-polluted natural sites with contrasting geological environments (limestone, granite, and carbonatite) and highly variable REE contents.
Results
REE concentrations in soils do not directly reflect bedrock concentrations, but depend largely on pedogenetic processes and on the mineralogy of bedrock and soil. The soils of all sites are with respect to bedrock enriched in heavy REE. The REE uptake by plants is not primarily controlled by the plant itself, but depends on the concentration and the speciation in the soil and the adsorbed soil water pool.
Conclusions
REE uptake by plant roots are linked with those of Fe. Roots absorb preferentially the light REE. Before translocation, REE are retained by the Casparian strip leading to much lower concentrations in the aerial parts. The transport of the REE within the xylem is associated with the general nutrient flux.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rare Earth Elements (REE) are trace metals including the lanthanide group and yttrium. They have become known to a wide public during the last few years because of supply shortages for the high-tech industry. In fact, a growing number of current or emerging alternative energy technologies (e.g. electric and hybrid vehicles, car catalysts, energy-efficient lighting, wind power) and digital equipment (e.g. flat panel displays, disk drives, digital cameras) contain REE-bearing components such as magnets, metal alloys, and phosphors (Haxel et al. 2002; Long et al. 2010). For example, a typical hybrid car comprises approximately 15 kg of REE, in particular neodymium (Nd; www.lynascorp.com; www.molycorp.com). REE are also used in medicine with gadolinium (Gd)-bearing contrast media for magnetic resonance imaging (Azoulay et al. 2007; Kümmerer and Helmers 2000) or lanthanum (La) carbonates for the treatment of renal disease (Bernard et al. 2005; D’Haese et al. 2003; Hutchison et al. 2004). In agriculture REE-doped food is used for pig and poultry fattening (He et al. 2001, 2003, 2010), and in China REE-doped phosphate fertilizers have been applied for several years on a massive scale for growing crops of maize, rice, wheat, potato and cabbage (Liang et al. 2005; Pang et al. 2002; Xiangsheng et al. 2006).
The rapidly growing demand for REE has led to an exponential increase of global REE mining production from about 50 kt/year in 1990 to 70 kt/year in 1995 and 130 kt/year in 2010 (Chen 2011; Haxel et al. 2002; Livergood 2010). This growing use leads to environmental contamination and REE are therefore considered as an emerging pollutant (Kulaksiz and Bau 2011; Yang et al. 2009) . The most frequently detected anthropogenic REE is Gd issued from magnetic resonance imaging and released into the environment through hospital effluents. The presence of anthropogenic Gd leads to a typical positive Gd-anomaly in the REE distribution patterns of surface waters, which has been reported from rivers, lakes and coastal environments in Europe (Bau and Dulski 1996; Elbaz-Poulichet et al. 2002; Hennebrüder et al. 2004; Knappe et al. 2005; Kulaksiz and Bau 2007, 2011; Möller et al. 2000, 2002, 2003; Rabiet et al. 2005), in the USA (Bau et al. 2006; Verplanck et al. 2005), and in Japan (Nozaki et al. 2000; Ogata and Terakado 2006). In China, elevated REE concentrations have been observed in soils adjacent to REE mines and refining plants (Wang et al. 1997; Zhenggui et al. 2001; Zhu et al. 1997) and in agricultural areas with intensive use of REE-enriched fertilizers (França et al. 2002).
REE have previously been considered to be non-toxic and to be readily excreted by animals and man after ingestion (Schwabe et al. 2012). However, recent publications have reported toxic effects of REE in bacteria (Wilde et al. 2002), plants (Babula et al. 2008) and animals (Briner et al. 2000; Che et al. 2010; Feng et al. 2006). In order to contribute to a better understanding of the behavior of the REE within the biosphere, we focus in the present study on the mechanisms controlling the transfer of REE at the soil-plant interface, which is one of the principal routes to introduce anthropogenic trace elements into the biosphere. Earlier studies have shown that solution complexation of the REE with organic and inorganic ligands plays an important role for REE absorption by plant roots (Ding et al. 2005a, b; Liang et al. 2008). However, these studies were based on hydroponic growth experiments and the results can therefore not be directly transposed to natural soil/root interfaces. As a consequence, these mechanisms remain poorly understood for natural systems (Tyler 2004).
In contrast to earlier studies we have therefore not chosen a laboratory (e.g. Ding et al. 2006a, c; Han et al. 2005; Hao et al. 1997; Semhi et al. 2009; Shtangeeva and Ayrault 2007) or field approach in cultivated plots enriched with anthropogenic REE (Ding et al. 2006b; Liang et al. 2005; Xu et al. 2002, 2003), but a field approach under undisturbed natural conditions investigating the REE of the pedo-geochemical background. To take into account environmental variability, 3 field sites with contrasting geo-pedological conditions were selected. All sites were under temperate continental climate conditions. On each site, REE transfer from soil to roots and aerial organs were studied in several tree species (spruce, beech and oak). The objectives of this comparative approach was to assess the roles of geo-pedological and environmental parameters in the transfer rates and transport mechanisms for the REE in soil-plant systems in order to contribute to a better characterization of the environmental availability of anthropogenic REE.
Materials and methods
Chemical properties, normalization, and natural occurrence of the REE
REE include the lanthanide group and yttrium. Within the lanthanide group the ionic radii decrease with increasing atomic number, which leads to a slightly variable behavior during chemical or biological processes (Henderson 1984; Tyler 2004). Under natural surface environments most REE occur as trivalent ions except cerium (Ce), which is transformed to Ce4+ under oxidizing conditions (>300 mV at pH 7). Europium (Eu) is reduced from Eu3+ to Eu2+ under extremely reducing conditions (<−350 mV at pH 7; Brookins 1989). The REE group can be separated into three sub-groups: the light REE (LREE, La-Sm), the medium REE (MREE, Eu-Td) and the heavy REE (HREE, Dy-Lu). Their particular chemical properties have made of the REE an important tracer for geochemical processes (Henderson 1984; Lipin and McKay 1989). They have also specific magnetic and optical properties, which is the basis for the rapidly growing number of technical applications.
REE data are typically presented as distribution patterns with the individual REE, listed in the order of their atomic number, as categories on the x-axis. The corresponding REE concentrations appear as normalized logarithmic values on the y-axis. Normalization is necessary to smooth the distribution patterns, which would otherwise have a zigzag shape. Typical normalizations for Earth’s surface processes are the NASC (North American Shale Composite; Gromet et al. 1984) or PAAS standards (Post Archean Australian Shales; Taylor and McLennan 1985), which both represent average upper continental crust. However, for more detailed studies it may be useful to normalize to a local standard, such as local bedrock, or to present the REE patterns as transfer factors between 2 subsequent reservoirs (e.g. soil/bedrock, root/soil). In the present paper we will use PAAS normalized REE patterns only for the bedrock and present all other data as REE transfer factors. The change of the shape of the REE patterns between 2 successive reservoirs is called “fractionation of the REE”, e.g. an enrichment or a depletion in light, medium, or heavy REE.
More specific fractionations are positive or negative peaks in the REE distribution patterns, which are most common for Ce and Eu, and referred to as positive or negative Ce and Eu anomalies. The magnitude of these anomalies is quantified by Ce/Ce* and Eu/Eu* ratios:
The subscript “norm” stands for normalized values. Anomalies with Ce/Ce* and Eu/Eu* ratios >1 appear as positive peaks in a REE pattern and are therefore referred to as “positive anomalies”. Anomalies with Ce/Ce* and Eu/Eu* ratios <1 yield negative peaks and are called “negative anomalies”. The occurrence of positive or negative Ce anomalies is typical for oxidizing conditions where Ce is present as Ce4+ and therefore less soluble than the other REE (McLennan 1989). By contrast, on the Earth’s surface Eu anomalies are, in most cases, related to the presence of feldspar or feldspar-derived alteration products such as clay minerals (Galan et al. 2007; McLennan 1989).
The average REE concentration of the upper Earth’s crust is about 150 mg/kg for the sum of the REE, and 25 mg/kg for neodymium (Nd; McLennan 1989). Typical concentrations in soils are a few tens of mg/kg for Nd and a few hundreds of mg/kg for the sum of the REE (Laveuf and Cornu 2009; Loell et al. 2011; Tyler 2004). The REE concentrations of vegetation are at least one order of magnitude lower than for soils, but vary over several orders of magnitude as a function of plant species and soil concentrations. The highest concentrations are found in roots, where values may achieve a few hundred μg/kg for Nd and a few mg/kg for the sum of the REE (Fu et al. 2001; Liang et al. 2008). In the above-ground biomass of plants, concentrations decrease considerably in the order leaf > stem > grain/fruit and vary in the range of a few μg/kg to a few tens of μg/kg for Nd, and a few tens to a few hundred μg/kg for the sum of the REE (Tyler 2004; Liang et al. 2008). In this study, Nd concentrations were used rather than the sum of the REE to characterize the REE content of a sample, because the Nd concentrations give a more reliable measure for the overall REE content than the sum of the REE, which is strongly influenced by Ce and Eu anomalies.
