Abstract
This study highlights the heavy metals (HMs) distribution in soils and their uptake by wild plants grown in the soils derived from the mafic and ultramafic terrains. Plant and soil samples were analyzed for Cu, Pb, Zn, Cr, Ni and Cd using atomic absorption spectrophotometer. The data indicate that almost all the HMs in the soil samples collected from the study area exceeded the reference and normal agricultural soils. Greater variability was noticed in the uptake of HMs by various plants grown on the studied soils. High concentrations of Cu and Zn in Cannabis sativa L. (seft hemp), Pb in Ailanthus altissima (Mill.) (Ailanto), Ni and Cr in Indigofrra gerardiana Wall. ex Baker (sage), and Saccharum griffihii Munro ex Boiss. (plume grass) were noticed among the studied plants. The multifold enrichments of Cr and Ni in the Indigofrra gerardiana and Saccharum griffihii as compared to the other plants of the study area suggested that these plants have the ability to uptake and translocate high concentrations of Cr and Ni. The excessive concentrations of Cr and Ni in these plants can be used for mineral prospecting but their main concern could be of serious environmental problems and health risks in the inhabitants of the study areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil environment may be contaminated with high concentrations of heavy metals (HMs) naturally, as a result of proximity to mineral outcrops or weathering of parent rocks (Del Río et al. 2006). Environmental and human health problems are usually associated with soils contaminated with HMs and, therefore, much more attention has been given to these kinds of soils by the researchers from a wide range of disciplines. In order to reach to a better understanding of these metals as pollutants, it is important to understand their natural sources as well. In most cases the enrichment of HMs in soils is due to the hazardous waste pollution but there are many cases where soils derived form mineralized rocks are naturally enriched in HMs (Alloway 1990; McBride 1994; Del Río et al. 2002; Kifyatullah et al. 2001; Shah et al. 2004).
The geochemical characteristics of soils are helpful in identification of the existence of rocks of special characters in the uphill or underneath areas. The soils produced from the weathering of mafic and ultramafic rocks are of greater interest in regard to environmental and exploration studies. The mafic and ultramafic rocks are generally enriched in HMs such as Cu, Pb, Zn, Cr, Ni and Cd and similarly the weathered soils of these rocks especially the ultramafic rocks, often referred as serpentine soils, are also enriched in these metals (Brooks 1987; Dinelli et al. 1997; Lottermoser 1997). Many workers have suggested that Ni is highly accumulated in the thousands of plants growing on the serpentine soils (Brooks 1983; Robinson et al. 1997; Baker et al. 2000; Pollard et al. 2002). The HMs dissolution in water and uptake by plants could result in the environmental problems of the area and also helps in the identification of pathfinders for the various types of mineral deposits (Brooks 1987; Adriano 1992; Adriano et al. 1994; Legittimo et al. 1995; Robinson et al. 1997; Brooks 1998; Kifyatullah et al. 2001; Shah et al. 2004).
The accumulation of HMs in the soil environment is of increasing concern because of the food safety and potential health risks. Food chain contamination is one of the important pathways for the entry of HMs into the human body (Zhu et al. 2004; Khan et al. 2008). Previously, numerous studies have demonstrated that wild plant species have accumulated high concentrations of HMs, grown on contaminated sites (Del Río et al. 2002; Del Río et al. 2006; Mills et al. 2006; Xio et al. 2008; Hernández and Pastor 2008; Dwivedi et al. 2008). However, more information are needed to identify the natural sources of HMs and their uptake by wild plants.
This research work was undertaken to study geochemical characteristics of the soils and plants of the Mingora and Kabal areas of the Swat region in the northern parts of Pakistan, where the mafic and ultramfic rocks are exposed (Begum 2008). This study will help in understanding the environmental impact of the HMs’ enrichment in the soils and plants and their role in the geochemical exploration.
Geology of the study area
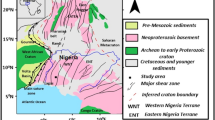
The present study area (Mingora and Kabal) is lying between latitude 30°44′ to 34°50′ north and longitude 72°15′ to 72°18′ east in the Swat region of northern Pakistan (Fig. 1). According to Afridi et al. (1995) the rocks exposed in the study area belong to the Indian plate, Mingora-Shangla mélange of the Indus Suture Zone and the Kohistan island arc. The Indian plate rocks are (1) Swat granite gneisses (Martin et al. 1962; Jan and Tahirkheli 1969; Di Pietro et al. 1999), (2) amphibolites with garnetifrrouces schist, biotite-schist hornblende and marbles of Marghazar Formation (Di Pietro 1991; Pogue et al. 1992) and (3) graphitic phyllites of the Saidu Formation (Kazmi et al. 1984; Treloar et al. 1989a, b; Lawrence et al. 1989; Di Pietro 1991, 1993). The Mingora-shangla mélange zone is composed of chaotic assemblages of mafic and ultramafic rocks such as serpentinite, greenschist, talc-carbonate schist and metabasalts (Kazmi et al. 1984; Jan and Jabeen 1990; Arif and Jan 1993). The Kohistan island arc rocks are mainly massive amphibolites of southern or Kamila amphibolite belt (Jan 1979, 1988; Bard et al. 1980; Treloar et al. 1990; Shah et al. 1992). Most part of the study area is covered by the Quaternary deposits mainly composed of stream channel deposits and also the weathering products of the aforementioned rocks in the area of study (Fig. 1).
