Abstract
In the present study, the tube well water quality and the associated health risks, emphasizing on arsenic contamination, were investigated in rural and urban samples from Tehsil Mailsi located in Punjab, Pakistan. Arsenic concentrations (μg/L) were ranged from 12 to 448.5 and which exceeded the WHO recommended limit (10 μg/L) in all cases. The calculated average daily dose (3.3 × 10−0.4 to 1.2 × 10−0.2 mg/kg day) and hazard quotient (1.1–40) reflected the potential health risk to local population due to tube well water consumption as drinking purpose. Sodium percent (Na%), sodium absorption ratio, residual sodium carbonate, Kelly’s index and magnesium absorption ratio were also determined to assess the suitability of tube well water for irrigation purpose. The resulting piper plot revealed the Na–Ca–HCO3 type water chemistry of the area and generally alkaline environment. The spatial distribution of arsenic in the tube well waters pinpoints the significant contribution of anthropogenic activities to arsenic pollution. Nevertheless, different statistical tools, including principal component analysis, hierarchical cluster analysis and correlation matrices, revealed the contribution of both natural and anthropogenic activities and alkaline type of aquifers toward the high level of arsenic contamination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) pollution in groundwater (GW) is a human health threat affecting millions of people worldwide (Katsoyiannis et al. 2015), particularly in Bangladesh, India, China, Argentina, Chile, Mexico and Pakistan (Bhowmik et al. 2015; Amini et al. 2008). The situation is much worse in semiarid and arid regions, where groundwater availability is critical for both drinking and irrigation purposes (Adhikary et al. 2010). In many semiarid and arid regions of Pakistan, groundwater accounts for more than 40 % of water used for irrigation (Briscoe and Qamar 2007), although arsenic contamination has already been demonstrated in the groundwater of Muzaffargarh (Nickson et al. 2005), Kalalanwala (Naseem et al. 2001; Farooqi et al. 2007), Jamshoro (Arain et al. 2009) and Khairpur (Fatmi et al. 2009). However, in the different areas of Indus plain, a large number (about one million) of tube wells are installed to fulfill the irrigation water demand of growing agricultural areas and have become important irrigation feature, which also has long-term effects on the quality and quantity of groundwater. Nevertheless, available data on arsenic contamination in tube well waters and its associated health risk effects in these areas are limited. For the assessment of suitability of the studied water for irrigation, SAR, RSC, %Na, KI and MAR are reported to be helpful proxies (Singh et al. 2012; Ramkumar et al. 2010). Base-exchange and meteoric genesis indices can also be used to evaluate sustainable water resources for irrigation. Possible sources influencing water systems were identified using different multivariate approaches, such as principal components analysis (PCA) and cluster analysis (CA). These approaches are reported as valuable tools for reliable management and rapid solutions of water resources and pollution problems, respectively (Reghunath et al. 2002; Zhang et al. 2011).
High arsenic concentrations have been reported from the surrounding areas of Sargana and Mailsi including Multan and Muzaffargarh (Nickson et al. 2005) and highlighted that arsenic concentrations are spatially variable. Therefore, monitoring of arsenic contamination in the As-rich tube well groundwater is essential in order to estimate the extent of arsenic exposure to the exposed population of about 700 thousand people. The objectives of the current study were to study: (1) the groundwater irrigation quality and the extent of arsenic contamination in Tehsil Mailsi area, (2) the source identification of As contamination using multivariate analysis and other statistical tools and (3) evaluation of health risk to the local population due to As contamination. The findings of this study highlight for first time the exposure levels of the local population to arsenic in the groundwater (tube well) of Mailsi Tehsil area and underline the associated human health risks, contributing to achieving sustainable water resources management, which is fundamental for a better economic growth of the country.
Materials and methods
Description of the study area
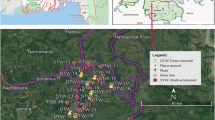
Tehsil Mailsi is located in southern Punjab, Pakistan (72°17′–72°19′E, 29°78′–29°92′N), covering an area of 14.88 km2, with an altitude of 126 m above sea level (Fig. 1). The southern Punjab, Pakistan, is located within an alluvial plain of the south-flowing Indus River and its major tributaries. The local population is 704,878 people according to census of Pakistan (2010). Farming is the basic occupation of population with major cash crops including cotton, wheat, sugarcane and rice. The groundwater obtained from tube wells serves as the main water supply for irrigation in these areas. From late June to August, the monsoon season starts, with heavy rainfall throughout the country. The average annual precipitation is 243 mm, and the mean temperature is 26 °C (DCR 1998). Mailsi is situated along river Sutlej; however, this river is dry in most part of the year that is why this area is facing shortage of good quality drinking water. Groundwater is the most important source of drinking water in this area. Unluckily, due to unplanned urbanization for the past few decades, the resource degraded in quality. The factors that are affecting water quality of Mailsi are extensive use of fertilizers, pesticides, sewerage system, drainage system, solid waste management and land fill/dumping sites. Among all these factors, inappropriate sewerage system and use of fertilizers are the main causes of water pollution where sewerage water is mixing with ground water and fertilizers are seeping down the groundwater (Abbas et al. 2014).
The study area is characterized by shallow aquifers, which is under alluvial plains, with thick layer of Holocene and Pleistocene sediments transported by the River Sutlej (Greenman et al. 1967), originating from the southern slopes of the holy mountain Kailash, near the lake of Mansarovar and flows parallel to the Himalayas. High percentage of silt, clay, fine sand and low organic matter is present in these sediments. The study area consists of a thickened sequence of unconsolidated flood plain fluvial deposits and eolian deposits of Pleistocene to present age. Calcium carbonate concretions of irregular shape, but of regular size and distribution, are associated with these sediments (Farooqi et al. 2007). The study area is toward the southwestern part of Bari Doab (the area between the two rivers, Sutlej and Ravi), which covers moderately older alluvial deposits that tend to coincide with zones of highly mineralized groundwater (Greenman et al. 1967). The geologic features that influence permeability and transmissibility are lateral lithological changes, disparity in sand thickness and grain size distribution. The eolian deposits occur above the water table and have no hydrological significance except at few places where the zone of saturation may be within them. The sediments formed as channel infill, levees and overbank flood plain deposits, show lateral and vertical variation. This is due to cyclic shifting in the course of Indus river and its tributaries, which laid down these sediments. The grain size decreases from northeast to southwest, which points to heterogeneous conditions of deposition and cause for variation in the permeability values of the layers. The absence of continuous clay layers, in general, is the indicative of the presence of unconfined aquifers in the fluvial sediments (Farooqi et al. 2007). The sedimentary formations along the Indus River system are similar to those that include the As-contaminated aquifers in the sedimentary basins associated with the Ganga and Brahmaputra River system in Bangladesh and West Bengal, composed of Quaternary alluvial-deltaic sediments derived from Himalayan source rocks. However, the sedimentary basins along the Indus River system, located at the western edge of the Asian monsoon area, are in a more arid climate than other sediments. The older Quaternary (i.e., Pleistocene) deposits are more widely distributed in the western sedimentary basin, probably promoting more aerobic aquifer conditions in the study area than in the other locations (Mahmood et al. 1998; Tasneem 1999).