Field sites
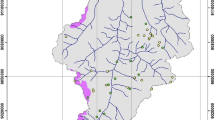
The 3 field sites are located in eastern France and SW Germany all within a distance of less than 200 km (Fig. 1). The climate at the 3 sites is temperate continental with oceanic influence. The geo-pedological conditions of the 3 sites are different: The first site is located on limestone bedrock, the second site on granite and the third site on carbonatite. Carbonatites are carbonates of volcanic origin and strongly enriched in REE. This third site therefore allows the study of soil-plant transfer at elevated REE concentrations. Carbonatites are mined for REE extraction (e.g. in the Mountain Pass area in California; www.molycorp.com) and the carbonatite site is therefore also representative for soil-plant transfers in such mining sites.
Localization of the 3 field sites in eastern France and southwestern Germany. The limestone site is located in the Jura mountains, about 20 km SSW of the city of Besançon (France). The granite site lies within the environmental observatory of the Strengbach catchment in the southern Vosges mountains, 20 km NW of the city of Colmar (France). The carbonatite site is situated on the flanks of the inactive volcano “Kaiserstuhl” in the Rhine plain, 15 km NW of the city of Freiburg im Breisgau (Germany)
The general soil parameters of the 3 sites are summarized in Table 1 and their major element composition in Table 2. The limestone site is located on a forested plateau of the Jura mountains close to Cussey-sur-Lison (France) at an elevation of 320 m. The mean annual precipitation is about 1,000 mm/year and the mean annual temperature 10.2°C. The plant cover consists in a natural deciduous forest, punctually interrupted by spruce stands planted for wood production. Three representative field plots were selected on the limestone site. The first plot, with beech, is situated directly on the plateau with eutric cambisol. The second plot is also located in a beech stand, but on a slope at the foot of a limestone cliff. The soil of this stand is as for the plateau an eutric cambisol. The third plot is situated in a spruce stand implanted in a dolina with a luvic cambisol. In the following these 3 plots of the limestone site will be referred to as “beech plot plateau”, “beech plot slope”, and “spruce plot” respectively.
The granite site is located in the Strengbach catchment in the southern Vosges mountains at altitudes ranging from 880 to 1,150 m. The mean annual precipitation is about 1,350 mm/year and the mean annual temperature 6°C (Probst et al. 1992). The Strengbach catchment is a completely equipped environmental observatory with permanent sampling and measuring stations maintained by the laboratory of hydrology and geochemistry of the CNRS/University of Strasbourg (LHyGeS, http://ohge.u-strasbg.fr). The catchment is almost completely covered with forest composed of 80 % spruce and of 20 % beech. The bedrock of the Strengbach catchment is a coarse-grained granite locally affected by hydrothermalism. The soils are coarse-grained and rich in bedrock boulders (Fichter et al. 1998). Two plots were studied, one in a spruce stand with dystric cambisol and the other in a beech stand with entic podzol. The plots are termed “spruce plot” and “beech plot” below and they correspond to the “VP” and “HP” plots, respectively, of earlier publications dealing with the Strengbach catchment (e.g. Stille et al. 2009).
The carbonatite site is located on the inactive volcano “Kaiserstuhl” in the Rhine plane near Freiburg im Breisgau, Germany. The site is located at 360 m altitude with an average annual precipitation of 600 to 700 mm/year and a mean annual temperature of 9.9°C. The site is not forested, but mainly occupied by vineyards with a few plots of grassland and trees. The soil is a superficial eutric cambisol about 30 cm deep. Beech and spruce are absent from the site and therefore parts of oaks were sampled.
Field sampling and analytical methods
For the limestone and carbonatite sites the bedrock samples were recovered in close vicinity of the field plots. For the granite site a bedrock outcrop was located at about 300 m from the beech plot. The chemical data of this outcrop were taken from Aubert et al. (2001). In contrast, no bedrock outcrop was available for the spruce plot. Therefore, 2 granite boulders recovered from a soil profile were analyzed. For all sites, soils were sampled in pits by taking 3–5 subsamples per horizon, which were bulked in the field. All sites were equipped with Teflon (granite site) or polypropylene (limestone and carbonatite sites) lysimeter plates for soil water sampling. The plates were installed at 20 to 30 cm of depth for the limestone and carbonatite sites. For the granite site the soil water data were taken from Stille et al. (2009) who sampled complete soil water profiles from 5 to 70 cm depth in the beech and spruce plots. Tree trunk samples were recovered from slices cut at the heights above ground specified in the supplementary data files. Roots and leaves were sampled on the same tree specimens as the trunk samples. Needle samples were of the current year. Roots were handpicked in pits and sorted by diameter (<2 mm, 2–10 mm, >10 mm). Sap samples of beech (limestone and granite sites) and oak (carbonatite site) were recovered during the rapid growth period at the end of spring (May/June) from small branches by squeezing with a portable nitrogen pressure chamber at about 30 bars.
Bedrock samples were crushed, milled in an agate mill, fused with Li2BO4 and dissolved in HNO3 for analysis by ICP-AES (Jobin Yvon) and ICP-MS (Thermo Xseries 2) at the LHyGeS laboratory at CNRS Strasbourg (France) according to the procedure described by Samuel et al. (1985). Soil samples were sieved (2 mm mesh size), followed by the same digestion and analytical procedure as for the bedrock samples. The detection limit for the REE in soil and bedrock samples was 0.01 mg/kg with a precision better than ±5 %. The CaCO3 and organic carbon content of the soil samples were determined at the laboratory of Chrono-Environnement in Besançon (France) by calcimetry and an Elementar Vario Max carbon analyzer, respectively. Soil pH was determined in distilled water according to ISO 10390.
Roots and leaves were first washed in 50 mL polypropylene tubes for 10 min with acidified ultrapure water from a Millipore Elix–Simplicity chain under continuous agitation in order to remove soil and airborne particles. Afterwards, the samples were rinsed with ultrapure water and dried during 2 weeks at room temperature, followed by 24 h at 40°C in a drying oven. Trunk samples were air-dried, then split into match-sized splints and calcined using a step-wise heating procedure (350, 450, and 500°C during 90, 90, and 120 min respectively).
For chemical analysis about 1–5 g of cleaned roots, leaves or calcined wood were completely digested in closed 120 mL Savillex teflon vessels under clean-lab conditions. Complete digestion was typically achieved after 3 days using a multi-step procedure with a mixture of distilled concentrated HNO3, ultrapure H2O2, and HF on a hot plate at 80°C. After evaporation to dryness the samples were taken up in 15 mL of 1 M HNO3 for ICP analysis. For sap samples, 1 mL of distilled concentrated HNO3 was added to about 3 mL of sap, evaporated to dryness and taken up in 15 mL of 1 M HNO3 for ICP analysis. Soil water was filtered immediately after sampling with 0.45 μm cellulose acetate filters and acidified to pH 2 with HNO3. The chemical analyses of vegetation and soil waters were as for bedrock and soils realized by ICP-AES (Jobin Yvon) and ICP-MS (Thermo Xseries 2) at the LHyGeS laboratory at CNRS Strasbourg (France) with a precision of <±5 %. The detection limits for the REE was 0.01 μg/kg for vegetation samples and 0.001 μg/L for soil waters. Replicates of selected vegetation samples yielded identical REE patterns with absolute REE concentrations varying in a range of ±10 %.
Results
REE contents in bedrock, soil, and soil water
The bedrocks of the 3 sites were characterized by distinct REE patterns and concentrations (Fig. 2). The raw data of Fig. 2 are available as supplementary data file. For limestone and granite the REE concentrations were in the same range. In contrast, much higher values and a strong LREE enrichment were observed for carbonatite, which is to be expected for this type of rock (Hornig-Kjarsgaard 1998).