Geological and samples location map of the Mingora and Kabal areas, Swat, Pakistan (modified after Afridi et al. 1995)
Materials and methods
Soil and plant sampling
Twenty-eight representative soil samples at various sites, shown in Fig. 1, were collected from the upper horizon (0–20 cm) with the help of an auger. Each sample was stored in kraft paper bags. The growing flora of the soils derived from mafic and ultramafic rocks in the Mingora and Kabal areas is very rich but nine prominent plant species including Cannabis sativa L. (seft hemp), Ailanthus altissima (Mill.) (Ailanto), Indigofrra gerardiana Wall. ex Baker (Dyer,s indigo), Salvia moorcroftiana Wall. Ex Bth. (sage), Rumex hastatus D. Don (Dock sorrel), Xanthium strumarium L. (Bur weed), Dodonaea viscose (L.) Jacq. (Switch sorrel), Debergeasia sulicifolia (D. Don) Rendle (Dutchman pipe) and Saccharum griffihii Munro ex Boiss. (plume grass) were also collected from the same site from where soil samples were collected (Fig. 1). All the plants were in seedling stage (<70 cm in height). After collection, the plants were identified and the botanical names were given to every plant with the help of a plant taxonomist of the Botany Department, University of Peshawar. Both the plant and soil samples were brought to the Geochemistry Laboratory of the National Centre of Excellence in Geology, University of Peshawar for further preparation and analysis.
The reference soil and plant samples were collected from an area (not shown on the map) about 50 km SW of the study area where meta-sediments and granite-gneisses were exposed. There was no input of the mafic and ultramafic rocks in the soils of this area. Therefore, in the following text, reference soils and plants samples will represent the background values for comparison purpose.
Samples’ preparation and analyses
After transportation to the laboratory, each soil sample was air-dried and sieved through a <2 mm mesh, and then sealed in Kraft paper envelopes until analysis. Sub-samples were used to measure the physico-chemical properties according to standard procedures. For HM analyses, 0.5 g of each moisture-free powdered soil sample was taken in triplicate in a teflon beaker and treated with hydrofluoric acid (HF) and aqua regia (HNO3:HCl ratio 3:1) and the final volume of 30 ml was prepared with 2 N HCl by following the method of Jeffery and Hutchison (1986) and Ryan et al. (2001). The extracts were analyzed using atomic absorption spectrometer, Perkin Elmer, Analyst 700, USA, (AAS-PEA-700).
All the plant samples were properly washed with tap water and finally with double de-ionized water (DDW) to remove all visible soil particles. The washed plant samples were oven-dried at 70°C for 48 h to a constant weight and were ground with an electric grinder. About 2 g of each ground plant sample was taken in triplicate in Pyrex beakers separately and was digested with HNO3–HClO4 and aqua regia. The digestion procedure was adopted from Ryan et al. (2001). The extract was made to the final volume of 50 ml with DDW. Concentrations of HMs in the digested samples were determined using AAS-PEA-700. A reagent blank and standard reference plant and soil materials were included to verify the accuracy and precision of the digestion and subsequent analysis procedure. All the chemicals were of analytical grade. All the reagents and the calibration standards were prepared using the DDW. The results obtained were in triplicate.
The data were statistically analyzed using the statistical package SPSS 11.5. The measures were expressed in terms of mean, while the figures presented the mean values and standard deviation of triplicate. Statistical significance was computed using Duncan’s multiple range test and Paired-samples t test, with a significance level of P < 0.01.
Results and discussion
Soil
Table 1 summarizes the results of HMs in the soil samples collected from the mafic and ultramafic rock terrains and reference sites. The concentration of Cu in the soils of Mingora and Kabal areas was ranged from 29 to 184 mg/kg with a mean value of 63 mg/kg (Table 1). Cu concentrations in the soil samples were significantly (P < 0.01) higher than the reference soil (Table 2). Cu in all the soil samples of Mingora and Kabal areas exceeded the normal agricultural soil value (20 mg/kg) of Bohn et al. (2001) and reference soil (Table 2). The occurrence of Cu in small amount (4–20 mg/kg) is generally beneficial for the normal growth of the plants while its amount less than 4 mg/kg is considered deficient and more than 20 mg/kg is considered toxic (Jones 1972; Adriano 1986, 2001). The high concentration (>20 mg/kg) of Cu in the soils of Mingora and Kabal areas could be hazardous as far as the normal growth of plants is concerned. The data indicate that the Cu concentrations in the soil samples were higher than the concentrations detected in the wastewater-contaminated soils (Mapanda et al. 2007). Furthermore, the concentrations of Cu were markedly high and generally similar to the values (83–162 mg/kg) detected in the soil contaminated with mine spill (Del Río et al. 2002).
Pb concentrations were in the range of 34–90 mg/kg with an average amount of 55 mg/kg in the soil samples collected from Mingora and Kabal areas (Table 1). Pb in almost all the soil samples of Mingora and Kabal areas were many folds higher than that of the normal limit set for agricultural soils (10 mg/kg) and the reference soils (Table 2). Like Cu, Pb concentrations in the soil samples were significantly (P < 0.01) higher than the reference soil samples. The bioavailability of HMs from soil to plants depends on various factors including soil properties, climatic conditions, plant genotype and agronomic management (Kabata-Pendias and Pendias 2001). Pb occurs mainly as Pb2+ and its primary form in nature is galena (PbS). During weathering, it slowly oxidized and form carbonates which incorporate in the stable phases such as clay minerals, Fe-oxides, Mn and Al hydroxides, phosphates and organic matters (Kabata-Pendias and Pendias 2001). Therefore, phyto-availability of Pb in the studied soils seems to be very low. Obviously, total Pb concentrations in the soil samples are not a good indicator for phyto-availability because the plant uptake depends on the water soluble fraction of the metals (Adriano 2001; Khan et al. 2006). The findings of this study indicated that the Pb contamination level is lower than the Pb values detected in the soil samples collected from silver mine areas (Figueroa et al. 2008).