Sampling and analysis
Mailsi city and Sargana were selected as sampling sites in Tehsil Mailsi (Fig. 1). Mailsi is situated in active flood plain area, around 3 km away from Sutlej River. All the samples were collected in 2012 by following the standard procedures (Khan et al. 2012). The study area has a semiarid climate with long and extremely hot summers, and dry and warm winters, monsoons and dust storms. A total of 44 tube well water samples, 22 from Sargana and 22 from Mailsi, were collected on the basis of geographical distribution with depth of 80–95 m. At each site, survey among the residents was also conducted to collect information about water sources, economic status, health, age, sex and dietary habits. In the study area, 57 % of the participating population used hand and electric pumps for drawing water to surface for potable use, 18 % was supplied with water from water treatment plants, 5 % of the people used canal water (surface water) and 20 % of participating population used tube well water.
All groundwater samples were filtered immediately upon sampling on-site using 0.45-μm cellulose acetate filters in Millipore Sterivex syringe capsules. Nalgene-1 bottles (60 mL) were used to take filtered samples, for anion and cation determination, and at every 11th sampling point, a field duplicate was also collected. On-site field measurements (i.e., pH, temperature, dissolved oxygen (DO) and electrical conductivity, EC) were conducted during sample collection. The latitudes and longitudes were recorded via global positioning system (GPS) at the time of sample collection. The pH, EC, DO and TDS values in all water samples were determined by W2015 pH/EC meter and dissolved oxygen meter (Sino well Company, Shanghai, China). The sealed samples were stored at 4 °C in portable coolers and then transported to laboratory for further analysis.
Calcium (Ca2+) and magnesium (Mg2+) of water samples were analyzed by volumetric titration with ethylene diamine tetra acetic acid (EDTA, 0.05 N). Alkalinity (quoted as HCO3) by 0.1 HCl (Sultana et al. 2014). NO3 − was determined spectrophotometrically using UV/VIS spectrophotometer (Shimadzu model UV 1601) at a wavelength of 220 nm. Measurement of UV absorption at 220 nm enables rapid determination of NO3 −. To overcome interferences by dissolved organic matter, which also absorbs at 220 nm, measurements for NO3 − at 275 nm, where DOC does not absorb, were conducted and NO3 − values were accordingly corrected. SO4 2– values were determined by gravimetric method as BaSO4. Chloride (Cl−) was determined by titration method (APHA 1998). Na+ and K+ were determined using graphite furnace atomic absorption spectrometer (GFAAS AAS-700 PerkinElmer, USA). For total As analysis, 2 mL of 12 M HCl was added to 15 mL of sample water together with 0.25 mL of a solution containing 10 % KI and 10 % ascorbic acid. Graphite furnace atomic absorption spectrometer (GFAAS AAS-700 PerkinElmer, USA) was employed to measure the total As. The quality control of measurements was performed by using standard reference solutions of analytical grade chemicals with 99.9 % spectroscopic purity (Merck Darmstadt, Germany). Twice distilled water was used throughout the analysis, blank and duplicate samples. Reproducibility of the analytical data was within 5 %, and the analytical error estimated to be <10 %, based on the analytical results of standard stock solutions independently prepared from the commercially distributed standard solution using a standard calibration line.
Human health risk assessment
Average daily dose (ADD)
A health risk assessment model derived from US-EPA (2011) was applied to assess health risk in individuals exposed to As. The average daily dose (ADD) of arsenic through water consumption was calculated using the following equation:
where C = concentration of As (μg/L) in water, IR = the daily average intake of water rate (L/d, assumed to be 2 L/d for adult) (US-EPA 2011), ED = the exposure duration (assumed 67 years), EF = the exposure frequency (365 days/year), BW = the body average weights (kg, assumed to be 72 kg for adult), and AT = the average life time (24,455 days), respectively (Khan et al. 2012; Muhammad et al. 2011).
Non-carcinogenic and carcinogenic risk
The ratio of ADD and the highest reference dose without any adverse effect risk is called hazard quotient (HQ) and is used to express the risk of non-carcinogenic effects (US-EPA 2011). Oral reference dose (Ro fD) of arsenic could be used to assess HQs through water exposure. Non-carcinogenic health risks in the study area, because of tube well water consumption, were assessed by hazard quotient (HQ) under standard assumptions (US-EPA 2011). When HQ value >1 represents potential health risk concern, while the value less than one is considered safe for consumers (Khan et al. 2008) and RefD equal to 0.0003 mg/kg day can be toxic (US-EPA 2011; Muhammad et al. 2010; Shah et al. 2012). THQ was calculated by using the following relationship:
We characterized the risk by estimating the risk of cancer using the Cancer Slope Factor index and lifetime average daily dose with a probabilistic approach. Carcinogenic risks (CRs) were estimated by ADD and exposure life time to arsenic (US-EPA 2011). The CSF is also called a “potency factor” and is used to calculate the Incremental Lifetime Cancer Risk by multiplying the CSF by the chronic daily intake (ADD). The ADD is the dose over a lifetime and is expressed in mg/kg-day (Chen et al. 1985). The Cancer Slope Factor is used to derive the risk-specific dose (RSD) (mg/kg-day) for direct acting carcinogenic agents. The RSD is often calculated based on a one-in-a-million extra risk (10−6 risk) or a one-in-a-hundred-thousand risk (10−4 risk) for other than highly exposed individuals (Chen et al. 1985). The range of risk levels for carcinogens is from 10−4 to 10−6 over a human lifetime. CR was calculated by the following equation:
where CSF = 1.5 mg/kg day is the cancer slope factor for arsenic (US-EPA 2011).
Tube well water quality parameters
In the current study, the discussion of water quality for irrigation is mainly based on the following factors: Sodium absorption ratio (SAR) was calculated using the following formula (Patterson 1994);
where all ionic concentrations are in meq/L. The sodium in tube well water is denoted as percentage of sodium (%Na) and calculated by the following formula:
The quantities of Ca2+, Mg2+, Na+ and K+ are expressed as meq/L. Residual sodium carbonate (RSC) was determined by the following formula:
Ion concentrations were taken in meq/L.
Base-exchange index was calculated by the following equation (Soltan 1998):
Equation (8) was used for estimating the meteoric genesis index (Singh et al. 2012):
The concentrations of Na+, K+, Cl− and SO4 2− were taken in meq/L. Kelly’s index (KI) was calculated as follows (Kelly 1940):
MAR was calculated using Eq. (10) (Szabolcs and Darab 1964):
Ion concentrations were expressed in meq/L.
Multivariate statistical analysis and maps
Hierarchical cluster analysis (HCA), principle component analysis (PCA) and correlation matrix (CM) were conducted using SPSS statistic software. The distribution maps of arsenic were made using Arc-GIS and Surfer software. Piper diagram (1994), Chadha (1999) and Stiff diagram (1951) were used to determine hydro-chemical facies and major ion composition, respectively.