Average REE distribution patterns in bedrock of the 3 study sites (limestone, granite, carbonatite). The patterns are normalized to PAAS (Post Archean Australian Shales), which represents average upper continental crust. The error bars correspond to the standard deviations of the means, which are based on the values given in the supplementary data files
The REE concentrations in soils are presented in Fig. 3 as soil/bedrock transfer factors to visualize REE fractionation during soil formation. Only average data are presented because significant depth-trends were absent. The raw data are as for the bedrock available as supplementary data file. The absolute REE concentrations were 3 to 7 times higher for the soils of the carbonatite site than for the other sites. However, the soil/bedrock transfer factors were about 0.5 for the carbonatite site, and thus markedly lower than for the limestone and granite sites with transfer factors of 2 and 1, respectively (Fig. 3). For all sites the soils were enriched in HREE with respect to bedrock. Furthermore, the soils of the limestone site showed strong positive Eu anomalies. A slight positive Eu anomaly occurred for the soils of the granite site, whereas no significant anomalies were found for the carbonatite site. REE concentrations were correlated with Fe and Al contents in the soils of the limestone site, and with Fe and Mn in the soils of the granite site (Table 3).
Average REE distribution patterns of soils of the 3 field sites. The patterns are normalized to local bedrock and represent soil/bedrock transfer factors. The averages and the corresponding standard deviations are those given in the supplementary data files. The average soil pH values are those given in Table 1
Soil water REE are presented in Fig. 4 as soil water/soil transfer factors in order to visualize REE fractionation during soil water/soil interaction. The raw data are available as supplementary data file. The soil data used for normalization are average values of the complete soil profiles for the limestone and carbonatite sites, which are less than 60 cm deep. For the granite site, average values over the uppermost 60 cm of the soil profiles were used for the spruce plot and over the uppermost 70 cm for the beech plot. The soil water/soil transfer factors of the limestone site showed slightly HREE-enriched distribution patterns with negative Ce anomalies. The patterns from the granite site showed similarly a HREE enrichment, completed by a positive Eu anomaly for the beech plot. Only one incomplete REE pattern was available for the carbonatite site. It was enriched in HREE and displayed a negative Ce anomaly as the soil waters of the limestone site.
REE distribution patterns of soil water of the 3 field sites. The patterns are normalized to local soil (<60 cm depth) and represent soil water/soil transfer factors. The soil pH values are the same as in Fig. 3
REE contents in vegetation
REE concentration data of vegetation are presented in Fig. 5. The raw data can be downloaded as supplementary data files. The average Nd concentrations of vegetation varied between 3,700 μg/kg for root and 4 μg/kg for trunk samples. They were lowest for the trunk, intermediate for needles, leaves, medium and large roots, and highest for small roots, which is in agreement with literature data (Fu et al. 2001; Hu et al. 2002; Liang et al. 2005, 2008; Tyler 2004; Wang et al. 2003). We consider that the REE content of leaves and needles represent essentially soil-derived REE, because air-borne particles were removed with dilute acid prior to digestion. However, a slight contribution of REE derived from foliar uptake from airborne particles cannot be completely excluded.
Range of Nd concentrations of vegetation samples. The raw data are available in the supplementary data files. Stars represent simple or average values according to the number of samples “n”. The bars associated with average values represent the absolute range of variation. Note the logarithmic scale of the x-axis
Limestone site
Nd concentrations in spruce varied between 40 and 3,700 μg/kg for the roots, and 0.3 to 15 μg/kg for the trunk (Fig. 5). The patterns of the REE plant/soil transfer factors were very similar for root, trunk and needles with a LREE enrichment and a slight negative Ce anomaly. Medium and large roots showed positive Eu anomalies, whereas the anomaly was negative for the trunk (Fig. 6). The beech samples had Nd concentrations between 600 and 3,500 μg/kg for the roots, and 4 to 10 μg/kg for the trunk (Fig. 5). The REE patterns showed, as for spruce, a slight LREE enrichment and negative Ce anomalies for some root samples (Fig. 6). Two sap samples were retrieved from small beech branches of the plateau plot. They showed strong positive Eu and Ce anomalies, and LREE enrichment.
REE distribution patterns of vegetation of the limestone site. The patterns are normalized to local soil in order to show fractionation at the plant/soil interface. The patterns represent average values. They are based on 27 samples for the spruce trunks, 6 samples for the beech trunks from the plateau, and 3 samples for the beech trunks from the slope and small beech and spruce roots. The data of sap, leaves and needles are based on 2 samples. Only 1 sample was available for medium and large roots
The REE root/soil transfer factors varied between 10−3 and 10−2 for spruce. For the beech roots from the plateau and slope plots the values were significantly higher, ranging between 10−2 and 10−1. This difference between spruce and beech disappeared in the trunk where the transfer factors varied between 10−4 and 10−3 for both species (Fig. 6).
Granite site
The Nd concentrations in the beech and spruce roots ranged between 30 and 220 μg/kg, which is distinctly lower than for the limestone site. In contrast, the Nd concentrations for trunk and branches ranged from 4 to 17 μg/kg for both sites (Fig. 5). The patterns of the REE plant/soil transfer factors of the spruce and beech organs from the granite site differed from those of the limestone site by the presence of positive Eu anomalies and the absence of negative Ce anomalies. Furthermore, LREE enrichment was less pronounced than for the limestone site and limited to roots (Fig. 7).
REE distribution patterns of vegetation of the granite site. The patterns are normalized to local soil (<60 cm depth for the spruce plot and <70 cm for the beech plot) in order to show fractionation at the plant/soil interface. The patterns represent average values, which are based on 12 samples for the spruce trunks and 2 samples for the beech branches. The patterns of leaves, needles and sap are based on 2 samples, whereas only 1 sample was available for small, medium, and large roots
The REE root/soil transfer factors varied between 10−3 and 10−2 for spruce and were thus comparable with spruce from the limestone site. The transfer factors were slightly higher for beech, which, however, remained below those for the beech of the limestone site. The sap samples retrieved from young beech were very similar to those from the limestone site with strong positive Ce and Eu anomalies.
Carbonatite site
The vegetation data from the carbonatite site are shown in Fig. 8. No spruce or beech were present on this site and therefore parts of oaks were sampled. The absolute REE concentrations were, in comparison to the other sites, less variable for the individual plant organs (Fig. 5). As for the limestone and granite sites, the patterns of the REE plant/soil transfer factors were slightly enriched in LREE. The patterns of roots and leaves displayed negative Ce anomalies. On the other hand, the branches showed a positive Eu anomaly, which was absent in the other organs. The sap samples were, like for the limestone and granite sites, characterized by a strong positive Eu anomaly. But in contrast to the other sites, there was no positive Ce anomaly. The REE root/soil transfer factors were with values between 10−3 and 10−2 comparable with the limestone and granite sites.
REE stock of vegetation and soil
The total REE stock of vegetation and soil was calculated for each site. The biomass of vegetation was determined for each field plot based on the height and the diameter of the trees on an area of 30*30 m following the procedure described in detail by Lambert et al. (2005). The REE stock in soil was calculated on the basis of the soil depths and densities, and the average REE concentrations given in the supplementary data files. We were unable to measure accurately the soil depths of the granite site and therefore an average depth of 1 m was used. The results are presented in Table 4 and show that, with respect to soil, vegetation represents a negligible REE reservoir.
Discussion
REE transfer from bedrock to soil
For the limestone site, the average soil/bedrock transfer factors were about 2 for most REE except for Eu with values of about 50. This REE enrichment of the soils is probably due to volume loss by decarbonatation during soil formation, which concentrates REE-bearing mineral particles. The strong positive Eu anomaly shows that this residual soil fraction contains Eu-rich feldspar particles or clay minerals resulting from feldspar weathering.
Previous studies on REE behavior in soils based on sequential extractions and electron microscopy have shown that a simple correlation analysis of bulk soil chemical data yielded a rough estimate for the mineralogical speciation of the REE (Aubert et al. 2001; Steinmann and Stille 1997; Stille et al. 2009). The strong correlation between REE and Fe (Table 3) suggests in the present case that the REE of the limestone soils are mainly located in Fe-oxyhydroxides. The additional correlation with Al may be due to Al located in Fe-oxyhydroxides or to an additional association of the REE with clay minerals of the clay-humus complex. Such a mixed speciation of the REE in Fe-oxyhydroxides and clays is typical for REE in soils (Land et al. 1999; Laveuf and Cornu 2009; Steinmann and Stille 1997).