A wide range of Zn concentration was observed among the soils collected from the study areas. The concentrations of Zn were ranged from 10 to 135 mg/kg with an average concentration of 48 mg/kg in the soil samples of Mingor and Kabal areas (Table 1). Zn concentrations in the soil samples were significantly (P < 0.01) higher than the reference soils and in some samples have exceeded the normal limit (50 mg/kg) set for agricultural soils (Tables 1, 2). Zn is an essential metal for plant growth, and its high mobility within the plant is responsible for highest concentration of Zn in wild plants (Bennetta et al. 2000). Zn deficiency can highly affect the nutrition quality of plants but its high concentration in the soils could be toxic for the plant growth and development (Adriano 2001). Therefore, it is suggested that the soils of Mingora and Kabal areas were generally safe, except at few places, and should have no toxic effects on plants.
In the study areas, Cr concentrations were ranged from 140 to 5,647 mg/kg with an average concentration of 863 mg/kg (Table 1). Cr concentrations in the soil samples were also significantly (P < 0.01) higher than the reference soil samples (Table 2). These highest concentrations of Cr suggest that this metal was highly enriched in the studied soils as compared to that of normal agricultural soil (20 mg/kg). The soil samples within the Mingora-Shangla mélange zone or in its vicinity in the Mingora area are having anomalous values (324–5,647 mg/kg) of Cr metal. This can be attributed to the formation of this soil, referred to as serpentine soil, due to the weathering of ultramafic rocks within the mélange zone where the Cr is generally associated with olivine, pyroxene and chromite (Arif and Jan 1993). Cr usually forms chromate ions (CrO4 and HCrO4) which are easily mobilized and sorbed by clays and hydrous oxides (Kabata-Pendias and Pendias 2001). Naturally occurring Cr compounds have principal valences of III (chromic) and VI (chromate). The Cr(VI) is much less stable than Cr(III) and can very easily be mobilized in both acid and alkaline soils. According to Bartlett and James (1979) and Gough et al. (1979), Cr in the soil is usually oxidized from Cr(III) to Cr(VI) and then it is available for plant uptake. However, the Cr(III) can only be mobilized in the acidic soils (Adriano 1986; Kabata-Pendias and Pendias 2001). Cr has stimulatory effects on the growth of the plants and could be toxic in these soils which are derived from the ultramafic rocks (Adriano 2001). The serpentine soils of the Mingora area, as having been derived from the ultramafic rocks, can produce toxicity in the plant species present in the study area. Furthermore, the concentrations of Cr in the soil samples were higher than the concentrations detected in the wastewater-contaminated soils (Khan et al. 2008). It means that the natural sources could equally be responsible for soil contamination with HMs.
A wide range of Ni concentrations were observed in the soil samples collected from Mingora and Kabal areas. Ni concentrations were ranged from 140 to 2,341 mg/kg with an average amount of 637 mg/kg (Table 1) and were significantly (P < 0.01) higher than the reference soil samples (Table 2). Ni concentrations in the soil samples of both Mingora and Kabal areas have shown the distribution patterns similar to Cr metal. Its concentration was many folds higher than the normal agricultural soil (40 mg/kg) and the reference soil samples (Table 2). This enrichment in the studied soils could also be due to the weathering of Cr–Ni bearing phases (i.e. olivine, pyroxene serpentine, etc.) in the ultramafic rocks of the mélange zone. However, the soils of Kabal area were less enriched as compared to that of Mingora area. Ni is beneficial for the plant growth if present in normal amount in soil; however, the soil with high concentration, especially derived from the ultramafic rocks, can produce toxicity symptoms in plants (Mishra and Kar 1974; Adriano 2001). The data indicate that the soils of Mingora area were highly contaminated with Ni and can be considered more toxic for the growth of plants.
Cd concentrations were ranged from 2 to 4 mg/kg in the soils of Mingora and Kabal areas (Table 1). Cd concentrations were significantly (P < 0.01) higher than the reference soil and exceeded the normal value set for agricultural soil (Table 2). Soil contamination with Cd is believed to be a most serious health risk. However, the Cd concentration (<4 mg/kg) in the studied soils was not considered as hazardous for the plant growth (Adriano 2001) and plants grown there had no symptoms of toxicity. The findings of this study indicated that the Cd concentrations in the soil samples were higher than the concentrations detected in the wastewater-contaminated soils and the soils contaminated with silver mine (Figueroa et al. 2008). However, the concentrations of Cd were lower than the values detected in some soil samples contaminated with mine spill (Del Río et al. 2002).