Results and discussion
Major ion composition
Table 1 summarizes the analytical determinations of major cations and anions in tube well water samples from Mailsi and Sargana. The results were compared with the corresponding permissible limits recommended by the World Health Organization (WHO). Ground waters generally showed a weak alkaline behavior with a pH range of 6.5–8.3 and 6.4–8.4 in Sargana and Mailsi, respectively (Table 1). The pH of tube well water indicated that the dissolved carbonates in groundwater were predominantly in the bicarbonate form (Lopez et al. 2010; Baig et al. 2009b). Total dissolved solids (TDS), pH, dissolved oxygen (DO) and EC values were within permissible limits (WHO 2008) except for one sample of Mailsi area. The samples contained high value of EC and TDS most likely due to domestic waste with high dissolved solids (Pradeep 1998; Shyamala et al. 2008). Concentration of SO4 2− ranged from 132 to 943.6 mg/L (Table 1). Higher concentrations of SO4 2− were observed in Mailsi area, and 59 % tube well water samples were exceeding the permissible limit of 250 mg/L (WHO 2008) in both Sargana and Mailsi. From both sites, 77 % of samples exceeded the NO3 − permissible concentration limit of 50 mg/L (WHO 2008). The higher concentration of NO3 − and SO4 2− in tube well water may be attributed to rock minerals, sewage discharges, animal manure, detergents and fertilizer-based agriculture practices in Tehsil Mailsi and its surrounding agricultural areas (Kahlown et al. 2006). The Cl− concentration in most samples was below the permissible limit of 250 mg/L (WHO 2008). Some water samples had higher Cl− concentrations possibly due to local inputs from fertilizers at orchards and/or domestic wastes disposals in these areas. In 59 % of the samples, the concentration of HCO3 − exceeded the standard value (500 mg/L, WHO 2008). Increased concentrations of HCO3 − salts are due to weathering of carbonate rocks (Ahmed et al. 2004).
In current studied area, the concentrations of K+, Fe2+ and Mn2+ were lower than the recommended limits (WHO 2008). However, relatively higher values of Na+, Ca2+ and Mg2+ concentration were measured in collected water samples. The major sources of K+ are the weathering of mafic and ultramafic rocks (Singh et al. 2004) as well as sewage and industrial effluents. Lower concentrations of K+ in all water samples can be justified due to the low disintegration rate on fixed clay minerals (Geleijnse et al. 2003). The higher values of Na+ (119.2–587 mg/L) in our study might be related to the precipitation and/or coating on the minerals and also due to extensive use of detergents, discharged municipal waste and effluent percolation into water pathways (Manzoor et al. 2006). Weathering of silicate minerals is the main cause for higher concentrations of Ca2+ and Mg2+ in tube well water in both areas (Ramkumar et al. 2010).
Evaluation of tube well water quality
Tube well water samples were classified on the basis of major groundwater ion chemistry (Piper 1994). Approximately 83 % of tube well water samples showed distribution of mixed type cations, 16 % were of (Na++K+) type, whereas one sample was of Mg2+ type, as shown in Piper diagram (Fig. S-1). Among the major anions, 64 % of samples fall within mixed type area and 25 % turned out to be of Cl− type. Na+–HCO3 − and Ca2+–HCO3 − were two major types of water, identified by Piper diagram (Fig. S-1). This indicates that the dominant anion is HCO3 − while the dominant cations are Na+ and Ca2+. High Na+, Ca2+ and HCO3 − concentrations also make the water improper for irrigation purposes. This suggested that concentrations of major ions Na+, Ca2+, Mg2+ increase due to water–rock interactions. The water–rock interactions generally include chemical weathering of rock-forming minerals, dissolution precipitation of secondary carbonates, and ion exchange between water and clay minerals (Moghaddam and Fijani 2008).
Chada (1999) proposes a hydro-geochemical scheme for drinking water purpose by plotting the difference between (CO3 2− + HCO3 −) and Cl− +SO4 2− concentrations in meq/L expressed as percent versus (Ca2+ + Mg2+) and (Na+ + K+) concentrations in meq/L as percent, based on Piper diagram (1994). The resulting diagram has four fields representing four types of hydro-geochemical processes (Fig. S-2). It can by seen by Figure S-2 that 16 % (N = 7) of the samples collected in the study area were of Na–Cl type, which showed the dominance of evaporation, while 84 % of the samples fall in the category of Ca–Mg–Cl, Na–HCO3 and Ca–HCO3 type. Similarly, Figure S-3 shows that 9 % (N = 4) were of Ca–Mg–Cl type, 50 % (N = 22) as Na–HCO3 type and only 25 % as Ca–HCO3 (N = 11). However, these samples showed the water quality of recharge and least affected by the evaporation. Water quality of Na–HCO3 type is produced because of the base ion exchange, and Ca–Mg–Cl type water quality represents the reverse ion exchange (Chae et al. 2006; Guo et al. 2007). Moreover, the average ionic composition analysis by stiff diagram has also shown in Fig. S-3, which represents the dominance of Na+–Cl−, Mg2+–SO42− and Ca2+–HCO3 −.
The suitability of GW for irrigation depends on their mineral composition. Criteria to determine tube well water quality include: EC, SAR, RSC, KI, MAR and %Na. Table 2 shows the classification of tube well water samples from Sargana and Mailsi. SAR values ranged from 2.6 to 13.56 and 3.3 to 8.3 in Sargana and Mailsi cities, respectively. Considering relative frequency distribution regarding SAR (Table 6), in Sargana, 7 samples of tube well water (32 %) were fit for irrigation, while in Mailsi, 11 samples (50 %) were suitable for irrigation (Richard 1954). However, 12 samples (54 %) were marginally appropriate in Sargana for irrigation purpose, while 11 samples (50 %) were marginally appropriate for irrigation purpose in Mailsi (Muhammad et al. 1996), and 3 samples (14 %) were found as improper in Sargana (Richard 1954). Increase in sodium absorption values was due to increase in Na+ proportion as compared to Ca2+ + Mg2+ in water samples (Emerson and Bakker 1973). Ability of soil to transmit water is severely reduced by excessive sodicity in Sargana as compared to Mailsi. The classification of tube well water samples according to % Na is also given in Table 2. The range of sodium percentage was 38.8–78.5 and 51.5–62.5 % in Sargana and Mailsi, respectively (FAO 1992). Considering relative frequency distribution regarding percentage of sodium (Table 6), in both study areas, 10 samples (45 %) in Sargana and 10 samples (45 %) in Mailsi were marginally suitable for irrigation (Muhammad 1996). The range of RSC values in water samples was from 6.6 to 8.21 and 6.7 to 8.24 meq/L in the study area (Table 2). Considering relative frequency distribution regarding RSC (Table 6), in Sargana 7 samples (32 %) were fit, while in Mailsi 16 samples (73 %) were fit for irrigation (Richard 1954), and 3 samples (14 %) were marginally fit in Sargana, while 3 samples (14 %) were marginally fit in Mailsi (Muhammad 1996). Good quality water has RSC values below 1.25 meq/L, and water is considered as harmful if RSC values exceed 2.5 meq/L (Table 6). Similarly, Kelly’s index (KI) and Meteoric genesis index (r2), calculated using Eq. (8) for each sample, are also given in Table 2 to further assess the water quality of studied areas.