For the granite site the soil/bedrock transfer factors were about 1, demonstrating that soil formation on granite has, in contrast to the limestone site, not led to a REE enrichment. The correlations between REE, Fe and Mn suggest that the REE are similarly to the limestone site mainly bound to Fe-Mn-oxyhydroxides. Aubert et al. (2001) furthermore identified igneous apatite and zircon as the principal REE source in the granite bedrock of the Strengbach catchment and showed that the REE budget of the soils is mainly controlled by dissolution of primary apatite. More recently, Stille et al. (2009) identified newly-formed rhabdophane (REE-PO4) in the surface soils of the Strengbach catchment and suggested that rhabdophane may, together with Fe-Mn-oxyhydroxides, represent an important secondary REE carrier phase of the surface soils.
The soils of the carbonatite presented a HREE enrichment with respect to the bedrock without specific anomalies. The REE soil/bedrock transfer factors were about 0.5, which is lower than for the other sites. Large amounts of REE, and in particular LREE, have thus been removed during soil formation. However, the REE concentrations of the resulting soils were still 5 to 10 times higher than for the other sites.
The soils of all sites were enriched in HREE with respect to bedrock. The fact that this HREE enrichment was common to the 3 sites, in spite of their completely different bedrock lithology, excludes preferential alteration of specific primary REE carrier phases of the bedrock as a fractionation process. A similar HREE enrichment has been described by Öhlander et al. (1996) for soils on moraines in northern Sweden. These authors showed that the HREE are enriched during soil formation, whereas the LREE are preferentially removed. Based on sequential extractions on the same soil material, Land et al. (1999) furthermore suggested that this HREE enrichment of soil is due to preferential scavenging of the HREE during precipitation of pedogenetic Fe-oxyhydroxides. This is in agreement with our observation that the REE of the LREE-enriched soils are mainly bound to Fe-oxyhydroxides. Land et al. (1999) also showed that the REE of the adsorbed soil pool as well as the particulate load of a nearby stream were enriched in the LREE, suggesting that there is a link between preferential scavenging of HREE in soils and preferential LREE export from a labile soil REE pool to surface and subsurface runoff. In a more recent study, carried out on soils on moraines in Wyoming, Harlavan et al. (2009) found similarly a higher mobility for the LREE during pedogenesis.
REE transfer from soil to soil water
The negative Ce anomaly of soil water of the limestone site can directly be related to the association of the soil-hosted REE with Fe-oxyhydroxides because Ce occurs under oxidizing conditions not in trivalent form as the other REE, but as Ce4+ in Fe-oxyhydroxides (Braun et al. 1990; Henderson 1984; Koeppenkastrop and De Carlo 1992). Ce is under these conditions less soluble than the other REE resulting in negative Ce anomalies in soil water and positive anomalies in the corresponding soil. The absence of such a negative Ce anomaly in the soil waters of the granite site may be related to soil pH, which is lower than for the limestone site. The positive Eu anomaly of soil water from the beech plot may be related to REE derived from feldspar alteration products.
The soil water/soil transfer factors were lower for the carbonatite site than for the two other sites, leading to similar REE concentrations for the soil waters of the 3 sites in spite of the very different concentrations in soil and bedrock. This shows that the REE concentrations of soil water were mainly controlled by solubility and not by the REE concentrations of soil. The slight HREE enrichments of soil water with respect to soil of the granite and carbonatite sites may similarly be solubility-controlled, because the HREE form more stable complexes with organic and inorganic ligands than the LREE (Brookins 1989; Ding et al. 2006b; Sonke and Salters 2006).
REE transfer at the soil/plant interface
Origin of the REE taken up by plant roots
The highest REE concentrations occurred in the oak roots from the carbonatite site, followed by beech and spruce roots from the limestone site. The lowest concentrations in roots were those of beech and spruce from the granite site (Fig. 5).
The REE patterns of the roots primarily reproduced the patterns of local soil particles, but with a slight enrichment of the LREE. The only similarities between roots and soil water were negative Ce anomalies for the limestone and carbonatite sites, and positive Eu anomalies for the granite site. Thus, one can infer that gravitational soil water, i.e. the soil water which percolates the soils vertically driven by gravity and which is sampled by lysimeter plates, was not the main source for REE uptake by plants. On the contrary, the close similarities between root and soil REE patterns strongly suggest that soil mineral particles present in the close vicinity of the root (rhizospheric zone) were the main source of REE for plants. Very likely, the rhizospheric acidification effect caused an enhanced physico-chemical alteration of the mineral particles present in the very vicinity of the root, resulting in the solubilization of REE which are then easily taken up by roots.
This hypothesis is supported by the correlation data from the limestone site (Table 3), where positive correlations occur between REE, Fe, and Al for soils and roots, but not for gravitational soil water. However, the negative Ce anomalies of the root samples resemble soil water and hint to the involvement of an aqueous phase during the transfer of the REE from soil to roots. As hypothesized above, this aqueous phase is most probably rhizospheric water. Indeed, it is well known that plants absorb poorly soluble elements mainly through the rhizospheric soil water pool (Hopkins and Hüner 2009).
Consequently, the comparison with the REE patterns of soil and soil water indicates that plant roots absorb the REE mainly through rhizospheric soil water adsorbed on REE-bearing soil particles. Based on the correlations between REE, Fe, and Al, these soil particles are mainly Fe-oxyhydroxides and possibly clay minerals. Unfortunately, it was not technically possible to sample this adsorbed soil water pool by suction cup lysimeters because our laboratory assays had shown that REE were fractionated by ceramic cups, while Teflon cups did not allow the recovery of sufficient volumes of water.
Origin of the LREE enrichment of plant roots
The REE patterns of the roots were slightly enriched in the LREE with respect to soil. This enrichment is strongest for the limestone site, followed by the carbonatite and granite sites (Figs. 6, 7 and 8). A similar LREE enrichment of vegetation has already been reported in the literature from field studies (Fu et al. 2001; Liang et al. 2008; Stille et al. 2006; Wyttenbach et al. 1998) and from laboratory studies with soil-grown plants (Aouad et al. 2006; Ding et al. 2006c).
We concluded in the discussion on the origin of the REE taken up by plant roots that they were mainly derived from the adsorbed soil water pool of Fe-oxyhydroxide and clay particles. The correlations between REE, Fe and Al in soils and plant roots show that uptakes of these elements are strongly linked. In contrast to Al and REE, Fe is known to be essential for plant growth and development. Plants have therefore developed specific strategies and processes to absorb Fe (Berner et al. 2003; Hinsinger 1998; Neilands 1981; Reichman and Parker 2005; Robin et al. 2008). The systematic association of the REE with an essential plant nutrient suggests that the REE are absorbed circumstantially during absorption of Fe. To check this relationship in more detail REE/Fe root/soil enrichment factors were calculated according to the following formula:
The resulting enrichment patterns are shown in Fig. 9. In the limestone site, except for spruce 1, plant roots absorbed preferentially the LREE with respect to Fe and there is almost no enrichment for the HREE. The plants of the limestone site thus favor LREE over Fe and do not discriminate between HREE and Fe. The enrichment patterns furthermore showed negative Ce-anomalies. For the granite site, the LREE enrichment was much less pronounced or absent, but there was systematically a positive Eu anomaly. For the carbonatite site the enrichment factors were negative without variations throughout the REE group.
Enrichment factors of REE/Fe ratios at the root/soil interface. The correlation data presented in Table 3 suggest that plant roots absorb the REE together with Fe. The REE/Fe enrichment factors shown here allow this relation with Fe to be detailed for the individual REE. Almost all roots at the limestone site absorb the LREE preferentially with respect to Fe and there is no similar enrichment for the other sites. Note the negative Ce anomalies for the limestone site and the positive Eu anomalies for the granite site. The enrichment factors were calculated according to the formula given in the text
In the discussion on REE transfer from bedrock to soil we hypothesize that the HREE enrichment of soil is probably due to preferential scavenging of the HREE in Fe-oxyhydroxides. The REE adsorbed on these Fe-oxyhydroxides are according to the sequential extraction data from Land et al. (1999) enriched in LREE. The LREE enrichment of the REE/Fe patterns is thus in agreement with the hypothesis that plant roots absorb the REE mainly from the adsorbed Fe-oxyhydroxide pool. However, this scenario cannot explain why the LREE enrichment of the REE/Fe patterns is much stronger for the limestone site than for the granite site.