Plants
Table 1 summarizes the results of HMs in the plant samples collected from the mafic and ultramafic rock terrains and reference sites, while Fig. 2 indicates the enrichment values for various HMs in the plant samples. Cu concentrations were ranged from 7 to 33 mg/kg in the plant samples of Mingora and Kabal areas (Table 1). Cu concentrations in the plants grown on soils derived from mafic and ultramafic rocks were higher than the reference soil grown plants. Cu concentrations in the selected plant samples were in the order of Cannabis sativa > Salvia moorcroftiana > Ailanthus altissima > Xanthium strumarium > Saccharum griffihii > Dodonaea viscose > Debergeasia sulicifolia > Rumex hastatus > Indigofrra gerardiana (Table 2). However, the concentrations of Cu were lower than the values (27–65 mg/kg) detected in the wild plants (belong to the family Poaceae but different species) grown on soil contaminated with mine spill (Del Río et al. 2002). Cu concentration <5 mg/kg is considered inadequate for the growth of many of plant species (Kabata-Pendias and Pendias 2001). Cu in very small amount from 5 to 20 mg/kg in plant tissues are adequate for their normal growth, while more than 20 mg/kg is considered as toxic for numerous plant species (Jones 1972). It is mobile within the plant’s body, which probably explains its highest concentrations in wild plants (Bennetta et al. 2000). The data indicated that in some of the studied plants samples, the concentrations of Cu exceeded the limit causing phyotoxicity.
Variable enrichment values of HMs in plant samples collected from Mingora and Kabal areas. Enrichment factor is calculated as HMs in plants of mafic-ultramafic terrain/HMs in reference plants. SG, S. griffihii; IG, I. gerardiana; RH, R. hastatu; SM, S. moorcroftiana; XM, X. strumarium; DV, D. viscose; DS, D. sulicifolia; CS, C. sativa; AA, A. altissima
Pb concentrations in the plants grown in the study area ranged from 2 to 8 mg/kg. The highest Pb value (8 mg/kg) was detected in the samples of Ailanthus altissima (Table 1). Pb concentrations in the plants, except Indigofrra gerardiana and Rumex hastatus, grown on soils derived from mafic and ultramafic rocks were higher than the reference soil grown plants. Pb concentrations in the selected plant samples were in the order of Ailanthus altissima > Debergeasia sulicifolia > Xanthium strumarium > Saccharum griffihii > Salvia moorcroftiana > Dodonaea viscose > Cannabis sativa > Rumex hastatus > Indigofrra gerardiana (Table 1). In few cases, Pb contents in the studied plants were found >6 mg/kg (Table 1). As the whole plant, including roots, have been treated for the HMs concentration, therefore, it was not possible to find out that which parts of the plants have greater accumulation of Pb. However, previous studies (e.g. Zimdahl and Koeppe 1977; Kabata-Pendias and Pendias 2001) have shown that Pb is generally accumulated in the roots of many plants during the translocation of Pb in the plants. Pb concentrations in studied plant samples were inconsistent with the results in literature for both wild and domesticated plants (Del Río et al. 2002; Del Río et al. 2006). The concentrations of Pb ranging from 2 to 6 μg/kg are sufficient for the normal growth of plants while its background level in the forage plants is reported as 2.5 mg/kg (Broyer et al. 1972; Kabata-Pendias and Pendias 2001). Natural Pb concentration in plants grown in uncontaminated and unmineralized soils is generally ranging from 0.1 to 10 mg/kg with average amount of 2 mg/kg (Cannon 1976; Kabata-Pendias and Pendias 2001). Pb concentrations in the plant samples of Mingora and Kabal areas generally found within the permissible limit for the normal growth of various plants.
Highly variable concentrations of Zn, ranged from 4 to 33 mg/kg, were noticed in the plants of the study area (Table 1). Zn concentrations in the plants grown on soils derived from mafic and ultramafic rocks were many folds higher than the plants grown on the reference soils (Table 1). Zn contents in the studied plants were in the order of Cannabis sativa > Ailanthus altissima > Xanthium strumarium > Saccharum griffihii > Salvia moorcroftiana > Dodonaea viscose > Debergeasia sulicifolia > Rumex hastatus > Indigofrra gerardiana. Its accumulation in various plant species, especially in Calamine flora, has also been reported by many workers (Zalecka and Wierzbicka 2002; Kupper et al. 2000; Whiting et al. 2000; Hajiboland and Manafi 2007; Olko et al. 2008). Zn plays an important role in the plant metabolism and is not considered to be highly phytotoxic (Lindsay1972; Price et al. 1972). However, Zn toxicity limit depends on the plant species, physiology, genotypes and growth rate, and its uptake was different among the plant communities depending on their ability to accumulate and detoxify it like other HMs (Gupta et al. 2002; Jala and Goyal 2006). Upper toxic limit of Zn in most of the plants is ranged from 100 to 500 mg/kg (Brooks 1983; Macnicol and Beckett 1985). According to Jones (1972), the optimum requirement of Zn for plants varies greatly from species to species and, therefore, it is difficult to establish a single critical value. However, plants with Zn contents below 20 mg/kg are suspected of Zn deficiency with normal values ranging from 25 to 150 mg/kg of Zn (Jones 1972). Therefore, Zn contents of plants in Mingora and Kabal areas are generally low in Zn and, therefore, no environmental threats can be expected in the region in this regard.