Spatially distributed arsenic in active flood plain
Arsenic concentrations in tube well waters ranged from 12 to 448.5 μg/L (Fig. 2) in the study area (Table 5). In both sites, all tube well water samples exceeded the WHO permissible limit of As (10 μg/L) in tube well water intended for human consumption. In the study area, As concentrations in tube well water were higher than those reported by Arain et al. (2009) in Manchar lake water (35–157 μg/L) and the nearby ground waters (23.3–96.3 μg/L), as well as in Jamshoro surface and groundwater (3–106 μg/L) (Baig et al. 2009a, b) but lower than the previous studies conducted in East Punjab (Farooqi et al. 2007) and Muzaffargarh (Nickson et al. 2005). Total arsenic concentration in unfiltered samples was higher near the Sutlej River areas than far ones (Fig. 2), and similar pattern was also recorded in the districts Multan and Muzaffargarh (Nickson et al. 2005). In south East Asia, As-affected areas include Red River Delta, Vietnam, Mekong basin, Cambodia (Berg et al. 2007), Ganga–Meghna–Brahmaputra plain, India (Chakraborti et al. 2002) Bengal delta, Bangladesh (Berg et al. 2001) and Western Snake River Plain, Idhao, USA (Busbee et al. 2009).
Natural enrichment of tube well water by As can arise in numerous ways such as hydrothermal volcanism, agricultural activities, oxidation of arsenical sulfide minerals (Singh and Jangveer 2011; Schreiber et al. 2000), reduction of FeOOH and the release of its sorbed load in groundwater (Ravenscroft et al. 2009), desorption of As from mineral sorption sites in response to increase in pH (Robertson 1989), and finally evaporative enrichment (Nicolli et al. 1989). In order to understand the possible mechanism for groundwater As release in the study area, earlier studies can be followed where different mechanisms have been suggested (Nickson et al. 2005; Farooqi et al. 2007; Halim et al. 2009). A study by Katsoyiannis et al. (2007) reported oxidative dissolution mechanism for the enrichment of As in groundwater on the basis of following indicators: high pH, NO3 − and SO4 2− and As are present in the form of AsV. On the other hand, oxidative dissolution mechanism has been suggested when high concentrations of HCO3 − (>500 mg/L) and SO4 2− (>250 mg/L) and pH (>7.5) occurred in groundwater (Smedley and Kinniburgh 2002). In the present study, higher concentrations of HCO3 −, SO4 2−, NO3 −, and high DO values along with alkaline pH (Table 1) are the indicative of oxidative environment. Hence, our findings are well supported by oxidative dissolution and to some extent by evaporative enrichment. In arid environments, evaporative concentration of dissolved materials can produce elevated As in groundwater (Bhattacharya et al. 2006). In these systems, evaporation enhances the concentrations of all ions in residual waters, a process likely to be occurred in regions where water table is very close to the surface and affected by evaporation (Nickson et al. 2005). Additionally, human activities can promote evaporative enrichment of As by decreasing water table to the near surface or by groundwater pumping for irrigation (Nickson et al. 2005). The main anthropogenic activities that may release As into the environment include smelting, fossil fuel processing and combustion, wood preserving, pesticide production, agricultural activities, disposal, incineration of municipal and industrial wastes (Popovic et al. 2001; Prosun et al. 2002). The use of arsenical pesticides presents a nonpoint anthropogenic source of arsenic. Arsenical pesticides include lead arsenate [Pb3(AsO4)2], calcium arsenate [Ca3(AsO4)2], magnesium arsenate [Mg3(AsO4)2], zinc arsenate [Zn3(AsO4)2], zinc arsenite [Zn(AsO2)2] and Paris green [Cu(CH3CCOO)2Cu(AsO2)2], (Martin et al. 2000).
In the present study, high Cl− in some samples and high Na+ concentrations are the indicators of high evaporation rates. This is consistent with dendrogram (Fig. S-4), as Cl− and As are in the same group. The ground waters from the basins are greatly affected by evaporative concentrations, resulting in high TDS values (Fujii and Swain 1995). Likewise, high EC in some samples is also related to high As concentrations in the study area (Table 1). This could be inferred from our results that As is released by oxidative dissolution under the influence of high alkaline water and high pH and to some extent by high evaporation rates.
As(V) can be effectively adsorbed on Fe-oxyhdroxide/oxide in pH less of equal to 7. At pH values higher than 7.5, arsenic starts to get desorbed from iron oxides and released into the groundwater (Katsoyiannis and Katsoyiannis 2006; Katsoyiannis and Zouboulis 2006). Natural evaporation over long periods can cause solute concentrations in shallow groundwater to increase (Welch et al. 2000), and effects of evaporative concentration under toxic conditions will be sorption of As to soils (Jones et al. 2009) and aquifer sediments (Nimick 1998).
Multivariate analysis for source apportionment
Table 3 shows correlation matrices (CM) for selected parameters, which were measured in tube well waters. The Pearson’s correlation coefficients and their significance levels (p < 0.05) were employed between all the variables presented (Table 3). The correlation matrix showed that various parameter pairs had significant positive correlations such as EC–TDS (r = 0.99), Ca2+–Mg2+ (r = 0.47), Mg2+–SO4 2− (r = 0.42), Mg2+–HCO3 −(r = 0.43), SO4 2−–Na+ (r = 0.46), SO4 2−–As (r = 0.39), NO3 −–HCO3 − (r = 0.45), Na+–As (r = 0.28), Mg2+–As (r = 0.38), HCO3 −–As (r = 0.49), Mn−–As (r = 0.31). These relationships were supplementary supported by dendrogram of CA in tube well water (Fig. S-4) and highlighted both natural and anthropogenic source of arsenic in the study area (Bhowmik et al. 2015).
For example, the use of arsenical pesticides in the study is already reported and may cause arsenic contamination in different ecological settings of Pakistan (Alamdar et al. 2016). Thus, As contamination is likely to occur due to the leaching process of pesticides and fertilizers from soils to groundwater (Sharma 2006). Exogenous introduction of As into the environment through the use of pesticides in the form of calcium arsenate, arsenic acid, lead arsenate and sodium arsenate (Alloway 1970; Woolson et al. 1971) is considered a major source of metal pollution in water. Water-soluble As in fertilizers (DAP) is estimated to be 5–10 mg/kg with an average value of 7.4 mg/kg (Farooqi et al. 2007b). Such a high concentration of As in fertilizers would be a pollutant source of soil surface and underlying groundwater associated with cultivation in the study area (Farooqi et al. 2007b). However, arsenical pesticides have infrequently been migrated with depth (Welch et al. 2000). Similarly, Hudak (2000) reported that groundwater pollution with As was associated with extensive use of arsenical agro-chemicals and other agricultural activities.