This discrepancy may be explained by solution complexation of the REE in the adsorbed soil water pool. Dissolved organic and inorganic REE complexes are more stable for the HREE than for the LREE at low REE/humic acid ratios (Ding et al. 2006b; Sonke and Salters 2006; Marsac et al. 2010). Ding et al. (2006c) have shown, for hydroponically grown soybean plants, that increasing concentrations of organic ligands in the hydroponic solution led to the progressive enrichment of the LREE in the leaves. Ding et al. (2006c) concluded from this that plant roots preferentially absorb free LREE ions rather than dissolved HREE complexes. Similarly, Fu et al. (2001) found for the roots of soil-grown plants an enrichment of the LREE with respect to the labile soil reservoir. They related this enrichment, like Ding et al. (2006c), to the dominant speciation of the LREE as free ions, whereas the HREE were mainly present as dissolved complexes and therefore less absorbed. The stronger LREE enrichment of the REE/Fe patterns of the limestone site with respect to the granite site may be related to the higher soil pH of the limestone site, which leads to higher percentages of complexed HREE in the adsorbed soil water pool.
Moreover, cation transport across plant cell membranes is mediated through ion channels, carriers and pumps located in plant cell membranes (Taiz and Zeiger 2006). These membrane transport proteins usually display selectivity and are able to discriminate between different kinds of ions. Gao et al. (2003) have shown that Eu and La can be absorbed into the plant cell and bound to the inner membranes of the different organelles. Little is known about the transport proteins that can mediate REE uptake by plant cells, but we can readily hypothesize that ion channels may be involved in the passive diffusion of REE across the plasmalemma. The present results showed that plants preferentially absorb LREE. This indicates that plants may discriminate between free and complexed cations differing by diameter and charge. Further research is still needed in this field to better understand the underlying mechanisms.
In summary, we conclude that the LREE enrichment of plant roots may be related to 2 different factors: 1) The adsorbed soil water pool from where plant roots absorb the REE is enriched in LREE because pedogenic Fe-oxyhydroxides include preferentially HREE in their mineral structure. 2) The resulting LREE enrichment of plant roots is amplified by the preferential uptake of free LREE ions compared to HREE essentially present as complexed ions. Consequently, the HREE will preferentially be transferred towards the gravitational soil water pool, which could contribute to the HREE enrichment that we observed for gravitational soil water.
Specific behavior of europium during absorption by plant roots
Vegetation of the granite site is characterized by positive Eu anomalies, which are absent for most samples of the other sites. These Eu anomalies appear also in the REE/Fe enrichment factors presented in Fig. 9. Very similar positive Eu anomalies were described by Ding et al. (2006b) for soil-grown wheat. The anomaly was absent in the roots, appeared in the leaves, and was strongest in stem and grains. Ding et al. (2006b) related this Eu anomaly to precipitation of Eu-enriched phosphate particles in the different plants organs. However, phosphate precipitates with positive Eu-anomalies are not known from literature and we suggest therefore rather a link with the strong Ca depletion of the soils of the granite site where plants suffer from a marked Ca-deficit (Stille et al. 2009). Calcium and Eu have almost identical ionic radii (Shannon 1976) and previous studies have shown that Eu can substitute Ca in plants, in particular in soils with elevated Eu/Ca ratios (Shtangeeva and Ayrault 2007; Zeng et al. 2003). The soils of the granite site have average Eu/Ca ratios of about 3.4 * 10−6 against 1 * 10−6 for the limestone site. Therefore positive Eu anomalies found in vegetation grown in the granite site by Ca deficiency may correspond to such Ca deficit. Whether or not Eu may be a Ca physiological substitute remains unknown.
Accumulation of the REE by the root system
REE concentrations are higher in small than in medium or large roots (Fig. 5). A similar preferential accumulation in small roots has been previously described by Thiry et al. (2005) for uranium. Complementary and non-exclusive mechanisms can be proposed to explain this enrichment. Absorption of mineral nutrients by plants is mainly localized in small roots (Hopkins and Hüner 2009), the higher concentrations may thus directly reflect higher nutriment fluxes. Moreover, the highest REE contents observed in small roots are consistent with the hypothesis that REE in plant tissues mainly accumulate in the primary cell wall during the early stages of cell growth (Bayer and Bayer 1991; Dong et al. 2009; Gao et al. 2003). Indeed, small roots display only primary cell walls which are thin and characteristic of young growing cells, whereas older roots have cells with thick lignin-rich secondary walls that are deposited when most cell enlargement has ended. Primary cell walls represent a larger fraction of the total root dry weight than in large roots, which may explain the higher REE concentrations of small roots. Furthermore it should be noted that most of the mass of small roots corresponds to active “living” tissues, with active growth and metabolism while large roots are mainly composed of inactive “dead” tissues.
REE transfer to the aerial plant organs
The REE concentrations of the aerial tree organs are about 10 to 100 times lower than in roots. Concentrations are similar for all field sites, in spite of the high variations observed in roots and soils. This indicates that the REE were not efficiently translocated from roots to shoots. The concentrations in the aerial organs seem to be independent of REE concentrations in soils and roots.
After absorption by the roots, trace elements may follow 2 different routes to reach the aerial plant organs: the apoplastic pathway (“through the cell wall”) or the symplastic pathway (“through the cytoplasm”) (Shan et al. 2003). Along the apoplastic pathway, REE moved into the cell wall by passive transport with respect to the diffusion gradient. In contrast, along the symplastic way, the transport of solutes occurred across the plasmalemma and ions entered into the cell within the cytoplasm (Hirano and Suzuki 1996; Hong et al. 2002; Klüsener et al. 1995). Both, apoplastic and symplastic routes are interrupted by the Casparian strip, which is an ion-selective cell wall located in the endoderm of the roots. Our results are in agreement with what is known about the role of the Casparian strip, which blocks or at least modulates the transfer of specific ions to the aerial parts of the plant. The large difference in REE concentrations between roots and aerial organs is very likely due to efficient filtering of the REE by the endoderm thanks to the Casparian strip. However, the REE/Fe ratios found in the aerial plant organs and sap remain similar to those observed in the roots except for Ce and Eu. This indicates that the endoderm similarly affects the REE and Fe fluxes. Furthermore, the shoot/root interface displays no systematic fractionation between LREE and HREE as for the root/soil interface.
The REE concentrations in the sap samples are strongly correlated with Fe (r = 0.95, n = 6), but also with other elements such as Al, Mg, K and P (r > 0.9, n = 6, Table 3). This demonstrates that the link observed between REE and Fe for root absorption still persists in sap. However, the new correlations with Mg, K, and P suggest that the transport of the REE and Fe in xylem sap is related to a mechanism which is common for macronutrients and trace metals. Cations taken up by plant cells form complexes with organic compounds (for instance organic acids) in which the cation becomes bound to the complex by non-covalent bonds (Taiz and Zeiger 2006). Plants are known to assimilate K and Ca, as well as micronutrients including Fe in this manner. The close link between sap concentrations of REE and mineral nutrients strongly suggests that REE form similar complexes in the xylem sap. This is in agreement with the studies of Ding et al. (2005a, 2006a) on hydroponic wheat who showed that the transport of REE in the xylem is mainly controlled by solution complexation with organic ligands. These authors furthermore found that some REE were immobilized during transport by chemical precipitation (mainly phosphate particles) and cell wall absorption.
The sap samples are also characterized by strong positive Ce and Eu anomalies (Figs. 7 and 8). Positive Ce anomalies in water samples are typical for REE transport by colloidal Fe-oxyhydroxide particles and incompatible with a transport as dissolved free ions (Steinmann and Stille 2008). So, REE in sap could also be associated to similar REE-oxyhydroxide particles. As previously said for the granite site, the positive Eu anomaly in sap could be related to the similarity of the ionic radii of Eu and Ca leading to an enrichment of Eu in sap with respect to the other REE.
The xylem is the principal route for the transport of metals from the roots to the aerial plant organs (Hopkins and Hüner 2009). For all tree species of our study we observed that the REE concentrations were lower for the trunk than for the roots or leaves, indicating that the trunk is a site of REE transfer rather than of REE accumulation. However, a more detailed look at the trunk data revealed no systematic evolution of the REE patterns, whether between individual growth rings, or with height above ground. This may be related to the fact that the REE signature of each trunk portion reflects the REE signature of sap during growth, which has been variable through time.
Leaves display higher REE concentrations than trunk samples. This is consistent with the behavior of other mineral nutrients: growing leaves are sinks for nutrients and known to accumulate them. This is also in agreement with the study of Ding et al. (2005a) who showed that REE concentrations in leafs of wheat increased with leaf age. Such effects may of course be enhanced by transpiration fluxes. As previously seen in other organs, REE contents of leaves are also correlated with Ca and Na (Shtangeeva and Ayrault 2007; Zeng et al. 2003).