Cr concentrations were ranged from 35 to 358 mg/kg in the plant samples collected from the study area (Table 1). The highest Cr concentration was detected in Saccharum griffihii, while lowest concentration in Dodonaea viscose (Table 1). Cr concentrations in the plants grown on soils derived from mafic and ultramafic rocks were higher than the reference soil grown plants. This can be attributed to the phyto- availability of Cr(VI) from the studied serpentine soils (Bartlett and James 1979; Gough et al. 1979). Cr on average was in the order of Indigofrra gerardiana > Saccharum griffihii > Debergeasia sulicifolia > Rumex hastatus > Xanthium strumarium > Cannabis sativa > Salvia moorcroftiana > Dodonaea viscose > Ailanthus altissima. Cr accumulation in the plants grown on the ultramafic terrains elsewhere has also been reported by Mertz et al. (1974), Petrunina (1974) and Kifyatullah et al. (2001). The toxicity limit of Cr in plants is generally reported from 1 to 10 mg/kg (Macnicol and Beckett 1985; Adriano 1992). In this regard, the Cr concentrations in all selected plants were manifolds higher than the recommended level for toxicity in plants. This high level of Cr could be hazardous for plant community in the study areas.
Ni concentrations were ranged from 20 to 289 mg/kg in the plant samples collected from the study areas. The highest Ni concentration was found in Saccharum griffihii, while lowest value was noticed in Debergeasia sulicifolia (Table 1). Like other HMs, variable concentrations of Ni were accumulated in these wild plants. Furthermore, Ni concentrations in the plants grown on soils derived from mafic and ultramafic rocks were higher than the reference soil grown plants. However, Indigofrra gerardiana and Saccharum griffihii showed greater accumulation of Ni among all the studied plants (Table 1). Ni concentrations were in the order of Indigofrra gerardiana > Saccharum griffihii > Ailanthus altissima > Salvia moorcroftiana > Xanthium strumarium > Cannabis sativa > Rumex hastatus > Dodonaea viscose > Debergeasia sulicifolia (Table 1). Ni accumulation in many plant species of serpentine flora has also been reported elsewhere (Brooks and Yang 1984; Reeves 1992; Robinson et al. 1997; Reeves et al. 1996; 1999; Brooks 1998; Kifyatullah et al. 2001; Hajiboland and Manafi 2007). The results indicated that the Ni concentrations were markedly higher and inconsistent with the values in the plants grown on wastewater-contaminated soils (Khan et al. 2008). The phytotoxic Ni concentrations are varied widely among plant species and are generally ranging from 40 to 246 mg/kg (Gough et al. 1979). It was noticed that the amount of Ni in all the studied plants was within the toxic range and could be hazardous for the plants growth.
This study shows that variable concentrations of Cd were accumulated in the wild plant samples and its concentrations were ranged from 0.09 to 0.40 mg/kg (Table 1). Cd concentrations in the plants grown on soils derived from mafic and ultramafic rocks were higher than the reference soil grown plants. However, the concentrations of Cd in plant samples were low as compared to other HMs and were not considered to cause toxicity (Adriano 2001; Kabata-Pendias and Pendias 2001). Del Río et al. (2002) reported the Cd concentrations higher than our values in the plants grown on site contaminated by mine spillage. The variable concentrations of various metals in the plant samples were due to their different uptake rates for these elements from the soils (Kifyatullah et al. 2001; Shah et al. 2004).
HMs’ enrichment
Figure 2 shows the enrichment levels of the various HMs in the plants grown on the soils derived from mafic and ultramafic rocks of the Mingora and Kabal areas. The data showed the highest enrichment of Cu in C. sativa, Pb in D. sulicifolia, Zn in C. sativa, Cr and Ni in I. gerardiana, and Cd in S. moorcroftiana. However, the plant enrichment rates were highly varied from metal to metal and from species to species. Furthermore, it is suggested that the wild plants were highly contaminated with HMs and their enrichments were multifold.
Relationships between metals
Tables 3 and 4 showed the inter-elemental relationship for HMs in the studied soils and plants, respectively. No significant inter-element correlation existed in the soils and plants. However, Ni and Cr showed good correlation in both the soils (r = 0.83) and plants (r = 0.97), while Zn and Cu (r = 0.73) and Zn and Cd (r = 0.77) also showed good correlation in plants (Tables 1, 2). Regression analysis was performed to identify the relationships between the metal concentrations in soil samples as well as in plant samples. The data indicated that these relationships were not strong, may be because of the different soil characteristics and plant physiologies (Khan et al. 2008; Xio et al. 2008). Linear regression analysis was used to examine the relationships between the concentrations in soils and plants (Fig. 3). The findings indicated that the weak positive correlations (r < 0.33) for Cu, Zn and Cd and a little stronger positive correlation for Cr (r = 0.66) and Ni (r = 0.80) existed in the studied soils and plants. The weak correlations of HMs in plants and soils can be attributed to the greater variation of these elements in the soils of the area and also the variable uptake rates of the different plants (Kifyatullah et al. 2001; Shah et al. 2004).
Environmental perspectives
The biogeochemical studies show that the ability of various plants to uptake greater amount of various HMs from the soils has provided significant clues for both mineral prospecting and environmental degradation (Hawkes and Webb 1962; Levinson 1974; Rose et al. 1979; Brooks 1987; Robinson et al. 1997; Brooks 1998; Kabata-Pendias and Pendias 2001; Bohn et al. 2001; Kifyatullah et al. 2001; Shah et al. 2004; Hernández and Pastor 2008). It has been noticed during this study that most of the HMs were highly enriched in the soils but their toxicities on the plant species cannot be considered hazardous. However, the variable enrichment of Cr and Ni in the plant species, especially in I. gerardiana and S. griffihii grown on the soils derived from ultramafic rocks of the mélange zone in Mangora area can be applicable not only to mineral exploration but also to environmental and toxicological concerns. The Cr and Ni toxicity and their carcinogenic effects on both animals and human beings are well reported (Hernández and Pastor 2008; Xio et al. 2008; Khan et al. 2008).