According to cluster analysis, the first group of parameters, such as Fe, pH, K+, EC, Mn, DO, Mg2+ and NO3 − (Fig. S-4), suggested that all these parameters originate from agricultural activities, industrial activities and weathering of mafic and ultramafic rocks (Shah et al. 2010).
The second group of parameters, such as As, Ca2+ and Cl− (Fig. S-4), possibly originated from parent rock material, agricultural and human activity. High As concentration in the region near to Sutlej River was compared to the area located far from it (Fig. 1) due to weathering and erosion of mafic and ultramafic rocks (Shah et al. 2010; Kavcar et al. 2009) and anthropogenic contribution of extensive use of fertilizers, pesticide, coal combustion, acid rain, sewerage system, drainage system, solid waste management, and landfills/dumping sites (Abbas et al. 2014; Khan et al. 2012; Shah et al. 2010; Nickson et al. 2005). This similar pattern was also observed in previous study of district Muzaffargarh and Multan (Nickson et al. 2005). The third group of parameters, consist of Na+, TDS, HCO3 − and SO4 2− (Fig. S-4), were considered as the result of agricultural waste: sewage waste and weathering of parent rock material. High SO4 2− concentrations were from household waste, fertilizer leaching and animals dung (Kahlown et al. 2006). HCO3 − salts are added to water sources from weathering of carbonaceous rocks (Ahmed et al. 2004). High Na+ concentrations may be derived from leaching of chemical fertilizer, household waste and animals manure (Kahlown et al. 2006).
Factor analyses (FA) that include principal component analysis (PCA) are used to further explain the source of arsenic and other studied variables (Table 4). Factor-1 contributed 24 % with moderately strong positive loading on EC (r = 0.613), TDS (r = 0.622), Mg2+ (r = 0.789), NO3 − (r = 0.532), SO4 2− (r = 0.576), HCO3 − (r = 0.587), Na+ (r = 0.582). The sources of EC, TDS, NO3 −, Mg2+, HCO3 −, SO4 2− and Na+ could be anthropogenic due to domestic waste water, agriculture activities and geogenic due to weathering minerals and Calc-silicate rocks (Farooqi et al. 2007a, b). The total variance is contributed by 14 % by factor-2 and 11 % by factor-3 with high value of Cl− (r = 0.484), K+ (r = 0.425) and DO (r = 0.775), Fe (r = 0.257), which is due to the local geochemical conditions, influenced by mafic and ultramafic rocks weathering (Shah et al. 2000). Factor-4 contributing 8.1 % shows high concentration of Mn (r = 0.751), demonstrating adulteration from both natural and anthropogenic sources. Factor-5 has contributed by 7.2 % to the total variance with high loading of As (r = 0.331), which indicates the involvement of both geogenic and anthropogenic sources (Khan et al. 2012). Factor-6 has contributed by 6.8 % to the total variance with a high loading on pH (r = 0.458), due to same reason as for factor-2 and factor-3 (Shah et al. 2000). The overlapped plotting on rotated space diagram (Fig. S-5) between Ca2+ and Mn2+, and between K+ and Fe3+ is very important elements for the evaluation of their relation with groundwater (Jones et al. 2009). Some parameters like Na+ and SO4 2− appeared in left component, while other parameters were present in right component, which shows small variations of physiochemical parameters in both areas, presumably due to climatic and agricultural effect.
Human health risk due to high arsenic in tube well water
Toxic risk index is higher in Mailsi than Sargana (Table 5). Average daily doze (ADD) calculations of As are summarized in Table 5. The results suggest that in Mailsi and Sargana areas, where people are consuming groundwater contaminated with As, the ADD values ranged from 3.3E−04 to 9.4E−03 and 3.4E−04 to 1.2E−02 mg/kg day, respectively. In our study area, the ADD values of As were lower than those reported (Karim 2000) for Bangladeshi groundwater (5.00E−02–5.00E−01 mg/kg day) and Vietnam groundwater (5.00E−03–4.9E−01 mg/kg day) (Nguyen et al. 2009). However, they are higher than reported values in Turkey (2.3E−05–5.21E−03 mg/kg day) in groundwater (Caylak and Halifeoglu 2010) and were much higher than (0.00–5.56E−07 mg/kg day) for other areas in Pakistan (Muhammad et al. 2010). Hazard quotient values (HQs) in tube well water ranged from 1.1 to 31.1 and 1.13 to 40 in Sargana and Mailsi areas, respectively (Table 5). The results showed that residents of both areas have a higher toxic risk index in Mailsi than in Sargana. Notably, 100 % of tube well water samples are exceeding the typical toxic risk index of 1.00. The highest HQ value was found in Mailsi (Max HQ = 40). In this area, the groundwater is used for irrigation and domestic purposes, and therefore, people (60 %) of both areas are under risk as arsenic in this water is more than permissible limit given by USEPA (USEPA 2011). In Sargana and Mailsi sites, the potential cancer risk (CR) value ranged from 4.9E−04 to 1.4E−02 and 5.1E−04 to 1.8E−02 in tube well water samples (Table 5). Cancer risk values lower than 10−6 are considered to be negligible, but CR values higher than 10−4 are considered deplorable (USEPA 2010), and risk values lying between 10−6 and 10−4 are generally considered as acceptable (Islam et al. Islam et al. 2014). In the study area, the CR values in all tube well water samples were greater than one in a million (10−6), considered significant by the USEPA (2010). Arsenic concentrations in all groundwater sampled, as well as all other relevant values (ADD, HQ and CR), were elevated, due to anthropogenic activity such as agricultural activities, industrial activities, climatic bustle and household waste. This constitutes an urgent situation, which requires immediate remedial action for protecting the health of local population. These results designate that study area has an elevated contamination level of arsenic, which is not safe for agricultural and other domestic uses (Table 6).
Concluding annotations and recommendations
The present study investigated the geochemistry of tube well waters with Na+ > Ca2+ > Mg2+ > K+ and HCO3 − > SO4 2− > Cl− > NO3 − inclination. The predominating water type in the study area is of Ca2+–Na+–HCO3 −, reflecting the geology and climate of the area. Indexes such as SAR and MAR revealed good quality of tube well water for irrigation purposes, whereas RSC, %Na and KI values showed that this water is not suitable for agriculture and domestic use. The maximum concentration of As was 12–448.5 μg/L with mean value 80.87 μg/L. Phosphatic fertilizers are extensively used in the area. In many studies, elevated arsenic concentrations in groundwater have been found due to application of phosphatic fertilizers containing high concentrations of sodium (Campos 2002; Davenport and Peryea 1991). Therefore, positive relationship between As and Na+ (r = 0.283, p < 0.05) in the study area indicates that the source of As is application of phosphatic fertilizers and industrial waste and weathering of sodium arsenite and sodium arsenate. However, the possibility for arsenic release due to phosphate competition is possible, although it usually takes place in anoxic environments and reductive dissolution of ferric oxides. The statistical approaches PCA and HCA traced out the possible sources of contamination of As and other physicochemical parameters in tube well waters, which included local geochemistry, weathering of rocks and agricultural activity in the study area. Toxic risk index of As is in the order of Mailsi > Sargana, with all samples exceeding the typical toxic risk index 1.00, indicating that residents are under threat. Environmental isotopes technique is suggested to trace the sources of high arsenic and nitrate concentration in groundwater. Precise and high efficiency irrigation systems and practices must be adopted to reduce the use of contaminated water for growing crops.