Conclusions
The REE concentrations in soils are primarily controlled by pedogenetic parameters and the mineralogy of the REE carrier phases in the bedrock and in the soil rather than by the REE concentrations of the bedrock. For all the soils whatever the site enrichment in HREE was observed with respect to bedrock. In agreement with earlier studies, this fractionation can be related to preferential scavenging of the HREE by pedogenic minerals, most probably Fe-oxyhydroxides. LREE are consequently more mobile and preferentially exported by surface and subsurface runoff. This also implies that the LREE are more available for root absorption than the HREE.
The REE concentrations of gravitational soil water are similar for all sites, in spite of the strongly variable concentrations in soil. The REE content of soil water is therefore probably mainly solubility-controlled. The REE patterns of soil water are slightly enriched in the HREE with respect to soil as a consequence of the higher stability of dissolved organic and inorganic HREE complexes.
Plant roots absorb the REE from the adsorbed soil water pool rather than from gravitational soil water. The REE concentrations in roots vary with the REE contents in soil, but there was a slight enrichment of the LREE. This enrichment is probably related to the higher mobility of the LREE in soils mentioned before and amplified by the higher stability of dissolved HREE complexes in soil water. In fact, earlier studies have shown that roots preferentially absorb free ions rather than dissolved complexes. The preferential speciation of the HREE as dissolved complexes could therefore contribute to the LREE enrichment of plant roots. This is mainly the case for the limestone site.
The REE absorbed by the roots are efficiently filtered by the Casparian strip and only a small amount is transported by sap flow to the aerial plant organs. This leads to decreasing REE concentrations in the order roots > leaves/needles > trunk. Within the sap, the REE are, as in the roots, correlated with Fe, but further correlations occur with macronutrients (Mg, K, P), suggesting that the REE transport in sap is related to a mechanism which controls the general nutrient flux. The sap samples are additionally characterized by positive Ce anomalies suggesting that the REE may be associated with colloidal Fe-oxyhydroxide particles.
From the above observations it can be concluded that the transfer of the REE at the soil/root interface is not primarily controlled by the plant itself, but REE concentration and speciation in soil and the adsorbed soil water pool play significant roles. To absorb the REE, plant roots seem to use the same mechanisms as for Fe, which is an essential plant nutrient and like the REE a poorly soluble trace metal. The detailed analysis of REE/Fe ratios shows that LREE/Fe ratios are higher in roots than in the adjacent soil, pointing to preferential absorption of the LREE. Within the xylem, the REE continue to follow the pathway of Fe, but now together with macronutrients such as Mg, K, and P. The transfer of the REE in soil-plant systems thus seems to be intimately coupled with the Fe cycle. The REE could therefore in the future, like Fe and other stable metal isotopes, be used to characterize the transport mechanisms during trace metal cycling within soil-plant systems.
References
Aouad G, Stille P, Crovisier J, Geoffroy VA, Meyer J, Lahd-Geagea M (2006) Influence of bacteria on lanthanide and actinide transfer from specific soil components (humus, soil minerals and vitrified municipal solid waste incinerator bottom ash) to corn plants: Sr-Nd isotope evidence. Sci Total Environ 370:545–551
Aubert D, Stille P, Probst A (2001) REE fractionation during granite weathering and removal by waters and suspended loads: Sr and Nd isotopic evidence. Geochim Cosmochim Acta 65:387–406
Azoulay R, Alison M, Sekkal A, Tilea B, Chalard F, Sebag G (2007) RP-WP-32 IRM Osteo-articulaire de l’enfant: apports de l’injection de chelate de gadolinium. J Radiol 88:1597–1597
Babula P, Adam V, Opatrilova R, Zehnalek J, Havel L, Kizek R (2008) Uncommon heavy metals, metalloids and their plant toxicity: a review. Environ Chem Lett 6:189–213
Bau M, Dulski P (1996) Anthropogenic origin of positive gadolinium anomalies in river waters. Earth Planet Sci Lett 143:245–255
Bau M, Knappe A, Dulski P (2006) Anthropogenic gadolinium as a micropollutant in river waters in Pennsylvania and in Lake Erie, northeastern United States. Chem Erde Geochem 66:143–152
Bayer ME, Bayer MH (1991) Lanthanide accumulation in the periplasmic space of Escherichia coli B. J Bacteriol 173:141–149
Bernard L, Anthony L, Daniel A, Nadya R, Natalie M, Tilman BD (2005) Chronic renal failure is associated with increase tissue depletion of lanthanum after 28-day oral administration. Kidney Int 67:1062–1069
Berner EK, Berner RA, Moulton KL (2003) Plants and mineral weathering: present and past. In: Drever JI, Turekian KK (eds) Surface and ground water, weathering and soils. Elsevier, pp 169–188
Braun JJ, Pagel M, Muller JP, Bilong P, Michard A, Guillet B (1990) Ce anomalies in lateritic profiles. Geochim Cosmochim Acta 54:781–795
Briner W, Rycek RF, Moellenberndt A, Dannull K (2000) Neurodevelopmental effects of lanthanum in mice. Neurotoxicol Teratol 22:573–581
Brookins DG (1989) Aqueous geochemistry of the rare earth elements. In: Lipin BR, McKay GA (eds) Geochemistry and mineralogy of rare earth elements. Mineral. Soc. Am, Washington, pp 201–225
Che Y, Xing R, Zhu Y, Cui Y, Jiang X (2010) Effects of lanthanum chloride administration on detouring learning in chicks. Biol Trace Elem Res: 1–7
Chen Z (2011) Global rare earth resources and scenarios of future rare earth industry. J Rare Earths 29:1–6
D’Haese PC, Spasovski GB, Sikole A, Hutchison A, Freemont TJ, Sulkova S, Swanepoel C, Pejanovic S, Djukanovic L, Balducci A et al (2003) A multicenter study on the effects of lanthanum carbonate (Fosrenol) and calcium carbonate on renal bone disease in dialysis patients. Kidney Int 64:2327–2328
Ding S, Liang T, Zhang C, Yan J, Zhang Z (2005a) Accumulation and fractionation of rare earth elements (REEs) in wheat: controlled by phosphate precipitation, cell wall absorption and solution complexation. J Exp Bot 56:2765–2775
Ding S, Liang T, Zhang C, Yan J, Zhang Z, Sun Q (2005b) Role of ligands in accumulation and fractionation of rare earth elements in plants. Biol Trace Elem Res 107:73–86
Ding S, Liang T, Zhang C, Huang Z, Chen T (2006a) Fractionation mechanisms of rare earth elements (REEs) in hydroponic wheat: an application for metal accumulation by plants. Environ Sci Technol 40:2686–2691
Ding S, Liang T, Zhang C, Wang L, Sun Q (2006b) Accumulation and fractionation of rare earth elements in a soil-wheat system. Pedosphere 16:82–90
Ding S, Liang T, Zhang C, Yan J, Zhang Z (2006c) Effects of organic ligands on fractionation of rare earth elements (REEs) in hydroponic plants: an application to the determination of binding capacities by humic acid for modeling. Chemosphere 65:1942–1948
Dong S, Zhao Y, Liu H, Yang X, Wang K (2009) Duality of effect of La3+ on mitochondrial permeability transition pore depending on the concentration. Biometals 22:917–926
Elbaz-Poulichet F, Seidel J, Othoniel C (2002) Occurrence of an anthropogenic gadolinium anomaly in river and coastal waters of Southern France. Water Res 36:1102–1105
Feng L, Xiao H, He X, Li Z, Li F, Liu N, Zhao Y, Huang Y, Zhang Z, Chai Z (2006) Neurotoxicological consequence of long-term exposure to lanthanum. Toxicol Lett 165:112–120
Fichter J, Turpault M, Dambrine E, Ranger J (1998) Mineral evolution of acid forest soils in the Strengbach catchment (Vosges mountains, N-E France). Geoderma 82:315–340
França EJ, De Nadai Fernandes EA, Bacchi MA, Tagliaferro FS (2002) Pathway of rare-earth elements in a Brazilian forestry fragment. J Alloys Compd 344:21–26
Fu F, Akagi T, Yabuki S, Iwaki M (2001) The variation of REE (rare earth elements) patterns in soil-grown plants: a new proxy for the source of rare earth elements and silicon in plants. Plant Soil 235:53–64