The high concentrations of HMs in the soils and plants can be transferred directly (through inhalation of contaminated dusts and dermal contacts with contaminated soils) or from forage plants to animals and then to the inhabitants of the area through meat and milk of these animals. This food chain contamination is one of the major pathways through which HMs enter into the human beings. It has been found during discussion with the inhabitants and the physicians of the area that the cases of cancer, especially breast cancer in women and the intestinal and liver cancer in both genders, are increasing rapidly in the whole region. In this regard, a detailed study involving the biochemist, geochemist and epidemiologists is needed to be carried out in order to unravel the toxicity caused by these elements in the area of study.
Conclusions
It is concluded from this study that the lithologies of the area have played a major role in the enrichment of various HMs in the soils and plants. The high concentrations of Cr and Ni in soils and plants can be attributed to the mafic and ultramafic rocks of the Mingora-Shangla mélange zone and Kohistan island arc in the Mingora and Kabal areas. Furthermore, the elevated concentrations of HMs in soils and plants can cause serious health risks as these HMs reach to human beings through ingestion of contaminated food. This study suggests that detail investigations are needed to assess the level of daily intake of HMs by inhabitants through various pathways.
References
Adriano DC (1986) Trace elements in the terrestrial environment. Springer, New York
Adriano DC (1992) Biogeochemistry of trace metals. Lewis, Boca Raton
Adriano DC (2001) Trace elements in terrestrial environments; biogeochemistry, bioavailabilty and risk of metals, 2nd edn. Springer, New York
Adriano DC, Hen ZS, Yang SS (1994) Biogeochemistry of trace elements. Sci Tech Lett 60:1–613
Afridi AG, Khan RN, Shah H, Waliullah (1995) Regional geological map of the Charbagh quadrangle, District Swat, NWFP, Pakistan. Geol Surv Pak Peshawar
Alloway BJ (1990) Heavy metals in soils. Halstead Press, New York
Arif M, Jan MQ (1993) Chemistry of chromite and associate phases from the Shangla ultramafic body in the Indus suture zone of Pakistan. In: Treloar PJ, Searle MP (eds) Himalayan tectonics. Geological Society, London, Special Publication 74, pp 101–112
Baker AJM, McGrath SP, Reeves RD, Smith JAC (2000) Metal hyperaccumulator plants: a review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In: Terry N, Banuelos G (eds) Phytoremediation of contaminated soil and water. Lewis Publishers, Boca Raton
Bard JP, Maluski H, Matte P, Proust F (1980) The Kohistan sequence; Crust and mantle of an abducted island arc. Geol Bull Univ Peshawar 13:87–93
Bartlett RJ, James B (1979) Behavior of chromium in soils. III. Oxidation. J Environ Qual 8:31
Begum S (2008) Environmental studies of soils, waters and plants associated with the mafic and ultramafic roks in Kabal-Mingora area, Swat, northern Paksitan. Unpub M.Phil Thesis, University of Peshawar
Bennetta JP, Chiriboga E, Coleman J, Waller DM (2000) Heavy metals in wild rice from northern Wisconsin. Sci Total Environ 246:261–269
Bohn HL, McNeal BL, O’Connor GA (2001) Soil chemistry, 3rd edn. Wiley, New York
Brooks RR (1983) Biogeochemical methods of prospecting for minerals. Wiley, New York
Brooks RR (1987) Serpentine and its vegetation—a multidisciplinary approach. Dioscorides Press, Portland
Brooks RR (1998) Biogeochemistry and hyperaccumulators. In: Brooks RR (ed) Plants that hyperaccumulate heavy metals. CAB International, USA
Brooks RR, Yang XH (1984) Elemental level and relationships in the endemic serpentine flora of the Great Dykes, Zimbabwe and their significance as controlling factors in the flora. Taxon 33:392–399
Broyer TC, Johnson CN, Paull RE (1972) Some aspects of lead in plant nutrition. Plant Soil 36:301
Cannon HL (1976) Lead in vegetation. In: Lorering TG (ed) Lead in environment, US Geol Surv Prof Pap 957, p 23
Del Río M, Font R, Almela C, Vélez D, Montoro R, Bailón ADH (2002) Heavy metals and arsenic uptake by wild vegetation in the Guadiamar river area after the toxic spill of the Aznalcóllar mine. J Biotechnol 98:125–137
Del Río M, Font R, Moreno-Rojas R, Bailón ADH (2006) Uptake of lead and zinc by wild plants growing on contaminated soils. Indust Crops Prod 24:230–237
Di Pietro JA (1991) Metamorphic pressure-temperature conditions of Indian plate rocks South of the Main Mantle Thrust, Lower Swat, Pakistan. Tectonics 10:742–757
Di Pietro JA, Pogue KR, Lawrence RD, Baig MS, Hussain A, Ahmad I (1993) stratigraphy south of the Main Manle Thrust, Lower Swat, Pakistan. In: Treloar PJ, Searle MP (eds) Himalayan Tectonics. Geological Society, London, Special Publication 73, pp 207–220