References
Abbas, S. R., Sabir, S. M., Ahmad, S. D., Boligon, A. A., & Athayde, M. L. (2014). Phenolic profile, antioxidant potential and DNA damage protecting activity of sugarcane (Saccharum of ficinarum). Food Chemistry, 147, 10–16.
Adhikary, P. P., Chandrasekharan, H., Chakraborty, D., & Kamble, K. (2010). Assessment of groundwater pollution in West Delhi, India using geo statistical approach. Environmental Monitoring and Assessment, 167, 599–615.
Ahmed, K. M., Bhattacharya, P., & Hasan, M. A. (2004). Arsenic contamination in groundwater of alluvial aquifers in Bangladesh: An overview. Applied Geochemistry, 19, 181–200.
Alamdar, A., Eqani, S. A. M. A. S., Ali, S. W., Sohail, M., Bhowmik, A. K., Subhani, M., et al. (2016). Human Arsenic exposure via dust across different ecological zones throughout the Pakistan. Ecotoxicology and Environmental Safety, 126, 219–227.
Alloway, W. H. (1970). Agronomic control over the environmental cycling of trace elements. Advances in Agronomy, 20, 235–274.
Amini, M., et al. (2008). Statistical modeling of global geogenic arsenic contamination in groundwater. Environmental Science and Technology, 42, 3669–3675.
APHA. (1998). Standard methods for the examination of water and wastewater, American Public Health Association Washington D.C., Ed.19.
Arain, M., Kazi, T., Baig, J., Jamali, M., Afridi, H., Shah, A., et al. (2009). Determination of arsenic levels in lake water, sediment, and foodstuff from selected area of Sindh, Pakistan: estimation of daily dietary intake. Food and Chemical Toxicology, 47, 242–248.
Baig, D. N., Bukhari, D., & Shakoori, A. R. (2009a). Cry genes profiling and the toxicity of isolates of Bacillus thuringiensis from soil samples against American bollworm, Helicoverpaarmigera. Journal of Applied Microbiology, 109(6), 1967–1978.
Baig, J. A., Kazi, T. G., Arain, M. B., Afridi, H. I., Kandhro, G. A., Sarfraz, R. A., et al. (2009b). Evaluation of arsenic and other physico-chemical parameters of surface and ground water of Jamshoro, Pakistan. Journal of Hazardous Material, 166, 662–669.
Berg, M., Stengel, C., Trang, P. T. K., Hung Viet, P., Sampson, M. L., Leng, M., et al. (2007). Magnitude of arsenic pollution in the Mekong and Red River Deltas-Cambodia and Vietnam. Science of the Total Environment, 372(2), 413–425.
Berg, M., Tran, H. C., Nguyen, T. C., Pham, H. V., Schertenleib, R., & Giger, W. (2001). Arsenic contamination of groundwater and drinking water in Vietnam: a human health threat. Environmental Science and Technology, 35, 2621–2632.
Bhattacharya, P., Ahmed, K. M., Hasan, M. A., Broms, S., Fogelstorm, J., & Jacks, G. (2006). Mobility of arsenic in groundwater in a part of Brahmanbaria district, NE Bangladesh. Managing Arsenic in the Environment: From Soil to Human Health (pp. 95–115). Melbourne: CSIRO Publishing.
Bhowmik, A. V., et al. (2015). Mapping human health risks from exposure to trace metal contamination of drinking water sources in Pakistan. Science of Total Environment, 538, 306–316.
Briscoe, J., & Qamar, U. (2007). Pakistan’s Water economy running dry. Karachi: Oxford University Press. Commissioned by World Bank.
Busbee, M. W., Kocar, B. D., & Benner, S. G. (2009). Irrigation produces elevated arsenic in the underlying groundwater of a semi-arid basin in Southwestern Idaho. Applied Geochemistry, 24, 843–859.
Campos, V. (2002). Arsenic in groundwater affected by phosphate fertilizers at Sao Paulo, Brazil. Environmental Geology, 42, 83–87.
Caylak, E., & Halifeoglu, I. (2010). Cocuk Sagligive Hastaliklari Dergisi (in Turkish), 53(2), 159–173.
Chadha, D. K. (1999). A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeology Journal, 7, 431–439.
Chae, G. T., Yun, S. T., Kim, K., & Mayer, B. (2006). Hydrogeochemistry of sodium-bicarbonate type bedrock groundwater in the Pocheon spa area, South Korea. Journal of Hydrology, 321, 326–343.
Chakraborti, D., Rahman, M. M., Paul, K., Chowdhury, U. K., Sengupta, M. K., Lodh, D., et al. (2002). Arsenic calamity in the Indian subcontinent: what lessons have been learned? Talanta, 58, 3–22.
Chen, C. J., Chuang, Y. C., Lin, T. M., & Wu, H. Y. (1985). Malignant neoplasms among residents of a blackfoot disease-endemic area in Taiwan: High arsenic artesian well water and cancers. Cancer Research, 45, 5895–5899.
Davenport, J., & Peryea, F. (1991). Phosphate fertilizers influence leaching of lead and arsenic in a soil contaminated with lead arsenate. Water, Air, and Soil pollution, 57, 101–110.
DCR. (1998). District Census Report of Tehsil Mailsi. Population Census Organization, Pakistan. http://www.census.gov.pk/publications.php Accessed 10. 05. 10.
Emerson, W. W., & Bakker, A. C. (1973). The comparative effect of exchangeable Ca, Mg and Na on some soil physical properties of red brown earth sub soils. Australian Journal of Soil Research, 11, 151–157.
FAO. (1992). The use of saline waters for crop production. Irrigation and drainage paper 48. FAO, Rome.
Farooqi, A., Masuda, H., & Firdous, N. (2007a). Toxic fluoride and arsenic contaminated water in Lahore and Kasur districts, Punjab, Pakistan and possible contaminant sources. Environmental Pollution, 145, 839–849.
Farooqi, A., Masuda, H., Kusakabe, M., Naseem, M., & Firdous, N. (2007b). Distribution of highly arsenic and fluoride contaminated groundwater from east Punjab, Pakistan, and the controlling role of anthropogenic pollutants in the natural hydrological cycle. Geochemical Journal, 41(4), 213–234.
Farooqi, A., Yousafzai, M. A., & Jan, M. Q. (2007c). Hydro-geochemistry of the Indus Basin in Rahim Yar Khan District, Central Pakistan. Journal of the Chemical Society of Pakistan, 29, 525–537.
Fatmi, Z., Azam, I., Ahmed, F., Kazi, A., Gill, A. B., Kadir, M. M., et al. (2009). Health burden of skin lesions at low arsenic exposure through groundwater in Pakistan. Environmental Research, 109, 575–581.