Galan E, Fernandes-Caliani JC, Miras A, Aparicio P, Marquez MG (2007) Residence and fractionation of rare earth elements during kaolinization of alkaline peraluminous granites in NW Spain. Clay Miner 42:341–352
Gao Y, Zeng F, Yi A, Ping S, Jing L (2003) Research of the entry of rare earth elements Eu3+ and La3+ into plant cell. Biol Trace Elem Res 91:253–265
Gromet LP, Dymek RF, Haskin LA, Korotev RL (1984) The “North American shale composite”: its compilation, major and trace element characteristics. Geochim Cosmochim Acta 48:2469–2482
Han F, Shan X, Zhang J, Xie Y, Pei Z, Zhang S, Zhu Y, Wen B (2005) Organic acids promote the uptake of lanthanum by barley roots. New Phytol 165:481–492
Hao S, Xiaorong W, Qin W, Huating W, Liansheng W, Chen Yijun Dai Lemei C (1997) The effects of chemical species on bioaccumulation of rare earth elements in wheat grown in nutrient solution. Chemosphere 35:1699–1707
Harlavan Y, Erel Y, Blum JD (2009) The coupled release of REE and Pb to the soil labile pool with time by weathering of accessory phases, Wind River Mountains, WY. Geochim Cosmochim Acta 73:320–336
Haxel GB, Hedrick JB, Orris GJ (2002) Rare earth elements: critical resources for high technology. U.S. Geological Survey Fact Sheet 087-02: http://pubs.usgs.gov/fs/2002/fs087-02/
He ML, Ranz D, Rambeck WA (2001) Study on the performance enhancing effect of rare earth elements in growing and fattening pigs. J Anim Physiol Anim Nutr (Berl) 85:263–270
He ML, Wang YZ, Xu ZR, Chen ML, Rambeck WA (2003) Effect of dietary rare earth elements on growth performance and blood parameters of rats. J Anim Physiol Anim Nutr (Berl) 87:229–235
He ML, Wehr U, Rambeck WA (2010) Effect of low doses of dietary rare earth elements on growth performance of broilers. J Anim Physiol Anim Nutr (Berl) 94:86–92
Henderson P (1984) Rare earth element geochemistry. Elsevier
Hennebrüder K, Wennrich R, Mattusch J, Stark H, Engewald W (2004) Determination of gadolinium in river water by SPE preconcentration and ICP-MS. Talanta 63:309–316
Hinsinger P (1998) How do plant roots acquire mineral nutrients? chemical processes involved in the rhizosphere. Adv Agron 64:225–265
Hirano S, Suzuki KT (1996) Exposure, metabolism, and toxicity of rare earths and related compounds. Environ Health Perspect 104:85–95
Hong F, Wang L, Meng X, Wei Z, Zhao G (2002) The effect of cerium (III) on the chlorophyll formation in spinach. Biol Trace Elem Res 89:263–276
Hopkins WG, Hüner NPA (2009) Introduction to plant physiology. Wiley
Hornig-Kjarsgaard I (1998) Rare earth elements in sovitic carbonatites and their mineral phases. J Petrol 39:2105–2121
Hu X, Ding Z, Chen Y, Wang X, Dai L (2002) Bioaccumulation of lanthanum and cerium and their effects on the growth of wheat (Triticum aestivum L.) seedlings. Chemosphere 48:621–629
Hutchison AJ, Speake M, Al-Baaj F (2004) Reducing high phosphate levels in patients with chronic renal failure undergoing dialysis: a 4-week, dose-finding, open-label study with lanthanum carbonate. Nephrol Dial Transplant 19:1902–1906
Klüsener B, Boheim G, Liss H, Engelberth J, Weiler EW (1995) Gadolinium-sensitive, voltage-dependent calcium release channels in the endoplasmic reticulum of a higher plant mechanoreceptor organ. EMBO J 14:2708–2714
Knappe A, Möller P, Dulski P, Pekdeger A (2005) Positive gadolinium anomaly in surface water and ground water of the urban area Berlin, Germany. Chem Erde Geochem 65:167–189
Koeppenkastrop D, De Carlo E (1992) Sorption of rare-earth elements from seawater onto synthetic mineral particles: an experimental approach. Chem Geol 95:251–263
Kulaksiz S, Bau M (2007) Contrasting behaviour of anthropogenic gadolinium and natural rare earth elements in estuaries and the gadolinium input into the North Sea. Earth Planet Sci Lett 260:361–371
Kulaksiz S, Bau M (2011) Anthropogenic gadolinium as a microcontaminant in tap water used as drinking water in urban areas and megacities. Appl Geochem 26:1877–1885
Kümmerer K, Helmers E (2000) Hospital effluents as a source of gadolinium in the aquatic environment. Environ Sci Technol 34:573–577
Lambert MC, Ung CH, Raulier F (2005) Canadian national tree aboveground biomass equations. Can J For Res 35:1996–2018
Land M, Öhlander B, Ingri J, Thunberg J (1999) Solid speciation and fractionation of rare earth elements in a spodosol profile from northern Sweden as revealed by sequential extraction. Chem Geol 160:121–138
Laveuf C, Cornu S (2009) A review on the potentiality of Rare Earth Elements to trace pedogenetic processes. Geoderma 154:1–12
Liang T, Zhang S, Wang L, Kung H, Wang Y, Hu A, Ding S (2005) Environmental biogeochemical behaviors of rare earth elements in soil–plant systems. Environ Geochem Health 27:301–311
Liang T, Ding S, Song W, Chong Z, Zhang C, Li H (2008) A review of fractionations of rare earth elements in plants. J Rare Earths 26:7–15
Lipin B, McKay G (1989) Geochemistry and mineralogy of the rare earth elements. Mineralogical Society of America, Washington
Livergood R (2010) Rare earth elements: a wrench in the supply chain. Center for Strategic and International Studies: http://csis.org/files/publication/101005_DIIG_Current_Issues_no22_Rare_earth_elements.pdf
Loell M, Albrecht C, Felix-Henningsen P (2011) Rare earth elements and relation between their potential bioavailability and soil properties, Nidda catchment (Central Germany). Plant Soil 349:303–317
Long KR, Van Gosen BS, Foley NK, Cordier D (2010) The principal rare earth elements deposits of the United States—a summary of domestic deposits and a global perspective. U.S. Geological Survey Scientific Investigations Report 2010–5220: 1–96. http://pubs.usgs.gov/sir/2010/5220/
Marsac R, Davranche M, Gruau G, Dia A (2010) Metal loading effect on rare earth element binding to humic acid: experimental and modelling evidence. Geochim Cosmochim Acta 74:1749–1761
McLennan S (1989) Rare earth elements in sedimentary rocks: influence of provenance and sedimentary processes. In: Lipin B, McKay G (eds) Geochemistry and mineralogy of rare earth elements. Mineral. Soc. Am, Washington, pp 169–225
Möller P, Dulski P, Bau M, Knappe A, Pekdeger A, Sommer-von Jarmersted C (2000) Anthropogenic gadolinium as a conservative tracer in hydrology. J Geochem Explor 69–70:409–414
Möller P, Paces T, Dulski P, Morteani G (2002) Anthropogenic Gd in surface water, drainage system, and the water supply of the City of Prague, Czech Republic. Environ Sci Technol 36:2387–2394
Möller P, Morteani G, Dulski P (2003) Anomalous gadolinium, cerium, and yttrium contents in the Adige and Isarco river waters and in the water of their tributaries (Provinces Trento and Bolzano/Bozen, NE Italy). Acta Hydrochim Hydrobiol 31:225–239
Neilands JB (1981) Microbial iron compounds. Annu Rev Biochem 50:715–731
Nozaki Y, Lerche D, Alibo DS, Tsutsumi M (2000) Dissolved indium and rare earth elements in three Japanese rivers and Tokyo Bay: evidence for anthropogenic Gd and In. Geochim Cosmochim Acta 64:3975–3982
Ogata T, Terakado Y (2006) Rare earth element abundances in some seawaters and related river waters from the Osaka Bay area, Japan: significance of anthropogenic Gd. Geochem J 40:463–474
Öhlander B, Land M, Ingri J, Widerlund A (1996) Mobility of rare earth elements during weathering of till in northern Sweden. Appl Geochem 11:93–99
Pang X, Li D, Peng A (2002) Application of rare-earth elements in the agriculture of China and its environmental behavior in soil. Environ Sci Pollut Res 9:143–148
Probst A, Viville D, Fritz B, Ambroise B, Dambrine E (1992) Hydrochemical budgets of a small forested catchment exposed to acid deposition: the Strengbach catchment case study (Vosges massif, France). Water Air Soil Pollut 62:337–347
Rabiet M, Brissaud F, Seidel J, Pistre S, Elbaz-Poulichet F (2005) Deciphering the presence of wastewater in a medium-sized Mediterranean catchment using a multitracer approach. Appl Geochem 20:1587–1596