Di Pietro JA, Pogue KR, Hussain A, Ahmad I (1999) Geological map of the Indus syntaxis and surrounding area, northwest Himalaya, Pakistan. Geol Soc Am Sp Pap 328:159–178
Dinelli E, Lombini A, Simoni A, Ferrari C (1997) Heavy Metals in serpentine soils of selected outcrops of Piacenza and Parma provinces (northern Apennines, Italy). Miner Petrogr Acta 40:241–255
Dwivedi S, Srivastava S, Mishra S, Dixit B, Kumar A, Tripathi RD (2008) Screening of native plants and algae growing on fly-ash affected areas near National Thermal Power Corporation, Tanda, Uttar Pradesh, India for accumulation of toxic heavy metals. J Hazard Mater. doi:10.1016/j.jhazmat
Figueroa JAL, Wrobel K, Afton S, Caruso JA, Corona JFG, Wrobel K (2008) Effect of some heavy metals and soil humic substances on the phytochelatin production in wild plants from silver mine areas of Guanajuato, Mexico. Chemosphere 70:2084–2091
Gough LP, Shacklette HT, Case AA (1979) Element concentrations toxic to plants, animals and man. US Geol Surv Bull 1466:80
Gupta DK, Rai UN, Tripathi RD, Inouhe M (2002) Impacts of flyash on soil and plant responses. J Plant Res 115:401–409
Hajiboland R, Manafi MH (2007) Flora of heavy metal-rich soils in NW Iran and some potential hyper-accumulator and accumulator species. Acta Bot Croat 66(2):177–195
Hawkes HE, Webb JS (1962) Geochemistry in mineral exploration. Harper and Row, New York
Hernández AJ, Pastor J (2008) Relationship between plant biodiversity and heavy metal bioavailability in grasslands overlying an abandoned mine. Environ Geochem Health 30:127–133
Jala S, Goyal D (2006) Fly-ash as a soil ameliorant for improving crop production—a review. Biores Technol 97:1136–1147
Jan MQ (1979) Petrography of the amphibolites of Swat and Kohistan. Geol Bull Univ Peshawar 11:51–64
Jan MQ (1988) Geochemistry of amphibolites from the southern part of the Kohistan arc, N. Pakistan. Miner Mag 52:147–159
Jan MQ, Jabeen N (1990) A review of mafic-ultramafic plutonic complexes in the Indus suture zone of Pakistan. Phys Chem Earth 17:93–113
Jan MQ, Tahirkheli RAK (1969) The geology of the lower part of Indus Kohistan, Swat. Geol Bull Univ Peshawar 4:1–13
Jeffery PG, Hutchison D (1986) Chemical methods of rock analysis. Pergamon Press, USA
Jones JB (1972) Plant tissue analysis for micronutrients. In: Mortvedt JJ, Giorando PM, Lindsay WL (eds) Micronutrients in agriculture. Soil Science Society of America, Madison, p 319
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants, vol 3rd. CRC Press LLC, New York
Kazmi AH, Lawrence RD, Dawood H, Snee LW, Hussain SS (1984) Geology of the Indus suture zone in the Mingora-Shangla area of Swat, northern Pakistan. Geol Bull Univ Peshawar 17:127–143
Khan S, Cao Q, Chen BD, Zhu YG (2006) Humic acids increase the phytoavailability of Cd and Pb to wheat plants cultivated in freshly spiked, contaminated soil. J Soils Sed 6(4):236–242
Khan S, Lin A, Zhang S, Hu Q, Zhu YG (2008) Accumulation of polycyclic aromatic hydrocarbons and heavy metals in lettuce grown in the soils contaminated with long-term wastewater irrigation. J Hazard Mater 152:506–515
Kifyatullah Q, Shah MT, Irfan M (2001) Biogeochemical and environmental study of the Chromite-rich ultramaffic terrain of Malakand area, Pakistan. Environ Geol 40:1482–1486
Kupper H, Lombi E, Zhao F, McGrath SP (2000) Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 212:75–84
Lawrence RD, Kazmi AH, Snee LW (1989) Geological setting of the emerald deposits. In: Kazmi AA, Snee LW (eds) Emeralds of Pakistan: geology, gemology and genesis. Van Nostrand Reinhold, New York, pp 13–38
Legittimo PC, Dueshi L, Martini M (1995) Plant species as indicators of geochemical anomalies: experiences on aquifoliu (Holly). Environ Geol 25:114–118
Levinson AA (1974) Introduction to exploration geochemistry. Applied Publishing, Wilmette
Lindsay WL (1972) Inorganic phase equilibria of micronutrients in soils. In: Lindsay WL, Mortveddt JJ, Giordano PM (eds) Micronutrients in agriculture. Soil Science Society of America, Madison
Lottermoser BG (1997) Natural enrichment of top-soils with chromium and other heavy metals, Port Macquarie, New South Wales, Australia. Aust J Soil Res 35:165–176
Macnicol RD, Beckett PHT (1985) Critical tissue concentrations of potentially toxic elements. Plant Soil 85:107
Mapanda F, Mangwayana EN, Nyamangara J, Giller KE (2007) Uptake of heavy metals by vegetables irrigated using wastewater and the subsequent risks in Harare, Zimbabwe. Phys Chem Earth 32:1399–1405
Martin NR, Siddiqui SFA, King BH (1962) A geological reconnaissance of the region between the lower Swat and Indus river of Pakistan. Geol Bull Punjab Univ 2:1–13
McBride MB (1994) Environmental geochemistry of soils. Oxford University Press, New York