Fujii, R., & Swain,W.C. (1995). Areal distribution of selected trace elements, salinity, and major ions in shallow ground water, Tulare Basin, Southern San Joaquin Valley, California. US Geol. Surv. Water-Resour. Investig. Rep. 95–4048.
Geleijnse, J. M., Kok, F. J., & Grobbee, D. E. (2003). Blood pressure response to changes in sodium and potassium intake: A met regression analysis of randomized trials. Journal of Human Hypertension, 17, 471–480.
Greenman, D.V., Swarzenski, W.V., & Benet, D.G. (1967). Groundwater hydrology of Punjab, West Pakistan with emphasis on problems caused by canal irrigation. USGS Water Supply paper, 1608(H).
Guo, Q., Wang, Y., Ma, T., & Ma, R. (2007). Geochemical processes controlling the elevated fluoride concentrations in groundwaters of the Taiyuan Basin, Northern China. Journal of Geochemical Exploration, 93, 1–12.
Halim, M. A., Ratan, K. M., Rasul, G., Hirosiro, Y., Sasaki, K., Shimada, J., et al. (2009). Groundwater contamination with arsenic in Sherajdikhan, Bangladesh: geochemical and hydrological implications. Environmental Geology, 58, 73–84.
Hudak, P. F. (2000). Distribution and sources of arsenic in the southern high plains aquifer, Texas, USA. Journal of Environmental Science and Health, A., 35, 899–913.
Islam, M. S., Ahmed, M. K., & Al-Mamun, M. H. (2014). Determination of heavy metals in fish and vegetables in Bangladesh and health implications. Human and Ecological Risk Assessment International Journal,. doi:10.1080/10807039.2014.950172.
Jones, B. F., Naftz, D. L., Spencer, R. J., & Oviatt, C. G. (2009). Geochemical evolution of great salt lake, Utah, USA. Aquatic Geochemistry, 15, 95–121.
Kahlown, M.A., Ashraf, M., Hussain, M., Salam, H.A., & Bhatti, A.Z. (2006). Impact assessment of sewerage and industrial effluents on water resources, soil, crops and human health in Faisalabad. Pakistan Council of Research in Water Resources Khyabane-Johar, H-8/1, Islamabad.
Karim, M. M. D. (2000). Arsenic in groundwater and health problems in Bangladesh. Water Research, 34, 304–310.
Katsoyiannis, I. A., Hug, S. J., Amman, A., Zikoudi, A., & Hatziliontos, C. (2007). Arsenic speciation and uranium concentrations in drinking water supply wells in Northern Greece: Correlations with redox indicative parameters and implications for groundwater treatment. Science of the Total Environment, 383, 128–140.
Katsoyiannis, I. A., & Katsoyiannis, A. I. (2006). Arsenic and other metal contamination of groundwaters in the industrial area of Thessaloniki, Northern Greece. Environmental Monitoring and Assessment, 123(393), 406.
Katsoyiannis, I. A., Mitrakas, M., & Zouboulis, A. I. (2015). Arsenic occurrence in Europe. Emphasis in Greece and description of the applied full scale treatment plants. Desalination and Water Treatment, 54, 2100–2107.
Katsoyiannis, I. A., & Zouboulis, A. I. (2006). Comparative evaluation of conventional and alternative methods for the removal of arsenic from contaminated groundwaters. Reviews Environmental Health, 21, 25–41.
Kavcar, P., Sofuoglub, A., & Sofuoglub, S. C. (2009). A health risk assessment for exposure to heavy metals via drinking water ingestion pathway. International Journal of Environmental Health, 212, 216–227.
Kelly, W.P. (1940). Permissible composition and concentration of irrigated waters. In: Proceedings of the ASCF66, pp 607.
Khan, S., Cao, Q., Zheng, Y. M., Huang, Y. Z., & Zhu, Y. G. (2008). Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environmental Pollution, 152, 686–692.
Khan, S., Shahnaz, M., Jehan, N., Rehman, S., Shah, M. T., & Din, I. (2012). Water quality and human health risk in Charsadda district, Pakistan. Journal of Cleaner Production, 10, 10–16.
Lopez Pazos, S. A., Arias, A. C. R., Ospina, J. S., & Ceron, J. (2010). Activity of Bacillus thuringiensis hybrid protein against a lepidopteron and a coleopteran pest. FEMS Microbiology Letters, 302(2), 93–98.
Mahmood, S. N., Naeem, S., Siddiqui, I., & Khan, F. A. (1998). Studies on physico-chemical nature of ground water of Korangi/Landhi (Karachi). Journal of the Chemical Society of Pakistan, 19, 42–48.
Manzoor, S., Shah, M. H., Shaheen, N., Khalique, A., & Jaffar, M. (2006). Multivariate analysis of trace metals in effluents from textile industry in relation to soil and ground water. Journal of Hazardous Material, 137, 31–37.
Martin, A., Tomlin, R. R., & Arsello, B. M. (2000). Arsenic uptake in orchard trees: Implications for dendroanalysis. Chemosphere, 41, 635–637.
Moghaddam, A. A., & Fijani, E. (2008). Distribution of fluoride in groundwater of Maku area, northwest of Iran. Environmental Geology, 56, 281–287.
Muhammad, S., Rashid, A., & Memon, K. S. (1996). Soil salinity sodicity and water logging in Soil Science (pp. 472–506). Pakistan: National Book Foundation Islamabad.
Muhammad, S., Shah, M. T., & Khan, S. (2010). Arsenic health risk assessment in drinking water and source apportionment using multivariate statistical techniques in Kohistan region, northern Pakistan. Food and Chemical Toxicology, 48, 2855–2864.
Muhammad, S., Shah, M. T., & Khan, S. (2011). Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Microchemical Journal, 98, 334–343.
Naseem, M., Farooqi, A., Masih, D., & Anwar, M. (2001). Investigation of toxic elements in the ground water of Kalalanwala area near Lahore, Punjab, Pakistan. Proceedings of GEOSAS-III (pp. 23–27). Pakistan: Lahore.
Nguyen, V. A., Bang, S., Viet, P. H., & Kim, K. W. (2009). Contamination of groundwater and risk assessment for arsenic exposure in Ha Nam province, Vietnam. Environmental International, 35, 466–472.
Nickson, R., McArthur, J., Shrestha, B., Kyaw-Myint, T., & Lowry, D. (2005). Arsenic and other drinking water quality issues, Muzaffargarh District Pakistan. Applied Geochemistry, 20, 55–68.
Nicolli, H. B., Suriano, J. M., & Gomez, P. (1989). Groundwater contamination with arsenic and other trace element sinan area of the Pampa, Province of Cordoba, Argentina. Environmental Geology and Water Sciences, 14, 3–16.
Nimick, D. A. (1998). The fate of geothermal arsenic in the Madison and Missouri Rivers, Montana and Wyoming. Water Resources Research, 34, 3051–3067.