Reichman S, Parker D (2005) Metal complexation by phytosiderophores in the rhizosphere. In: Huang P, Gobran G (eds) Biogeochemistry of trace elements in the rhizosphere. Elsevier, Amsterdam, pp 129–156
Robin A, Vansuyt G, Hinsinger P, Meyer J, Briat J, Lemanceau P (2008) Iron dynamics in the rhizosphere: consequences for plant health and nutrition. In: Advances in agronomy. Academic Press, pp 183–225
Samuel J, Rouault R, Besnus Y (1985) Analyse multi-élémentaire standardisée des matériaux géologiques en spectrométrie d’émission par plasma par couplage inductif. Analysis 13:312–317
Schwabe A, Meyer U, Grün M, Voigt K, Flachowsky G, Dänicke S (2012) Effect of rare earth elements (REE) supplementation to diets on the carry-over into different organs and tissues of fattening bulls. Livest Sci 143:5–14
Semhi K, Chaudhuri S, Clauer N (2009) Fractionation of rare-earth elements in plants during experimental growth in varied clay substrates. Appl Geochem 24:447–453
Shan X, Wang H, Zhang S, Zhou H, Zheng Y, Yu H, Wen B (2003) Accumulation and uptake of light rare earth elements in a hyperaccumulator Dicropteris dichotoma. Plant Sci 165:1343–1353
Shannon R (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A32:751–767
Shtangeeva I, Ayrault S (2007) Effects of Eu and Ca on yield and mineral nutrition of wheat (Triticum aestivum) seedlings. Environ Exp Bot 59:49–58
Sonke JE, Salters VJM (2006) Lanthanide-humic substances complexation. I. Experimental evidence for a lanthanide contraction effect. Geochim Cosmochim Acta 70:1495–1506
Steinmann M, Stille P (1997) Rare earth element behavior and Pb, Sr, Nd isotope systematics in a heavy metal contaminated soil. Appl Geochem 12:607–623
Steinmann M, Stille P (2008) Controls on transport and fractionation of the rare earth elements in stream water of a mixed basaltic-granitic catchment basin (Massif Central, France). Chem Geol 254:1–18
Stille P, Steinmann M, Pierret MC, Gauthier-Lafaye F, Chabaux F, Viville D, Pourcelot L, Matera V, Aouad G, Aubert D (2006) The impact of vegetation on REE fractionation in stream waters of a small forested catchment (the Strengbach case). Geochim Cosmochim Acta 70:3217–3230
Stille P, Pierret MC, Steinmann M, Chabaux F, Boutin R, Aubert D, Pourcelot L, Morvan G (2009) Impact of atmospheric deposition, biogeochemical cycling and water-mineral interaction on REE fractionation in acidic surface soils and soil water (the Strengbach case). Chem Geol 264:173–186
Taiz L, Zeiger E (2006) Plant physiology. Sinauer
Taylor SR, McLennan S (1985) The continental crust: its composition and evolution. Blackwell
Thiry Y, Schmidt P, Van Hees M, Wannijn J, Van Bree P, Rufyikiri G, Vandenhove H (2005) Uranium distribution and cycling in Scots pine (Pinus sylvestris L.) growing on a revegetated U-mining heap. J Environ Radioact 81:201–219
Tyler G (2004) Rare earth elements in soil and plant systems—a review. Plant Soil 267:191–206
Verplanck P, Taylor H, Nordstrom D, Barber L (2005) Aqueous stability of gadolinium in surface waters receiving sewage treatment plant effluent, boulder creek, Colorado. Environ Sci Technol 39:6923–6929
Wang Y, Sun J, Chen H, Guo F (1997) Determination of the contents and distribution characteristics of REE in natural plants by NAA. J Radioanal Nucl Chem 219:99–103
Wang H, Shan X, Zhang S, Wen B (2003) Preliminary characterization of a light-rare-earth-element-binding peptide of a natural perennial fern Dicranopteris dichotoma. Anal Bioanal Chem 376:49–52
Wilde EW, Berry CJ, Goli MB (2002) Toxicity of gadolinium to some aquatic microbes. Bull Environ Contam Toxicol 68:420–427
Wyttenbach A, Furrer V, Schleppi P, Tobler L (1998) Rare earth elements in soil and in soil-grown plants. Plant Soil 199:267–273
Xiangsheng L, Jiachen W, Jun Y, Yubin F, Yanping W, He Z (2006) Application of rare earth phosphate fertilizer in Western Area of China. J Rare Earths 24:423–426
Xu X, Zhu W, Wang Z, Witkamp G (2002) Distributions of rare earths and heavy metals in field-grown maize after application of rare earth-containing fertilizer. Sci Total Environ 293:97–105
Xu X, Zhu W, Wang Z, Witkamp G (2003) Accumulation of rare earth elements in maize plants (Zea mays L.) after application of mixtures of rare earth elements and lanthanum. Plant Soil 252:267–277
Yang J, Liu Q, Zhang L, Wu S, Qi M, Lu S, Xi Q, Cai Y (2009) Lanthanum chloride impairs memory, decreases pCaMK IV, pMAPK and pCREB expression of hippocampus in rats. Toxicol Lett 190:208–214
Zeng F, Tian H, Wang Z, An Y, Gao F, Zhang L, Li F, Shan L (2003) Effect of rare earth element europium on amaranthin synthesis in Amarathus caudatus seedlings. Biol Trace Elem Res 93:271–282
Zhenggui W, Ming Y, Xun Z, Fashui H, Bing L, Ye T, Guiwen Z, Chunhua Y (2001) Rare earth elements in naturally grown fern Dicranopteris linearis in relation to their variation in soils in South-Jiangxi region (Southern China). Environ Pollut 114:345–355
Zhu W, Xu S, Shao P, Zhang H, Wu D, Yang W, Feng J (1997) Bioelectrical activity of the central nervous system among populations in a rare earth element area. Biol Trace Elem Res 57:71
Acknowledgments
We would like to thank P. Deleule (ONF–French National Forests Office) for his constructive collaboration during field work on the limestone site at Cussey-sur-Lison. R. Boutin (LHyGeS Strasbourg) is thanked for major and trace element analyses, and Ch. Loup (Chrono-Environnement Besançon) for CaCO3 and organic carbon analyses. The financial support by the French CNRS program “EC2CO-Cytrix”is kindly acknowledged. L. Brioschi benefited from a French Ministry of National Education and Research Ph.D. grant. We are grateful to L. Pourcelot for his prereview of the manuscript and P. Winterton for English corrections. The constructive comments of two anonymous reviewers helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Robert Reid.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary data 1
REE concentrations of bedrock samples in mg/kg. (XLS 13 kb)
Supplementary data 2
REE concentrations of bulk soil samples in mg/kg. (XLS 18 kb)
Supplementary data 3
REE concentrations of soil water samples in μg/L. The soil water data from the granite site are from Stille et al. (2009). All soil waters were sampled in autumn. (XLS 12 kb)
Supplementary data 4
Bulk chemistry of spruce samples of the limestone site. The meter values given for the trunk samples correspond to the sampling height above ground. The letters following sampling height indicate the sampling position on an individual slice of trunk: “O” = outer, “M” = middle and “C” = central portion. Concentrations for the REE and U in μg/kg, all other elements in mg/kg. (XLS 38 kb)
Supplementary data 5
Bulk chemistry of beech samples from the limestone site. The meter values given for the trunk samples correspond to the sampling height above ground. The letters following sampling height indicate the sampling position on an individual slice of trunk: “O” = outer, “M” = middle and “C” = central portion. Concentrations for the REE and U in μg/kg, all other elements in mg/kg. (XLS 27 kb)
Supplementary data 6
Bulk chemistry of spruce and beech samples from the granite site. The meter values given for the trunk samples correspond to the sampling height above ground. The letters following sampling height indicate the sampling position on an individual slice of trunk: “O” = outer, “M” = middle and “C” = central portion. Concentrations for the REE and for U in μg/kg (μg/L for sap), all other elements in mg/kg (mg/L for sap). (XLS 31 kb)
Supplementary data 7
Bulk chemistry of oak samples from the carbonatite site. Concentrations for the REE and for U in μg/kg (μg/L for sap), all other elements in mg/kg (mg/L for sap). (XLS 13 kb)
Rights and permissions
About this article
Cite this article
Brioschi, L., Steinmann, M., Lucot, E. et al. Transfer of rare earth elements (REE) from natural soil to plant systems: implications for the environmental availability of anthropogenic REE. Plant Soil 366, 143–163 (2013). https://doi.org/10.1007/s11104-012-1407-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1407-0