Mertz W, Angino EF, Cannon HI, Mambidge KM, Voors AW (1974) Chromium. In: Mertz W (ed) Geochemistry and environment. NAS, Washington DC
Mills T, Arnold B, Sivakumaran S, Northcott G, Vogeler I, Robinson B, Norling C, Leonil D (2006) Phytoremediation and long-term site management of soil contaminated with pentachlorophenol (PCP) and heavy metals. J Environ Manage 79:232–241
Mishra D, Kar M (1974) Nickel in plant growth and metabolism. Bot Rev 40:395
Olko A, Abratowska A, Zylkowska J, Wierzbicka M, Tukiendorf A (2008) Armeria maritime from a calamine heap—initial studies on physiologic metabolic adaptations to metal-enriched soil. Ecotox Environ Safety 69:209–218
Petrunina NS (1974) Geochemical ecology of plants from provinces of high trace element contents. In: Problems of geochemical ecology of organisms, Izd. Moscow
Pogue KR, DiPietro JA, Khan SR, Hughes SS, Dilles JH, Lawrence RD (1992) Late Paleozoic rifting in northern Pakistan. Tectonics 11:871–883
Pollard AH, Powel KD, Harper FA, Smith AC (2002) The genetic basis of metal hyperaccumulation in plants. Crit Rev Plant Sci 21:539–566
Price CA, Clark HE, Funkhouser EA (1972) Functions of micronutrients in plants. In: Giordano PM, Lindsay WL, Mortvedt JJ (eds) Micronutrients in agriculture. Soil Science Society of America, Madison
Reeves R (1992) The hyperaccumulator of nickel by serpentine plants. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic (serpentine) soils. Intercept Ltd, Andover, pp 253–277
Reeves R, Baker AJM, Borhidi A, Berazain R (1996) Nickle accumulating plants from the ancient serpentine soils of Cuba. New Phytol 133:217–224
Reeves R, Baker AJM, Borhidi A, Berazain R (1999) Nickle hyperaccumulation in the serpentine flora of Cuba. Ann Bot 83:29–38
Robinson BH, Chiarucci A, Brooks RR, Petit D, Kirkman JH, Gregg PEH, De Dominicis V (1997) The nickel hyperaccumulator plant Alyssum bertolonii as a potential agent for phytoremediation and phytomining of nickel. J Geochem Explor 59:75–86
Rose AW, Hawkes HE, Webb JS (1979) Geochemistry in mineral exploration. Academic Press, New York
Ryan J, Estefan G, Rashid A (2001) Soil and plant and analysis: laboratory manual. ICARDA, Islamabad
Shah MT, Majid M, Hamidullah S, Shervais JW (1992) Petrochemistry of amphibolites from the Shergarh Sar area, Allai Kohistan, N. Pakistan. Kashmir J Geol 10:123–139
Shah MT, Kifyattullah Q, Irfan M (2004) Pedo and biogeochemical study of zinc–lead deposits of the Besham area, northern Pakistan; its implication in mineral exploration and environmental degradation. Environ Geol 45:544–549
Treloar PJ, Broughton RD, Williams MP, Coward MP, Windley BF (1989a) Deformation, metamorphism and imbrication of the Indian plate south of the Main Mantle Thrust, North Pakistan. J Met Geol 7:111–125
Treloar PJ, Coward MP, Willams MP, Khan MA (1989b) Basement cover imbrication south of the Main Mantle Thrust, north Paksitan. In: Mailinconico LL, Lillee RJ (eds) Tectonics of the Western Himalayas. Geological Society of America, Special Paper 232, pp 137–152
Treloar PJ, Brodie KH, Coward MP, Jan MQ, Knipe RJ, Rex DC, Williams MP (1990) The evolution of the Kamila shear zone, Kohistan, Pakistan. In: Salisbury MH, Fountain DM (eds) Exposed cross-sections of the Continental crust. Kluwer Academic Press, Amsterdam, pp 175–214
Whiting SN, Leake J, McGrath SP, Baker AJM (2000) Positive response to Zn and Cdby roots of the Zn and Cd hyperaccumulator Thlaspi caerulescens. New Phytol 145:199–210
Xio WL, Luo CL, Chen YH, Shen ZG, Li XD (2008) Bioaccumulation of heavy metals by wild plants growing on copper mine spoils in China. Commun Soil Sci Plant Anal 39:315–328
Zalecka R, Wierzbicka M (2002) The adaption of Dianthus carthusianorum L. (Caryophyllaceae) to growth on a zinc-lead heap in southern Poland. Plant Soil 246:249–257
Zhu YG, Chen SD, Yang JC (2004) Effects of soil amendments on lead uptake by two vegetable crops from a lead-contaminated soil from Anhui, China. Environ Int 30:351–356
Zimdahl RL, Koeppe DE (1977) Uptake by plants. In: Bogges WR, Wixso BG (eds) Lead in the environment, Report Nation Sci Found, Washington DC, 99
Acknowledgments
The authors would like to thank Mr. Asadullah, taxonomist, Botany Department, University of Peshawar for plants identification and classification. This study was financially supported by the Director of NCE in Geology, University of Peshawar.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, M.T., Begum, S. & Khan, S. Pedo and biogeochemical studies of mafic and ultramfic rocks in the Mingora and Kabal areas, Swat, Pakistan. Environ Earth Sci 60, 1091–1102 (2010). https://doi.org/10.1007/s12665-009-0253-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-009-0253-8