Patterson, R. A. (1994). On-site treatment and disposal of septic tank effluent. Ph. D.thesis, University of New England.
Piper, A. M. (1994). A graphic procedure in the geochemical interpretation of water analysis. American Geophysical Union Transaction, 25, 914–923.
Popovic, A., Djordjevic, D., & Polic, P. (2001). Trace and major element pollution originating from coal ash suspension and transport processes. Environment International, 26, 251–255.
Pradeep, J. K. (1998). Hydrogeology and quality of ground water around Hirapur, District Sagar. Journal of Pollution Research, 17(1), 91–94.
Prosun, A. B., Mukherjee, J. G., & Nordqvist, S. (2002). Metal contamination at a wood preservation site: characterization and experimental studies on remediation. Science of the Total Environment, 36, 165–180.
Ramkumar, T., Venkatramanan, S., Mary, I. A., Tamilselvi, M., & Ramesh, G. (2010). Hydrogeochemical quality of groundwater in Vedaraniyam Town, TamilNadu, India. Research Journal of Environmental and Earth Sciences, 2(1), 44–48.
Ravenscroft, P., Brammer, H., & Richards, K. (2009). Arsenic in North America and Europe. In: Arsenic Pollution: A Global Synthesis, pp 387–454.
Reghunath, R., Murthy, T. R. J., & Raghavan, B. R. (2002). The utility of multivariate statistical techniques in hydrogeochemical studies: an example from Karnataka, India. Water Research, 36, 2437–2442.
Richards, L. A. (1954). Diagnosis and improvement of saline and alkali soils. USDA Agriculture Handbook No. 60, U.S. Government Printing Office, Washington, D.C., USA.
Robertson, F. N. (1989). Arsenic in ground-water under oxidizing conditions, south-west United States. Environmental Geochemistry and Health, 11, 171–185.
Schreiber, M. E., Simo, J. A., & Freiberg, P. G. (2000). Stratigraphic and geochemical controls on naturally occurring arsenic in groundwater, eastern Wisconsin, USA. Hydrogeology Journal, 8, 161–176.
Shah, M. T., Ara, J., Muhammad, S., Khan, S., & Tariq, S. (2012). Health risk assessment via surface water and sub-surface water consumption in the mafic and ultramafic terrain, Mohammed agency, northern Pakistan. Journal of Geochemical Exploration, 118, 60–67.
Shah, M. T., Begum, S., & Khan, S. (2010). Pedo and biogeochemical studies of mafic and ultramafic rocks in the Mingora and Kabal areas, Swat, Pakistan. Environmental and Earth Sciences, 60(5), 1091–1102.
Shah, M. T., Hussain, S. S., & Akbar, H. M. (2000). Mineralogy, chemistry and genesis of the Proterozoic base metal deposits at the northern margin of the Indian plate in Besham area, Himalaya, northern Pakistan (pp. 11–57). Pakistan Museum of Natural History: Economic Geology of Pakistan.
Sharma, A. K. (2006). Arsenic removal from water using naturally occurring iron, and the associated benefits on health in affected regions. Institute of Environment & Resources Technical University of Denmark, ISBN 87-91855-07-1.
Shyamala, R., Shanthi, M., & Lalitha, P. (2008). Physicochemical analysis of Bore well water samples of Telungupalayam area in Coimbatore district, Tamilnadu, India. E-Journal of Chemistry, 5, 924–929.
Singh, J. (2011). Water table falls alarmingly in Central Punjab, Water table in 9,058 sq km drops by over 20 metres, The Tribune, 1st Feb 2011 (cited on 1stFeb 2011).
Singh, V. K., Bikundia, D. S., Sarswat, A., & Mohan, D. (2012). Groundwater quality assessment in village LutfullapurNawada, Loni, District Ghaziabad, Uttar Pradesh, India. Environmental Monitoring and Assessment, 184, 4473–4488.
Singh, K. P., Mohon, D., Sinha, S., & Dalwani, R. (2004). Impact assessment of treated/untreated wastewater toxicants discharge by sewage treatment plants on health, agricultural, and environmental quality in wastewater disposal area. Chemosphere, 55, 227–255.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behavior and distribution of arsenic in natural waters. Applied Geochemistry, 17, 517–568.
Soltan, M. E. (1998). Characterization, classification, and evaluation of some groundwater samples in Upper Egypt. Chemosphere, 37, 735–745.
Stiff, H. A. J. (1951). The interpretation of chemical water analysis by means of patterns. Journal of Petroleum Technology, 3(10), 15–17.
Sultana, N., Alimon, A. R., Haque, K. S., Sazili, A. Q., Yaakub, H., & Hossain, S. M. J. (2014). The effect of cutting interval on yield and nutrient composition of different plant fraction of Moringaoleifera tree. Journal of Food and Agriculture Environment, 12, 599–604.
Szabolcs, I., & Darab, C.(1964). The influence of irrigation water of high sodium carbonate content of soils. In: Proceeding of 8th International Congress of ISSS, Trans, vol. II, pp 803–812.
Tasneem, M. A. (1999). Impact of agricultural and industrial activities on groundwater quality in Kasur Area. The Nucleus. Quarterly Journal of the Pakistan Atomic Energy Commission 36.
US-EPA. (2010). Risk-based concentration table.United State, Environmental Protection Agency, Philadelphia, Pa, and Washington, DC. http://www.epa.gov/reg3hwmd/risk/human/index.htm.
US-EPA. (2011). Exposure Factors Handbook.United States Environmental Protection Agency, Washington, DC.EPA/600/R-09/052F.
Welch, H., Westjohn, D. B., Helsel, D. R., & Wanty, R. B. (2000). Arsenic in ground water of the United States: occurrence and geochemistry. Ground Water, 38, 589–604.
WHO. (2008). Guidelines for drinking water quality. In: Recommendations. World Health Organization; 3rd ed., vol. 1, Geneva.
Woolson, E. A., Axley, J. H., & Kearney, P. C. (1971). The chemistry and phytotoxicity of arsenic in soils: I. contaminated field soils. Soil Science Society of America Journal, 35, 938–943.
Zhang, X., Wang, Q., Liu, Y., Wu, J., & Yu, M. (2011). Application of multivariate statistical techniques in the assessment of water quality in the Southwest New Territories and Kowloon, Hong Kong. Environmental Monitoring and Assessment, 173, 17–27.
Acknowledgments
We are highly thankful to Geological Survey of Pakistan and Department of Environmental Sciences, Quaid-i-Azam University, Islamabad, for technical support and research facilities, respectively. We are also thankful to Dr. Sajid Masood, Department of Plant Sciences, Quaid-I-Azam University, for technical review of the manuscript. We wish to thank people of Tehsil Mailsi visited, for their cooperation at the time of sampling.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rasool, A., Xiao, T., Farooqi, A. et al. Quality of tube well water intended for irrigation and human consumption with special emphasis on arsenic contamination at the area of Punjab, Pakistan. Environ Geochem Health 39, 847–863 (2017). https://doi.org/10.1007/s10653-016-9855-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-016-9855-8